Intestinal Carriage of Extended Spectrum Beta-Lactamase-Producing Salmonella enterica from Chickens and Poultry Farmers in Dschang, in the Western Region of Cameroon
Abstract
1. Introduction
2. Results
2.1. Distribution of Salmonella enterica Isolates
2.2. Susceptibility of SE Isolates to Antimicrobials
2.3. Distribution of ESBL-Producing SE Carriage
2.4. Associated Factor Analysis for Intestinal Carriage of SE ESBL Producers
3. Discussion
4. Materials and Methods
4.1. Study Description, Sampling Method and Selection Criteria
4.2. Sample Collection and Processing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly prevalent multidrug-resistant salmonella from chicken and pork meat at retail markets in Guangdong, China. Front. Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Jajere, S. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Nzouankeu, A.; Fonkoua, M.; Wouafo, M.; Njine, T.; Aidara-Kane, A.; Ngandjio, A. Molecular characterization of multidrug resistant Salmonella from chicken and humans in Yaounde. KEI J. 2016, 4, 1–29. [Google Scholar] [CrossRef]
- FAO. Zoonotic Diseases Spotlight: The Case for an Expert Elicitation Protocol on Zoonoses in Egypt; Food and Agricultural Organisation of the United Nations: Rome, Italy, 2018; Available online: https://www.fao.org/3/i8476en/I8476EN.pdf (accessed on 18 December 2019).
- Monrad, C. Le Microbiote Intestinal et Risque Cardiovasculaire. Ph.D. Thesis, Université de Mohammed v de Rabat, Morocco, Rabat, 2019. Available online: http://ao.um5.ac.ma/xmlui/handle/123456789/17521 (accessed on 27 January 2020).
- Berger, D.; Smith, F.; Sabesan, V.; Huynh, A.; Norton, R. Paediatric salmonellosis differences between tropical and sub-tropical regions of Queensland, Australia. Trop. Med. Infect. Dis. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Runyon, M.; Herrman, T.; Phillips, R.; Hsieh, J. Review of Salmonella detection and identification methods: Aspects of rapid emergency response and food safety. Food Control 2015, 47, 264–276. [Google Scholar] [CrossRef]
- Eng, S.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Hardy, A. Salmonella: A continuing problem. Postgrad. Med. J. 2004, 80, 541–545. [Google Scholar] [CrossRef]
- Mouiche, M.; Moffo, F.; Akaochere, K.; Okah-Nnane, H.; Mapiefou, P.; Ndze, N.; Mapiefou, N.P.; Ndze, V.N.; Wade, A.; Djuikwo-Teukeng, F.F.; et al. Antimicrobial resistance from a one health perspective in Cameroon: A systematic review and meta-analysis. BMC Public Health 2019, 19, 1135. [Google Scholar] [CrossRef]
- Smoglica, C.; Angelucci, S.; Farooq, M.; Antonucci, A.; Marsilio, F.; Di Francesco, C. Microbial community and antimicrobial resistance in fecal samples from wild and domestic ruminants in Maiella National Park, Italy. One Health 2022, 15, 100403. [Google Scholar] [CrossRef]
- Rao, S.; Linke, L.; Magnuson, R.; Jauch, L.; Hyatt, D. Antimicrobial resistance and genetic diversity of Staphylococcus aureus collected from livestock, poultry and humans. One Health 2022, 15, 100407. [Google Scholar] [CrossRef]
- Metuor, D. Caractérisations Moléculaire et Cinétique des Types de β-Lactamases à Spectre Elargi (BLSE) de Souches Bactériennes Collectées au Centre Hospitalier Universitaire Pédiatrique Charles De Gaulle (CHUP-CDG) de Ouagadougou. Ph.D. Thesis, Université de Ouagadougou, Ouagadougou, Burkina Faso, 2014. Available online: http://www.Labiogene.org/spip.php?article365 (accessed on 31 January 2020).
- Malijan, G.; Howteerakul, N.; Ali, N.; Siri, S.; Kengganpanich, M.; Nascimento, R.; Booton, R.D.; Turner, K.M.E.; Cooper, B.S.; Meeyai, A. A scoping review of antibiotic use practices and drivers of inappropriate antibiotic use in animal farms in WHO Southeast Asia region. One Health 2022, 15, 100412. [Google Scholar] [CrossRef]
- Djuikoue, I.; Woerther, P.-L.; Toukam, M.; Burdet, C.; Ruppe, E.; Gonsu, H.; Fokunang, C.; El Mniai, A.; Larissa, K.; Constant Pieme, A.; et al. Intestinal carriage of Extended spectrum Beta-lactamase producing E. coli in women with urinary tract infections, Cameroon. J. Infect. Dev. Ctries. 2016, 10, 1135–1139. [Google Scholar] [CrossRef]
- Da Silva, L.; Cardoso, B.; Fontana, H.; Esposito, F.; Cortopassi, S.; Sellera, F. Human pandemic K27-ST392 CTX-M-15 extended-spectrum β-lactamase-positive Klebsiella pneumoniae: A one health clone threatening companion animals. One Health 2022, 15, 100414. [Google Scholar] [CrossRef]
- Mairi, A.; Touati, A.; Ait Bessai, S.; Boutabtoub, Y.; Khelifi, F.; Sotto, A.; Lavigne, J.-P.; Pantel, A. Carbapenemase-producing Enterobacteriaceae among pregnant women and newborns in Algeria: Prevalence, molecular characterization, maternal-neonatal transmission, and risk factors for carriage. Am. J. Infect. Control. 2019, 47, 105–108. [Google Scholar] [CrossRef]
- Wilson, H.; Török, E. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb. Genom. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Iossa, G.; White, P.C. The natural environment: A critical missing link in national action plans on antimicrobial resistance. Bull. World Health Organ. 2018, 96, 858–860. [Google Scholar] [CrossRef]
- Alonso, C.A.; Zarazaga, M.; Sallem, B.; Jouini, A.; Slama, B.; Torres, C. Antibiotic resistance in Escherichia coli in husbandry animals: The African perspective. Appl. Microbiol. 2017, 64, 318–334. [Google Scholar] [CrossRef]
- Karaiskos, I.; Giamarellou, H. Carbapenem-sparing strategies for ESBL producers: When and how. Antibiotics 2020, 9, 23. [Google Scholar] [CrossRef]
- Garcia, C.; Hinostroza, N.; Astocondor, L.; Ochoa, T.; Jacobs, J. Characterization of ESBL-Producing Salmonella enterica serovar Infantisinfection in humans, Lima, Peru. Am. Soc. Trop. Med. Hyg. 2019, 101, 746–748. [Google Scholar] [CrossRef]
- Shigemura, H.; Sakatsume, E.; Sekizuka, T.; Yokoyama, H.; Hamada, K.; Etoh, Y.; Carle, Y.; Mizumoto, S.; Hirai, S.; Matsui, M.; et al. Food workers as a reservoir of extended spectrum cephalosporin-resistant salmonella in Japan. Appl. Environ. Microbiol. 2020, 4, e00072-20. [Google Scholar] [CrossRef]
- Andoh, L.; Dalsgaard, A.; Obiri-Danso, K.; Newman, M.; Barco, L.; Olsen, J. Prevalence and antimicrobial resistance of Sal-monella serovars isolated from poultry in Ghana. Epidemiol. Infect. 2016, 144, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Orum, T.; Ishola, O.; Adebowale, O. Occurrence and antimicrobial susceptibility patterns of Salmonella species from poultry farms in Ibadan, Nigeria. Afr. J. Lab. Med. 2022, 11, a1606. [Google Scholar] [CrossRef] [PubMed]
- Adel, W.; Ahmed, A.; Hegazy, Y.; Torky, H.; Shimamoto, T. High Prevalence of ESBL and Plasmid-Mediated Quinolone Resistance Genes in Salmonella enterica Isolated from Retail Meats and Slaughterhouses in Egypt. Antibiotics 2021, 10, 881. [Google Scholar] [CrossRef] [PubMed]
- Raseala, C.; Ekwanzala, M.; Momba, M. Shared Extended-Spectrum β-Lactamase-Producing Salmonella Serovars between Agricultural and Aquatic Environments Revealed through invA Amplicon Sequencing. Microorganisms 2020, 8, 1898. [Google Scholar] [CrossRef]
- Igbinosa, E.; Beshiru, A.; Igbinosa, I.; Okoh, A. Antimicrobial resistance and genetic characterisation of Salmonella enterica from retail poultry meats in Benin City, Nigeria. LWT 2022, 169, 114049. [Google Scholar] [CrossRef]
- Ngogang, M.; Ernest, T.; Kariuki, J.; Mouliom, M.; Ngogang, J.; Wade, A.; van der Sande, M.A.B. Microbial contamination of chicken litter manure and antimicrobial resistance threat in an urban area setting in Cameroon. Antibiotics 2020, 10, 20. [Google Scholar] [CrossRef]
- Mia, M.; Hasan, M.; Pory, F. Occupational exposure to livestock and risk of tuberculosis and brucellosis: A systematic review and meta-analysis. One Health 2022, 15, 100432. [Google Scholar] [CrossRef]
- Société Française de Microbiologie. Référentiel en Microbiologie Médicale, 2nd ed.; Société Française de Microbiologie: Paris, France, 2019. [Google Scholar]
- Popoff, M.Y.; Le Minor, L. Formules Antigéniques des Sérovars de Salmonella; World Health Organisation (WHO Collaborating Centre for Reference and Research on Salmonella): Geneva, Switzerland, 1997. [Google Scholar]
- Strockbine, N.; Bopp, C.; Fields, P.; Kaper, J.; Nataro, J. Escherichia, Shigella, and Salmonella. Man. Clin. Microbiol. 2015, 15, 685–713. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- EUCAST. Recommendations 2020; Société Française de Microbiologie: Paris, France, 2020; Available online: https://www.sfm-microbiologie.org/2020/10/02/casfm-eucast-v1-2-octobre-2020/ (accessed on 1 August 2020).
- EUCAST. Recommendations Vétérinaires 2020. Société Française de Microbiologie: Paris, France, 2020; Available online: https://www.sfm-microbiologie.org/2020/09/09/casfm-veterinaire-2020/ (accessed on 1 August 2020).
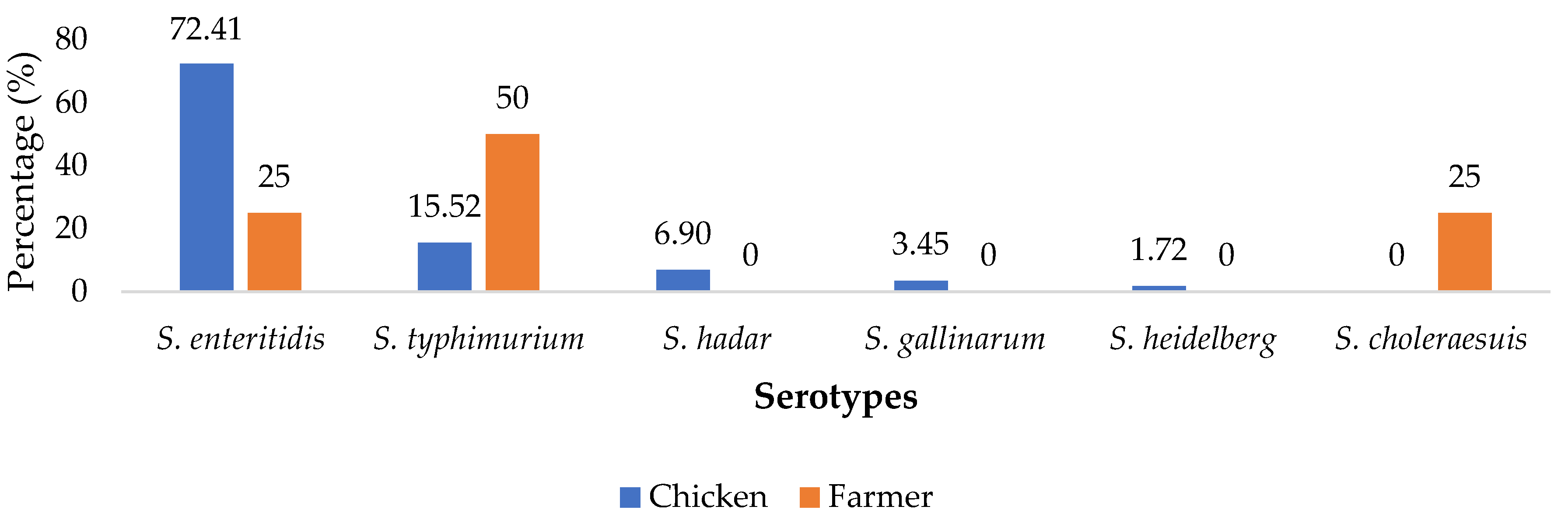
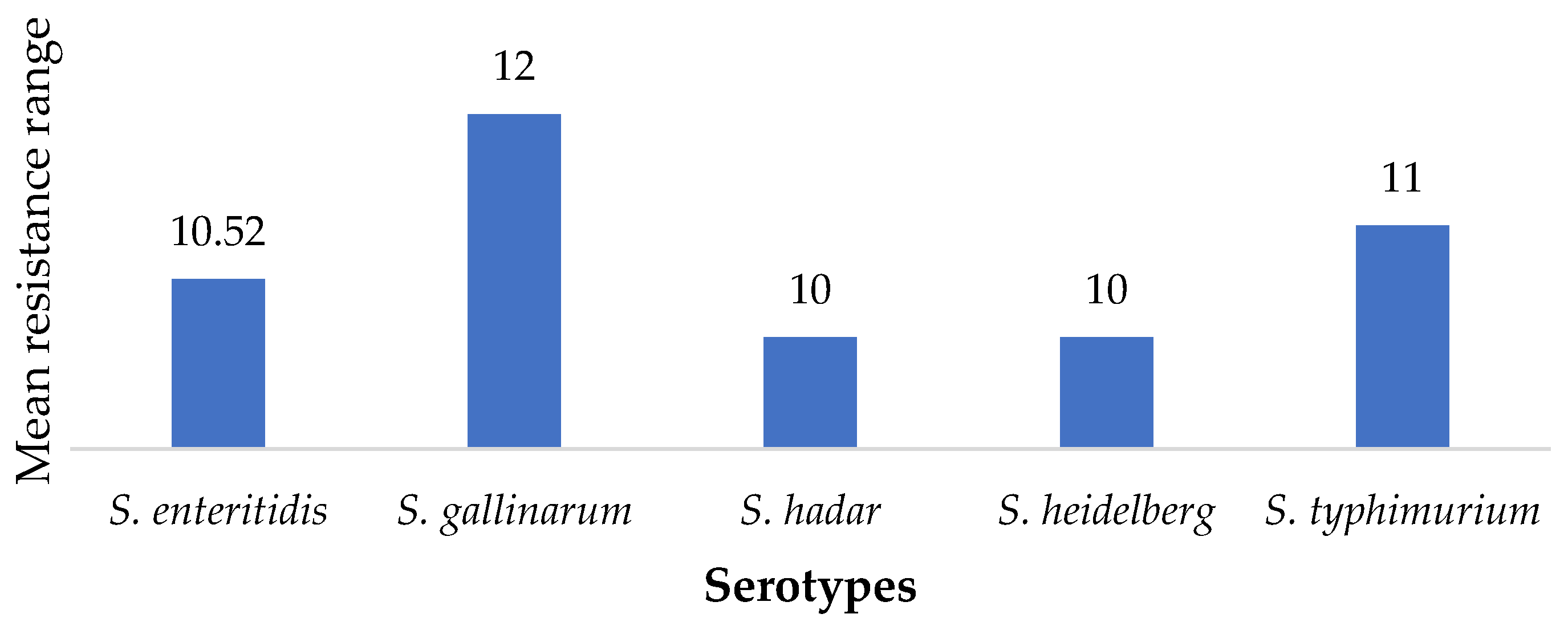
| Sample Units | Number Sampled | Number of Isolates | Percentage (%) | CI |
|---|---|---|---|---|
| Chicken | 416 | 232 | 55.77 | 51.00, 60.54 |
| Farmers | 72 | 16 | 22.22 | 12.62, 31.82 |
| Antibiotics | Population | Susceptibility to Antimicrobials (%) | ||
|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||
| Amoxicilline/clavulanic acid | Chicken | 53.45 | 0 | 46.55 |
| Farmers | 50 | 0 | 50 | |
| Amoxicilline | Chicken | 24.14 | 0 | 75.86 |
| Farmers | 25 | 0 | 75 | |
| Ceftazidime | Chicken | 67.24 | 3.45 | 29.31 |
| Farmers | 50 | 25 | 25 | |
| Cefoxitine | Chicken | 1.72 | 5.17 | 93.10 |
| Farmers | 25 | 0 | 75 | |
| Cefepime | Chicken | 1.72 | 0 | 98.28 |
| Farmers | 0 | 0 | 100 | |
| Cefotaxime | Chicken | 58.62 | 5.17 | 36.21 |
| Farmers | 25 | 0 | 75 | |
| Ticarcilline | Chicken | 32.76 | 5.17 | 62.07 |
| Farmers | 25 | 0 | 75 | |
| Imipenem | Chicken | 94.83 | 1.72 | 3.45 |
| Farmers | 75 | 0 | 25 | |
| Ceftriaxone | Chicken | 67.24 | 1.72 | 31.03 |
| Farmers | 50 | 0 | 50 | |
| Ciprofloxacine | Chicken | 3.45 | 6.90 | 89.66 |
| Farmers | 0 | 0 | 100 | |
| Nalidixic acid | Chicken | 12.07 | 0 | 87.93 |
| Farmers | 0 | 0 | 100 | |
| Aztreonam | Chicken | 53.45 | 0 | 46.55 |
| Farmers | 50 | 0 | 50 | |
| Gentamicine | Chicken | 50 | 0 | 50 |
| Farmers | 50 | 0 | 50 | |
| Amikacine | Chicken | 63.79 | 0 | 36.21 |
| Farmers | 75 | 0 | 25 | |
| Tetracycline | Chicken | 0 | 0 | 100 |
| Farmers | 0 | 0 | 100 | |
| Cotrimoxazole | Chicken | 3.45 | 6.90 | 89.66 |
| Farmers | 0 | 0 | 100 | |
| Ceftiofur | Chickens | 5.17 | 8.62 | 86.21 |
| Population | Size (N) | ESBL-Producing SE (ESBL-SE) | ||
|---|---|---|---|---|
| Number (n) | Frequency (%) | Confidence Interval (%) | ||
| Chichens | 416 | 96 | 23.08 | [13.76; 32.40] |
| Farmers | 72 | 4 | 5.55 | [0.26; 10.84] |
| Variable | ESBL (%) | OR | OR (CI 95%) | p-Value | |
|---|---|---|---|---|---|
| Feed | No | 24 (15.00) | 1 | Ref | |
| Yes | 72 (10.71) | 0.68 | 0.17–2.78 | 0.59 | |
| Veterinary doctor | No | 64 (20.51) | 1 | Ref | |
| Yes | 32 (6.15) | 0.25 | 0.07–0.91 | 0.03 | |
| Knowledge of antibioresistance | No | 88 (18.64) | 1 | Ref | |
| Yes | 8 (2.22) | 0.10 | 0.01–0.80 | 0.03 | |
| Crawlspace | No | 56 (15.91) | 1 | Ref | |
| Yes | 40 (8.33) | 0.27 | 0.14–1.63 | 0.06 | |
| Veterinary pharmacy | No | 56 (14.00) | 1 | Ref | |
| Yes | 40 (9.26) | 0.19 | 0.03–0.27 | 0.00 | |
| Litter aspect | Dried | 24 (7.50) | 1 | Ref | |
| Humid | 72 (14.06) | 2.02 | 0.51–7.96 | 0.31 | |
| Mean of antimicrobial administration | Food | 48 (31.58) | 1 | Ref | |
| Water | 48 (11.54) | 0.54 | 0.09–3.12 | 0.49 | |
| Formation in aviculture | No | 56 (10.94) | 1 | Ref | |
| Yes | 40 (12.50) | 1.16 | 0.34–3.95 | 0.80 | |
| Formation in biosecurity | No | 72 (14.06) | 1 | Ref | |
| Yes | 24 (7.50) | 0.50 | 0.08–0.33 | 0.31 | |
| Water | Drilling | 48 (31.58) | 1 | Ref | |
| Well | 48 (10) | 0.51 | 0.05–5.53 | 0.57 | |
| Use of antibiotics as growth factors | No | 40 (12.50) | 1 | Ref | |
| Yes | 56 (10.94) | 0.86 | 0.25–2.92 | 0.05 |
| Variable | No/Yes | Adj OR | OR (CI 95%) | p-Value |
|---|---|---|---|---|
| Veterinary Doctors | No | 1 | ||
| Yes | 1.20 | 0.17–8.27 | 0.85 | |
| Knowledge of Antibioresistance | No | 1 | ||
| Yes | 0.06 | 3.80 × 10−3–0.86 | 0.04 | |
| Veterinary pharmacy | No | 1 | ||
| Yes | 0.21 | 0.04–1.17 | 0.07 |
| Antimicrobial | Family | Abbr | Charge (µg) | Diameter | |
|---|---|---|---|---|---|
| Critical | |||||
| S≥ | R< | ||||
| Amoxicilline/clavulanic acid | Oxapenam | AMC | 20–10 | 19 | 19 |
| Amoxicilline | Amino-penicillin | AMX | 20 | 19 | 19 |
| Ceftazidime | C3G | CAZ | 10 | 22 | 19 |
| Cefoxitine | C2G | FOX | 30 | 19 | 15 |
| Cefepime | C3G | FEP | 30 | 27 | 24 |
| Cefotaxime | C3G | CTX | 5 | 20 | 17 |
| Ticarcilline | Carboxy-penicillin | TIC | 75 | 23 | 20 |
| Imipenem | Carbapenem | IMP | 10 | 22 | 17 |
| Ceftriaxone | C3G | CRO | 30 | 25 | 22 |
| Ciprofloxacine | Fluro-quinolones | CIP | 5 | 25 | 22 |
| Acide nalidixique | Quinolone | NA | 30 | 14 | 14 |
| Aztreonam | Monbactam | ATM | 30 | 26 | 21 |
| Gentamicine | Aminoside | GN | 10 | 17 | 17 |
| Amikacine | Aminoside | AK | 10 | 18 | 18 |
| Tetracycline | Cycline | TE | 30 | 25 | 22 |
| Cotrimoxazole | Diaminopyri-midine | SXT | 1.25–23.75 | 14 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djuikoue, C.I.; Nana, C.D.S.; Nzenya, J.; Tomi, C.; Chounna, N.; Pomte, O.; Pokam, B.D.T.; Apalata, T. Intestinal Carriage of Extended Spectrum Beta-Lactamase-Producing Salmonella enterica from Chickens and Poultry Farmers in Dschang, in the Western Region of Cameroon. Bacteria 2023, 2, 37-47. https://doi.org/10.3390/bacteria2010003
Djuikoue CI, Nana CDS, Nzenya J, Tomi C, Chounna N, Pomte O, Pokam BDT, Apalata T. Intestinal Carriage of Extended Spectrum Beta-Lactamase-Producing Salmonella enterica from Chickens and Poultry Farmers in Dschang, in the Western Region of Cameroon. Bacteria. 2023; 2(1):37-47. https://doi.org/10.3390/bacteria2010003
Chicago/Turabian StyleDjuikoue, Cecile Ingrid, Cedric Dylan Seugnou Nana, Joelle Nzenya, Charlene Tomi, Noemy Chounna, Olivier Pomte, Benjamin D. Thumamo Pokam, and Teke Apalata. 2023. "Intestinal Carriage of Extended Spectrum Beta-Lactamase-Producing Salmonella enterica from Chickens and Poultry Farmers in Dschang, in the Western Region of Cameroon" Bacteria 2, no. 1: 37-47. https://doi.org/10.3390/bacteria2010003
APA StyleDjuikoue, C. I., Nana, C. D. S., Nzenya, J., Tomi, C., Chounna, N., Pomte, O., Pokam, B. D. T., & Apalata, T. (2023). Intestinal Carriage of Extended Spectrum Beta-Lactamase-Producing Salmonella enterica from Chickens and Poultry Farmers in Dschang, in the Western Region of Cameroon. Bacteria, 2(1), 37-47. https://doi.org/10.3390/bacteria2010003






