Abstract
Seed germination, a pivotal stage in the plant life cycle, profoundly impacts crop growth and establishment. However, fluctuating environmental conditions like drought, salinity, severe temperatures, and heavy metal toxicity impede seed germination rates and seedling vigor. Seed priming is a pre-sowing seed treatment that involves the controlled hydration of seeds, proven to improve germination rate and stress resilience. It initiates pre-germinative metabolism, including enzyme activity, antioxidant accumulation, hormone modulation, and cellular repair, without radicle emergence. Recent advancements in seed priming, encompassing the application of nanoparticles, phytohormones, and beneficial microbes, have significantly broadened its potential. Despite its proven benefits, challenges such as reduced seed longevity post-priming and variability in species-specific responses remain. This paper revisits the principles and methodologies of seed priming, highlighting its physiological, biochemical, and molecular mechanisms that enhance germination under stress conditions. Additionally, it addresses current challenges and future research directions for optimizing seed priming as a low-cost, eco-friendly approach to improve crop establishment under adverse environments, thereby supporting resilient and sustainable agriculture.
1. Introduction
Seed germination marks the critical onset of the plant life cycle and plays a pivotal role in determining crop establishment, overall growth success, and productivity [1,2]. However, seed germination and early seedling development are highly susceptible to environmental stressors such as drought, salinity, extreme temperatures, and heavy metal contamination, which pose a significant challenge to global agriculture by impairing seed germination rates, reducing seedling vigor, and ultimately affecting crop yield and food security [3].
To mitigate these challenges, research has focused on the implementation of different strategies such as the application of exogenous substances and the method of application [4,5]. Among the utilized methods, seed priming has emerged as a promising and sustainable approach to improve seed performance under adverse environmental conditions [6,7]. Seed priming is a pre-sowing treatment in which seeds are hydrated under controlled conditions to initiate key metabolic processes required for germination. It is well-reported that priming allows the seed to initiate a series of pre-germinative physio-biochemical and molecular events that enhance its ability to germinate quickly and uniformly upon sowing, even under stress conditions [8].
Seed priming is associated with the early activation of metabolic pathways that are typically initiated only after the onset of germination. These include increased enzyme activity (e.g., α-amylase, proteases) [9], enhanced antioxidant defense systems (e.g., accumulation of superoxide dismutase, catalase, and glutathione) [10], improved hormonal regulation, and cellular repair mechanisms that mitigate damage from oxidative stress [8]. These mechanisms collectively provide a “primed state” in seeds, facilitating accelerated imbibition, effective mobilization of stored reserves, and enhanced tolerance to abiotic stresses. In the last 10 years, substantial progress has been achieved in optimizing seed priming methods to improve their effectiveness and applicability. Traditional techniques such as hydro-priming, osmo-priming, halo-priming, and thermo-priming have been extensively employed in diverse crops with promising results [6,7,8]. Recent advances utilizing nanoparticles (NPs), phytohormones, and plant growth-promoting rhizobacteria (PGPR) as seed priming agents have opened new avenues for improving seed vigor and stress resilience [6,11,12]. For instance, NPs-based priming has demonstrated potential in accelerating seed germination by enhancing nutrient absorption and reactive oxygen species (ROS) scavenging, whereas priming with exogenous hormones such as salicylic acid (SA) or jasmonic acid (JA) not only scavenges ROS but also modulates stress-responsive gene expression. Likewise, microbial priming introduces advantageous bacteria that can inhabit the rhizosphere, improve nutrient absorption, and stimulate systemic resistance to environmental stress [6,11,12,13].
Notwithstanding its advantages, seed priming possesses some limitations. It can diminish seed shelf-life, particularly under suboptimal storage conditions, due to premature metabolic activation and energy depletion [14]. The efficacy also differs among species, cultivars, and seed lots, necessitating crop-specific tuning of variables such as time, temperature, and priming agents [15,16]. Logistical and economic obstacles such as infrastructure deficiencies, farmer education, and alignment with existing practices can impede adoption, particularly in resource-constrained environments. Scalable and user-friendly protocols are essential for widespread adoption. To tackle these problems and fully exploit the advantages of seed priming, a comprehensive understanding of the fundamental physiological, biochemical, and molecular pathways is crucial. Progress in omics technologies, encompassing genomes, transcriptomics, proteomics, and metabolomics, has started to elucidate the intricate regulatory networks associated with seed priming and stress responses [17]. For instance, transcriptomic investigations have detected differentially expressed genes associated with stress signaling, antioxidant defense, and hormone pathways in primed seeds [18,19], whereas proteomic analysis has uncovered alterations in storage proteins, stress-related proteins, and metabolic enzymes [20,21]. These insights can inform the creation of more focused and effective priming tactics, potentially facilitating precision seed treatments customized for particular environmental circumstances and crop needs.
This review seeks to deliver a thorough examination of seed priming as a method to improve seed germination and seedling establishment in the presence of abiotic stressors. It revisits the ideas and methodology of seed priming, clarifying the physio-biochemical and molecular mechanisms that support its advantageous effects. Furthermore, it discusses recent advancements in priming technologies, existing problems in their implementation, and prospective research avenues for enhancing their efficacy and scalability. Finally, this review will enhance our comprehension of seed priming to foster the creation of resilient, sustainable, and climate-adaptive agricultural systems.
2. Methods of Seed Priming
Seed priming entails regulated pre-sowing hydration followed by re-drying to improve germination efficacy, utilizing various chemicals according to particular physiological or environmental circumstances. Seed priming techniques are classified according to the priming agent employed, varying from basic water to intricate biological substances. Established methods include hydro-priming (water-soaking), osmo-priming (utilizing osmotic agents such as PEG), halo-priming (salt solutions), hormo-priming (plant growth regulators), and bio-priming (beneficial microorganisms) [22] (Figure 1). Each strategy addresses distinct physiological pathways, providing customized solutions for enhanced germination, stress resilience, and crop establishment. This section provides a brief overview of various seed priming methods and their comparative advantages.
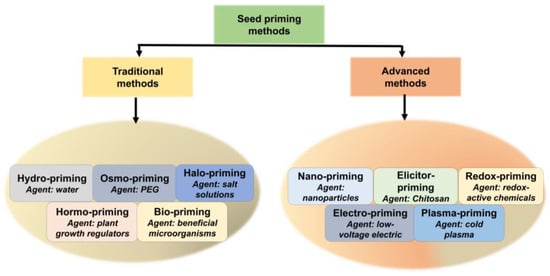
Figure 1.
Traditional and advanced methods of seed priming. Traditional methods encompass the utilization of agents such as water, PEG, saline solutions, plant growth regulators, and microorganisms, whilst advanced methods involve the application of nanoparticles, elicitors, low-voltage electricity, and cold plasma for seed treatment.
Hydropriming is the most straightforward technique of all available approaches. It involves immersing seeds in water for a specific duration, subsequently followed by desiccation. It is economical and extensively utilized, particularly in resource-constrained environments, although it provides restricted regulation of water absorption. Nonetheless, it necessitates no chemicals and is easily adaptable at the farm level. It diminishes metabolic lag time for seed germination and is appropriate for a diverse array of crops, particularly in low-input agricultural systems [23,24].
Osmo-priming employs osmotic solutions like polyethylene glycol (PEG) or mannitol to modulate water absorption [25]. This facilitates the gradual hydration of seeds, preventing premature germination and enhancing synchronization. This technique facilitates accurate control of water potential, inhibiting complete seed imbibition and radicle emergence while simultaneously initiating essential metabolic activities [25,26]. In contrast to hydropriming or biopriming, osmopriming often employs sterile, non-biological fluids, hence reducing the likelihood of microbial contamination during the priming procedure. Osmo-priming is relevant to numerous species, particularly those with desiccation-sensitive seeds or those cultivated in stress-prone conditions [27,28].
Halo-priming entails immersing seeds in saline solutions such as KNO3, NaCl, or CaCl2. This approach regulates water absorption and provides beneficial ions, enhancing germination and stress resilience, especially in saline or drought-prone environments [29]. In contrast to techniques necessitating specialized chemicals or apparatus (e.g., PEG in osmopriming), halo-priming employs easily available, inexpensive salts, rendering it suitable for extensive or resource-constrained applications [29,30]. Halo-priming is relevant to various crops and can be tailored by modifying salt kinds and concentrations to accommodate individual species and environmental conditions.
Hormo-priming is a prevalent priming technique that involves the application of plant growth regulators, including gibberellic acid (GA3), SA, auxins, and ABA, among others. Hormo-priming boosts germination speed and uniformity while also improving seedling vigour in both ideal and stressful situations, including drought, salinity, or low temperature [6]. Hormo-priming affects hormonal equilibrium and stimulates particular metabolic pathways to improve germination and vigor [31]. In contrast to other priming techniques such as hydropriming or osmopriming, hormo-priming provides more specific physiological responses through the modulation of endogenous hormone concentrations. It can activate defense-related pathways, enhance antioxidant enzyme activity, and augment stress resilience more efficiently [6,32].
Bio-priming is an environmentally sustainable technique that integrates seed hydration with advantageous bacteria, like as Trichoderma, Azospirillum, or Pseudomonas spp. to improve nutrient absorption and resilience to stress [13]. It enhances germination and seedling development while providing enduring advantages via rhizosphere colonization [33]. In contrast to hydro-priming or chemical priming and bio-priming offers both physiological and biological benefits, including the generation of phytohormones and the control of pathogens [34,35]. It is more sustainable than osmo-priming or halo-priming, rendering it suitable for organic and low-input agriculture.
Although various priming methods exist, the selection of an optimal seed priming method is contingent upon seed type, environmental circumstances, and resource availability. Factors such as seed coat attributes, dormancy, and moisture sensitivity affect the selection of the approach for priming [36]. Moreover, environmental factors such as salinity, drought, or severe temperatures also influence the selection process. Basic techniques such as hydro-priming may be appropriate for small-scale agriculture, whilst other methods like osmo-priming, bio-priming, or hormo-priming are preferable for commercial enterprises. However, it is crucial to optimize priming conditions, including time, temperature, and treating dosage, for each specific environment. In addition, synchronizing priming techniques with crop needs and regional conditions enhances germination, stress resilience, and seedling vigor.
3. Mechanisms of Seed Priming
Seed priming is acknowledged for improving germination efficacy and seedling vigor by influencing many physiological, biochemical, and molecular mechanisms (Figure 2). To fully understand how priming accelerates germination, it is essential to summarize the key mechanisms involved. This section provides a concise overview of the underlying mechanisms through which seed priming promotes faster germination and improves responses to environmental stress.
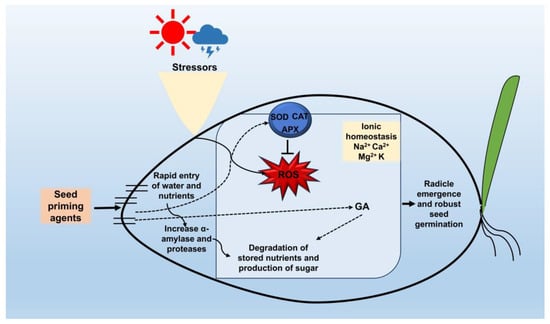
Figure 2.
Mechanisms by which seed priming agents enhance seed germination and seedling growth. Seed priming agents facilitate the fast absorption of water and nutrients, hence enhancing the activities of α-amylase and proteases. Moreover, stress-induced ROS are neutralized by priming-mediated antioxidant enzymes, and priming also modulates the gibberellin (GA) levels. Collectively, these actions diminish the stored nutrients and expedite sugar generation, hence enhancing seed germination and seedling growth. Straight or dotted arrows denote stimulation, enhancement, or activation of a given process. Inhibitory (T-shaped) arrows indicate inhibition of a given process.
3.1. Metabolic Activation
Priming reactivates metabolic processes crucial for germination, including respiration, protein synthesis, and enzyme activation [37]. Priming restores and reactivates mitochondria, enhancing ATP synthesis and supplying the energy necessary for early seed development. Furthermore, priming enhances the synthesis and activity of enzymes such as α-amylase and proteases [38]. These enzymes facilitate the degradation of stored nutrients in the endosperm or cotyledons, rendering them accessible to promote accelerated and robust seedling growth [8] (Figure 2). For example, seed priming enhances the α-amylase and proteases activities in rice under low and high temperatures [9]. In wheat, seed priming enhances carbohydrate and protein mobilization under salt stress by sustaining amylase and protease activities [39].
3.2. Osmotic Adjustment
Seed priming facilitates osmotic adjustment by enabling partial imbibition and controlled metabolic activation without radicle protrusion, thereby enhancing a seed’s capacity to withstand osmotic stress during germination. This controlled hydration phase allows the accumulation of osmo-protectants such as proline, soluble sugars, and inorganic ions, which contribute to maintaining cellular turgor under adverse conditions [40]. In osmo-priming and halo-priming treatments, seeds are exposed to low water potential solutions like PEG or saline water, which stimulate adaptive responses that modulate the seed’s internal osmotic potential. For example, salt-priming demonstrated promising effects on energy compartmentalization and photosystem II efficiency in maize, leading to improved physiological tolerance under salt stress [41]. In addition, sodium acts as an osmotic regulator during barley seed germination under saline conditions, enabling faster and more robust germination compared to purely osmotic stress environments [42]. Similarly, Irving and Zhang [43] showed that pre-soaking wheat seeds in water for 8 h before transferring them to PEG allowed seeds to build internal water reserves, improving their tolerance to subsequent osmotic stress. This suggests that early hydration plays a crucial mechanistic role by establishing favorable internal water gradients absent in unprimed seeds. Advanced imaging techniques such as micro-NMR have further confirmed the dynamic nature of seed hydration [44], yet such biophysical analyses remain underutilized in priming studies. Together, these findings underscore that priming-induced osmotic adjustment is a complex physiological process involving solute accumulation, water uptake regulation, and membrane stabilization, which are essential for enhancing seed performance under stress.
3.3. Hormonal Modulation
Seed priming influences the equilibrium of essential phytohormones that govern germination and initial seedling development. Abscisic acid promotes seed dormancy and suppresses germination [45]. However, priming decreases the ABA levels and has a positive impact on germination [46]. Concurrently, priming elevates GA levels, which facilitate the breakdown of seed storage reserves and stimulate radicle emergence [46,47] (Figure 2). Moreover, priming influences ethylene biosynthesis, which can antagonize ABA signaling and further facilitate germination, particularly under stress conditions [48]. Auxin modulation during priming may facilitate synchronized cell division and elongation, hence promoting consistent seedling development [49]. Collectively, these hormonal adjustments prepare the seed for faster, more synchronized germination and improved stress resilience. For example, studies in rice [50], lettuce [51], and stevia [31] have demonstrated that seed priming with GA and other phytohormones significantly enhances germination rate, seedling vigor, and tolerance to salt stress. This improved salt tolerance is primarily associated with elevated antioxidant enzyme activities and reduced lipid peroxidation, leading to decreased membrane damage. Such hormonal modulation facilitates rapid and uniform germination while enhancing the seedling’s capacity to withstand abiotic stress conditions.
3.4. Enhanced Antioxidant Defense
Seed priming augments the antioxidant defense mechanism by elevating the activity of enzymes like superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidases (APX) [6] (Figure 2). These enzymes collaborate to neutralize reactive oxygen species (ROS) produced after initial imbibition [52]. SOD converts superoxide radicals into H2O2, which is then decomposed by CAT and peroxidases. This enzymatic augmentation safeguards cellular constituents from oxidative damage and aids in preserving redox equilibrium [53]. Moreover, the regulated accumulation of ROS during priming functions as a signaling mechanism, activating genes associated with germination, hormone regulation, and stress responses, thereby facilitating accelerated and more robust seed germination [54].
3.5. Repair of Cellular Structures
Reports indicate that priming activates early repair mechanisms prior to germination, encompassing DNA repair and membrane reorganization [55]. These repairs reinstate genomic stability and augment membrane integrity, hence increasing solute transport and metabolic activity during rehydration [8]. Furthermore, priming facilitates protein quality control via chaperone activity and proteolysis [56]. Collectively, these mechanisms enhance seed viability, accelerate germination, and ensure more consistent seedling establishment.
3.6. Gene Expression and Epigenetic Changes
Seed priming induces notable gene expression and epigenetic modifications that improve seed performance in both stress and non-stress conditions [57]. During priming, regulated hydration initiates metabolic activities without completing germination. Therefore, this partial activation results in the overexpression of genes associated with stress responses, antioxidant defense, DNA repair, and hormone signaling [15,18]. For instance, ABA seed priming has been shown to activate IAA biosynthesis via upregulation of the YUCCA gene family, thereby enhancing auxin-mediated developmental responses and GA signaling pathways under drought stress in Sorghum bicolor. Furthermore, ABA priming modulated the expression of NAC transcription factors, with SbNAC21-1 identified as a key transcriptional activator intricately associated with auxin signaling [58]. Similarly, SA seed priming significantly increased the levels of GA3 in rice (Oryza sativa) seeds by upregulating Oryza sativa gibberellin 3-beta-dioxygenase 1 (OsGA3ox1) and OsGA20ox1, while concurrently reducing ABA accumulation through downregulation of Oryza sativa 9-cis-epoxycarotenoid dioxygenase 1 (OsNCED1). This hormonal reprogramming was linked to enhanced starch degradation capacity in primed seeds under chilling stress [59]. Concurrently, priming affects epigenetic processes like DNA methylation, histone changes, and small RNA activity, which can alter gene expression over extended periods [60]. These epigenetic modifications may establish a type of stress memory, enhancing resilience to subsequent stressors. For example, primed seeds frequently exhibit increased expression of genes associated with ABA and ROS detoxification [54]. This molecular reprogramming enhances germination rate, seedling robustness, and resilience. Consequently, gene expression and epigenetic alterations are pivotal to the improved physiological and adaptive responses facilitated by seed priming [60,61]. Recent studies have highlighted the pivotal role of epigenetic modifications in modulating soybean responses to salt stress. Salt stress-induced priming has been shown to trigger significant alterations in histone modifications, notably the enrichment of histone 3 lysine 4 dimethylation H3K4me2, H3K4me3, and H3K9ac marks, which are associated with transcriptional activation and enhanced salt tolerance [62]. Moreover, priming under salt stress conditions leads to global DNA hypomethylation, thereby contributing to chromatin relaxation and the upregulation of stress-responsive genes [60]. Repressive histone mark H3K27me3 exhibits dynamic redistribution during salt stress, correlating with the silencing of specific gene sets [63]. In addition, the activation of key salt-responsive transcription factors has been linked to epigenetic reprogramming involving both DNA demethylation and increased levels of active histone marks, such as H3K4me3 and H3K9ac [64].
In summary, seed priming prepares seeds for swift and robust germination via a complex array of mechanisms. Comprehending these fundamental processes facilitates the optimization of priming treatments, allowing for customization according to specific crops and environmental conditions, hence enhancing their implementation in sustainable agriculture.
4. Recent Advances and Applications in Seed Priming Agents
Recent years have witnessed substantial progress in seed priming technology, with an increasing focus on sustainability, climate resilience, and precision agriculture. These advancements have resulted in a variety of priming methodologies, the incorporation of molecular instruments, and increased implementation in agricultural systems, particularly in regions confronting abiotic stressors. This section explores cutting-edge developments in seed priming technologies and highlights their emerging applications and outcomes across diverse agricultural systems (Table 1).

Table 1.
Overview of different seed priming methods, their applications, and agronomic or physio-biochemical response across diverse crops.
4.1. Nano-Priming
Nano-priming has emerged as a widely adopted and promising seed priming technique, utilizing nanoparticles (NPs) such as zinc oxide, silver, and carbon-based materials to enhance germination and stress resilience [11]. This approach effectively improves seed germination, seedling vigor, nutrient uptake, antioxidant activity, and overall plant productivity by modulating morphological and physio-biochemical traits [11,79]. Numerous studies have demonstrated that NP-based priming enhances plant performance under both stress and non-stress conditions by mitigating oxidative damage and improving stress tolerance mechanisms [72,80,81]. For example, silver nanoparticles (AgNPs) have been reported to enhance seed germination, photosynthetic efficiency, and antioxidant defense mechanisms in rice [72]. Similarly, seed priming with iron nanoparticles significantly improved germination and yield performance in watermelon (Citrullus lanatus), primarily through the modulation of antioxidant activity [77]. However, despite its benefits, NPs priming may induce phytotoxic effects depending on nanoparticle type, size, concentration, and exposure duration. Reported adverse impacts include reduced germination, inhibited root and shoot growth, delayed flowering, and decreased yield [79,82,83]. Therefore, the safe application of NPs in agriculture necessitates rigorous assessment of their environmental and biological impacts. Establishing appropriate regulatory, ethical, and waste management guidelines is essential to ensure their sustainable use in seed priming.
4.2. Priming with Elicitors
Priming using elicitors involves the application of natural or synthetic substances to seeds, which stimulate the plant’s defensive mechanisms and improve stress resilience [84]. Recently, several elicitors such as SA, jasmonic acid, chitosan, β-aminobutyric acid (BABA), and an array of microbial or plant-derived compounds have been widely used to hasten seed germination. These chemicals enhance the plant’s immune system, resulting in more rapid and robust responses to subsequent biotic or abiotic challenges [85]. Elicitor-primed seedlings frequently demonstrate superior germination rates, augmented antioxidant activity, and heightened tolerance to infections, drought, salinity, and temperature extremes [84,86,87].
Notwithstanding its advantages, elicitor-based seed priming encounters constraints. The efficacy is dependent upon the type of elicitor, its concentration, the species of plant, and the surrounding conditions. Excess use may result in phytotoxicity, inhibited growth, or metabolic disturbances [87]. Furthermore, a deficient comprehension of the fundamental molecular pathways hinders the establishment of consistent methods. High cost, complex preparation procedure, and restricted stability in field situations further impede extensive utilization. Additional study is required to enhance formulations and application techniques for the secure, reliable, and cost-effective implementation in sustainable agriculture.
4.3. Redox Priming
Redox seed priming utilizes redox-active chemicals, including H2O2, NO, and ascorbic acid, to improve seed germination, vigor, and stress resilience. These chemicals modulate ROS levels, activate antioxidant defense mechanisms, and enhance cellular redox equilibrium, thereby equipping plants for environmental stress. For instance, H2O2 priming improves morpho-physiological characteristics in several crops such as wheat, maize, and lettuce under different abiotic stresses [88,89,90]. H2O2 priming enhances plant tolerance to abiotic stresses by activating multiple physiological and biochemical pathways. It improves seed germination under stress by modifying hydrotime parameters and enhancing early water uptake [91]. The priming effect is largely attributed to strengthened antioxidant defenses, better osmotic adjustment, and the regulation of stress-responsive genes. Additionally, H2O2 priming supports improved photosynthesis and stomatal function, contributing to better growth under adverse conditions [92]. These mechanisms collectively enhance seed germination and plant resilience by reducing oxidative damage. Nonetheless, redox priming possesses constraints. The efficacy is significantly dose-dependent, and excessive ROS may lead to oxidative damage instead of signaling advantages [92]. Moreover, responses differ according to species and environmental conditions. The absence of established protocols and insufficient comprehension of long-term impacts impede wider implementation in field environments.
4.4. Electro-Priming
Electro-priming is a seed treatment technique that uses low-voltage electric fields to activate physiological and biochemical processes in seeds, thereby improving germination, seedling vigor, and stress resilience [93]. For instance, electro-priming of wheat (Triticum aestivum) seeds has been shown to enhance seed germination, promote early seedling growth, and modulate phytohormone ABA and IAA levels [69]. The electric field affects ion mobility, enzyme function, and membrane permeability, facilitating accelerated water absorption and metabolic activation [94]. Although electro-priming is environmentally sustainable and free from synthetic or artificial chemicals, it possesses several restrictions. The ideal voltage, exposure duration, and seed variety must be meticulously calibrated, as improper settings may harm seed viability [95,96]. The scalability and expense of specialist equipment may hinder its adoption, particularly in resource-constrained environments. Additional research is required to establish standardized protocols for wider application.
4.5. Plasma-Priming
Plasma seed priming is an innovative, non-chemical method that subjects seeds to cold plasma, a partially ionized gas with reactive species, to enhance germination, seedling vigor, and stress resilience [97]. This technique improves water absorption, stimulates antioxidant enzymes, and perhaps sterilizes seed surfaces by diminishing microbial burden [97,98]. Nonetheless, its practical application is constrained by variables including high equipment expenses, the requirement for specialized knowledge, and variable responses among plant species. Excessive exposure to plasma can damage seed structure and reduce viability [99]. The underlying mechanisms are still poorly understood, impeding protocol standardization and widespread agricultural use. Therefore, additional research is necessary to rectify these shortcomings.
5. Challenges and Future Directions
Although seed priming has the potential to improve germination rates, seedling vigor, and stress tolerance, several challenges restrict its broad implementation. Scalability continues to be a significant obstacle, especially in extensive commercial agriculture, where the uniform processing of huge quantities of seeds presents logistical challenges [16]. In addition, cost-effectiveness is a significant issue, particularly for smallholder farmers in resource-constrained environments who may be deprived of essential infrastructure or resources. Furthermore, the formulation of crop-specific procedures is crucial, as various species and even cultivars within a species exhibit distinct responses to priming treatments. This necessitates a comprehensive study and validation, hence hindering the standardization and widespread implementation of seed priming technology.
Primed seeds typically have a diminished shelf life and may yield uneven outcomes under diverse environmental conditions [14]. The process can be labor-intensive and susceptible to mistakes such as over-priming, thereby compromising seed viability. Furthermore, the potential for microbial contamination during soaking, along with the necessity for specific equipment in certain techniques, introduces complexity.
Future studies should concentrate on the integration of seed priming with precision agriculture technology, including seed coating methods and intelligent delivery systems. Creating priming procedures customized for certain crops, regions, and agricultural systems will enhance adoption. Moreover, integrating seed priming with breeding techniques for stress tolerance can collaboratively enhance agricultural performance throughout climate change. Moreover, the effects of seed priming should be studied at the molecular level, particularly through single-cell analysis, to better understand the underlying mechanisms. As single-cell technologies are gaining increasing attention in plant science [17], applying them to seed priming research could reveal how individual cells respond to treatment, leading to more precise and effective priming strategies.
6. Conclusions
Seed priming has re-emerged as an effective and adaptable technique for improving germination efficiency, seedling vigor, and stress resilience in various crops. The incorporation of priming techniques into crop production systems has substantial potential for enhancing sustainability and food security, as global agriculture confronts unprecedented challenges from climatic variability, soil degradation, and resource constraints. Recent improvements in seed priming, including traditional hydro-priming and osmo-priming as well as innovative techniques like nano-priming, electro-priming, and hormo-priming, clearly exhibit the ability to influence physiological, biochemical, and molecular responses in seeds before germination. These approaches not only improve resistance to abiotic stresses but also favorably affect phytohormone regulation, antioxidant activity, nutrient mobilization, and gene expression. Recent data highlights the significance of priming in triggering epigenetic alterations, indicating possible transgenerational advantages and enduring resilience. In conclusion, current advancements in seed priming provide significant potential to improve crop yield, sustainability, and climate resilience. Ongoing research, innovation, and field validation will be crucial for converting these advancements into practical, farmer-centric solutions.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the Japan Society for the Promotion of Science (JSPS) for supporting the author’s research activities and academic stay at Okayama University, Japan, which significantly contributed to the successful completion of this study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Srivastava, L.M. Seed Germination, Mobilization of Food Reserves, and Seed Dormancy. In Plant Growth and Development; Elsevier: Amsterdam, The Netherlands, 2002; pp. 447–471. ISBN 978-0-12-660570-9. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed Vigour and Crop Establishment: Extending Performance beyond Adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Biswas, S. Influence of Abiotic Stresses on Seed Production and Quality. In Seed Biology Updates; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-813-4. [Google Scholar]
- Feng, D.; Liu, W.; Chen, K.; Ning, S.; Gao, Q.; Chen, J.; Liu, J.; Sun, X.; Xu, W. Exogenous Substances Used to Relieve Plants from Drought Stress and Their Associated Underlying Mechanisms. Int. J. Mol. Sci. 2024, 25, 9249. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Altaf-Un-Nahar, M.; Islam, M.R.; Roy, M.; Rahman, F.; Azam, M.G.; Brestic, M.; Rhaman, M.S.; Karim, M.R. Foliar and Root Applications of Salicylic Acid Alleviate Salinity Stress by Modulating Morpho-Physiological and Biochemical Aspects in Tomato (Solanum lycopersicum). Discov. Plants 2025, 2, 36. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef]
- Paul, S.; Dey, S.; Kundu, R. Seed Priming: An Emerging Tool towards Sustainable Agriculture. Plant Growth Regul. 2022, 97, 215–234. [Google Scholar] [CrossRef]
- Jatana, B.S.; Grover, S.; Ram, H.; Baath, G.S. Seed Priming: Molecular and Physiological Mechanisms Underlying Biotic and Abiotic Stress Tolerance. Agronomy 2024, 14, 2901. [Google Scholar] [CrossRef]
- Monajjem, S.; Soltani, E.; Zainali, E.; Esfahani, M.; Ghaderi-Far, F.; Chaleshtori, M.H.; Rezaei, A. Seed Priming Improves Enzymatic and Biochemical Performances of Rice During Seed Germination under Low and High Temperatures. Rice Sci. 2023, 30, 335–347. [Google Scholar] [CrossRef]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-Priming with H2O2 Alleviates Subsequent Salt Stress by Preventing ROS Production and Amplifying Antioxidant Defense in Cauliflower Seeds and Seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Tania, S.S.; Imran, S.; Rauf, F.; Kibria, M.G.; Ye, W.; Hasanuzzaman, M.; Murata, Y. Seed Priming with Nanoparticles: An Emerging Technique for Improving Plant Growth, Development, and Abiotic Stress Tolerance. J. Soil. Sci. Plant Nutr. 2022, 22, 4047–4062. [Google Scholar] [CrossRef]
- Meel, S.; Saharan, B.S. Enhancing Crop Resilience towards Drought: By Integrating Nanotechnology, Microbiomes, and Growth-Promoting Rhizobacteria. Discov. Agric. 2024, 2, 112. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-Priming of Seeds: Plant Stress Management and Its Underlying Cellular, Biochemical and Molecular Mechanisms. Plant Stress. 2022, 3, 100052. [Google Scholar] [CrossRef]
- Hussain, S.; Zheng, M.; Khan, F.; Khaliq, A.; Fahad, S.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Benefits of Rice Seed Priming Are Offset Permanently by Prolonged Storage and the Storage Conditions. Sci. Rep. 2015, 5, 8101. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of Seed Priming Strategies for Enhancing Salinity Tolerance in Plants: An Overview of the Progress and Achievements. Plant Stress. 2023, 9, 100186. [Google Scholar] [CrossRef]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular Dynamics of Seed Priming at the Crossroads between Basic and Applied Research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Ali, M.; Ye, W.; Li, B. Opportunities and Challenges in Advancing Plant Research with Single-Cell Omics. Genom. Proteom. Bioinform. 2024, 22, qzae026. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Mo, X.; Wang, Y.; Li, Q. Seed Priming with 2,4-Epibrassionolide Enhances Seed Germination and Heat Tolerance in Rice by Regulating the Antioxidant System and Plant Hormone Signaling Pathways. Antioxidants 2025, 14, 242. [Google Scholar] [CrossRef]
- Nguyen, D.-K.; Nguyen, T.-P.; Lin, C.-C.; Ly, T.-T.; Li, Y.-R.; Chang, C.-H.; Nguyen, V.-A.; Trinh, N.-N.; Huang, H.-J. Transcriptome Analysis Reveals the Role of Microbial Volatile 3-Methyl-1-Butanol-Induced Salt Stress Tolerance in Rice (Oryza sativa L.) Seedlings through Antioxidant Defense System. Plant Physiol. Biochem. 2025, 223, 109830. [Google Scholar] [CrossRef]
- Fercha, A.; Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Samperi, R.; Stampachiacchiere, S.; Laganà, A. Comparative Analysis of Metabolic Proteome Variation in Ascorbate-Primed and Unprimed Wheat Seeds during Germination under Salt Stress. J. Proteom. 2014, 108, 238–257. [Google Scholar] [CrossRef]
- Zhang, H.; Hui, G.; Gao, G.; Ali, I.; Tang, M.; Chen, L.; Zhong, X.; Jiang, L.; Liang, T.; Zhang, X. Physiological and Proteomic Analysis of Various Priming on Rice Seed under Chilling Stress. Plants 2024, 13, 2430. [Google Scholar] [CrossRef]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 1–10. ISBN 9789811386244. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-Priming Effects on Seed Germination and Field Performance of Faba Bean in Spring Sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ejaz, S.; Ahmad, M.; Jan, M.; Zafar, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; et al. Hydropriming for Plant Growth and Stress Tolerance. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 373–384. ISBN 9789811386244. [Google Scholar]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Han, G.; Sun, X.; Yang, X. Seed Osmopriming with Polyethylene Glycol (PEG) Enhances Seed Germination and Seedling Physiological Traits of Coronilla varia L. under Water Stress. PLoS ONE 2024, 19, e0303145. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.H.; Musa, Y.; Mustafa, M.; Sjahril, R.; Riadi, M. Comparison between hydro- and osmo-priming to determine period needed for priming indicator and its effect on germination percentage of aerobic rice cultivars (Oryza sativa L.). Agrivita J. Agr. Sci. 2016, 38, 222–230. [Google Scholar] [CrossRef]
- Sumbal, S.; Ali, A.; Nasser Binjawhar, D.; Ullah, Z.; Eldin, S.M.; Iqbal, R.; Sher, H.; Ali, I. Comparative Effects of Hydropriming and Iron Priming on Germination and Seedling Morphophysiological Attributes of Stay-Green Wheat. ACS Omega 2023, 8, 23078–23088. [Google Scholar] [CrossRef]
- Tolrà, R.; González-Cobo, C.; Corrales, I.; Padilla, R.; Llugany, M. Seed Halopriming as an Effective Strategy to Enhance Salt Tolerance in Cakile Maritima: Activation of Antioxidant and Genetic Responses. Antioxidants 2025, 14, 353. [Google Scholar] [CrossRef]
- Hmissi, M.; Krouma, A.; García-Sánchez, F.; Chaieb, M. Potential of Seed Halopriming in the Mitigation of Salinity Stress during Germination and Seedling Establishment in Durum Wheat (Triticum durum Desf.). Plants 2023, 13, 66. [Google Scholar] [CrossRef]
- Janah, I.; Elhasnaoui, A.; Abouloifa, H.; Ait-El-Mokhtar, M.; Ben Laouane, R. Hormonal Priming to Increase Germination of Stevia Rebaudiana Bertoni Seeds in Saline Environments. Int. J. Plant Biol. 2025, 16, 2. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Mohan, V.R. Chemical Seed Priming: Molecules and Mechanisms for Enhancing Plant Germination, Growth, and Stress Tolerance. Curr. Issues Mol. Biol. 2025, 47, 177. [Google Scholar] [CrossRef]
- Jyothi, C.; Masih, S.A.; Maxton, A. Efficiency of Seed Bio-Priming Technique for Drought Management in Mungbean. Environ. Ecol. 2023, 41, 2798–2804. [Google Scholar] [CrossRef]
- Sarkar, D.; Singh, S.; Parihar, M.; Rakshit, A. Seed Bio-Priming with Microbial Inoculants: A Tailored Approach towards Improved Crop Performance, Nutritional Security, and Agricultural Sustainability for Smallholder Farmers. Curr. Res. Environ. Sustain. 2021, 3, 100093. [Google Scholar] [CrossRef]
- Singh, P.; Vaishnav, A.; Liu, H.; Xiong, C.; Singh, H.B.; Singh, B.K. Seed Biopriming for Sustainable Agriculture and Ecosystem Restoration. Microb. Biotechnol. 2023, 16, 2212–2222. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X.; et al. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants 2024, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Elozeiri, A.A. Metabolic Processes During Seed Germination. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3621-7. [Google Scholar]
- Sen, A.; Puthur, J.T. Seed Priming-Induced Physiochemical and Molecular Events in Plants Coupled to Abiotic Stress Tolerance: An Overview. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–316. ISBN 978-0-12-817892-8. [Google Scholar]
- Sghayar, S.; Debez, A.; Lucchini, G.; Abruzzese, A.; Zorrig, W.; Negrini, N.; Morgutti, S.; Abdelly, C.; Sacchi, G.A.; Pecchioni, N.; et al. Seed Priming Mitigates High Salinity Impact on Germination of Bread Wheat (Triticum aestivum L.) by Improving Carbohydrate and Protein Mobilization. Plant Direct 2023, 7, e497. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought Stress Responses and Inducing Tolerance by Seed Priming Approach in Plants. Plant Stress. 2022, 4, 100066. [Google Scholar] [CrossRef]
- Ansari, H.H.; Siddiqui, A.; Wajid, D.; Tabassum, S.; Umar, M.; Siddiqui, Z.S. Profiling of Energy Compartmentalization in Photosystem II (PSII), Light Harvesting Complexes and Specific Energy Fluxes of Primed Maize Cultivar (P1429) under Salt Stress Environment. Plant Physiol. Biochem. 2022, 170, 296–306. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, L.J.; McGill, C.; Matthew, C.; Zhou, D.; Kemp, P. The Effects of Salinity and Osmotic Stress on Barley Germination Rate: Sodium as an Osmotic Regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef]
- Irving, L.J.; Zhang, H. Modelling the Effect of Salt and PEG on Water Uptake in Wheat Seeds. Agronomy 2021, 11, 1660. [Google Scholar] [CrossRef]
- Munz, E.; Rolletschek, H.; Oeltze-Jafra, S.; Fuchs, J.; Guendel, A.; Neuberger, T.; Ortleb, S.; Jakob, P.M.; Borisjuk, L. A Functional Imaging Study of Germinating Oilseed Rape Seed. New Phytol. 2017, 216, 1181–1190. [Google Scholar] [CrossRef]
- Kermode, A.R. Role of Abscisic Acid in Seed Dormancy. J. Plant Growth Regul. 2005, 24, 319–344. [Google Scholar] [CrossRef]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA Priming Improves the Germination and Seedling Growth in Cotton (Gossypium hirsutum L.) via Regulating the Endogenous Phytohormones and Enhancing the Sucrose Metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Tapfumaneyi, L.; Dube, P.; Mavengahama, S.; Ngezimana, W. Effect of Gibberellic Acid and Potassium Nitrate Seed Treatments on the Emergence and Seedling Vigor of Amaranth and Cleome Gynandra. Agrosyst. Geosci. Environ. 2023, 6, e20359. [Google Scholar] [CrossRef]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA Crosstalk with Ethylene and Nitric Oxide in Seed Dormancy and Germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Ellouzi, H.; Ben Slimene Debez, I.; Amraoui, S.; Rabhi, M.; Hanana, M.; Alyami, N.M.; Debez, A.; Abdelly, C.; Zorrig, W. Effect of Seed Priming with Auxin on ROS Detoxification and Carbohydrate Metabolism and Their Relationship with Germination and Early Seedling Establishment in Salt Stressed Maize. BMC Plant Biol. 2024, 24, 704. [Google Scholar] [CrossRef]
- Sour, V.; Sour, P.; Vorn, Y.; Sim, S. Effect of Hormonal Priming on Seed Germination and Initial Growth of Cambodian Rice in Salt Stress Condition. Int. J. Appl. Adv. Multidiscip. Res. 2024, 2, 369–380. [Google Scholar] [CrossRef]
- Hela, M.; Hanen, Z.; Imen, T.; Olfa, B.; Nawel, N.; Raouia, B.M.; Maha, Z.; Wissal, A.; Jun, H.; Abdelali, H.; et al. Combined Effect of Hormonal Priming and Salt Treatments on Germination Percentage and Antioxidant Activities in Lettuce Seedlings. Afr. J. Biotechnol. 2012, 11, 10373–10380. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Peng, J.; Li, F.; Ali, F.; Wang, Z. Regulation of Seed Germination: ROS, Epigenetic, and Hormonal Aspects. J. Adv. Res. 2025, 71, 107–125. [Google Scholar] [CrossRef]
- Aswathi, K.P.R.; Kalaji, H.M.; Puthur, J.T. Seed Priming of Plants Aiding in Drought Stress Tolerance and Faster Recovery: A Review. Plant Growth Regul. 2022, 97, 235–253. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H. Priming the Proteasome to Protect against Proteotoxicity. Trends Mol. Med. 2020, 26, 639–648. [Google Scholar] [CrossRef]
- Louis, N.; Dhankher, O.P.; Puthur, J.T. Seed Priming Can Enhance and Retain Stress Tolerance in Ensuing Generations by Inducing Epigenetic Changes and Trans-generational Memory. Physiol. Plant 2023, 175, e13881. [Google Scholar] [CrossRef]
- Yao, L.; Ni, Y.; Chen, C.; Xiong, W.; Gan, Q.; Jia, X.; Jin, S.; Yang, J.; Guo, Y. Unlocking the Synergy: ABA Seed Priming Enhances Drought Tolerance in Seedlings of Sweet Sorghum Through ABA-IAA Crosstalk. Plant Cell Environ. 2025, 1–18, pce.15575. [Google Scholar] [CrossRef]
- Nie, L.; Song, S.; Yin, Q.; Zhao, T.; Liu, H.; He, A.; Wang, W. Enhancement in Seed Priming-Induced Starch Degradation of Rice Seed Under Chilling Stress via GA-Mediated α-Amylase Expression. Rice 2022, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.; Wang, Q.; Chan, L.; Wang, Z.; Huang, M.; Li, M.; Wong, F.; Lam, H. DNA Hypomethylation Is One of the Epigenetic Mechanisms Involved in Salt-Stress Priming in Soybean Seedlings. Plant Cell Environ. 2024, 1–13, pce.15297. [Google Scholar] [CrossRef]
- Harris, C.J.; Amtmann, A.; Ton, J. Epigenetic Processes in Plant Stress Priming: Open Questions and New Approaches. Curr. Opin. Plant Biol. 2023, 75, 102432. [Google Scholar] [CrossRef]
- Yung, W.; Wang, Q.; Huang, M.; Wong, F.; Liu, A.; Ng, M.; Li, K.; Sze, C.; Li, M.; Lam, H. Priming-induced Alterations in Histone Modifications Modulate Transcriptional Responses in Soybean under Salt Stress. Plant J. 2022, 109, 1575–1590. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, G.; Guo, W.; Wang, W.; Zhao, H.; Gao, T.; Lv, Q.; Yang, X.; Xu, F.; Dong, Y.; et al. Dynamic Changes in Genome-Wide Histone3 Lysine27 Trimethylation and Gene Expression of Soybean Roots in Response to Salt Stress. Front. Plant Sci. 2019, 10, 1031. [Google Scholar] [CrossRef]
- Song, Y.; Ji, D.; Li, S.; Wang, P.; Li, Q.; Xiang, F. The Dynamic Changes of DNA Methylation and Histone Modifications of Salt Responsive Transcription Factor Genes in Soybean. PLoS ONE 2012, 7, e41274. [Google Scholar] [CrossRef]
- BiBi, R.; Elahi, N.N.; Danish, S.; Alahmadi, T.A.; Ansari, M.J. Enhancing Germination and Growth of Canola (Brassica napus L.) through Hydropriming and NaCl Priming. Sci. Rep. 2024, 14, 14026. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; De Man, W.L.; De Proft, M.; Prinsen, E.; Delcour, J.A. The Impact of Hydro-Priming and Osmo-Priming on Seedling Characteristics, Plant Hormone Concentrations, Activity of Selected Hydrolytic Enzymes, and Cell Wall and Phytate Hydrolysis in Sprouted Wheat (Triticum aestivum L.). ACS Omega 2019, 4, 22089–22100. [Google Scholar] [CrossRef]
- Hadia, E.; Slama, A.; Romdhane, L.; Cheikh M’Hamed, H.; Fahej, M.A.S.; Radhouane, L. Seed Priming of Bread Wheat Varieties with Growth Regulators and Nutrients Improves Salt Stress Tolerance Particularly for the Local Genotype. J. Plant Growth Regul. 2023, 42, 304–318. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Samarah, N.H.; Rasheed, E.I. The Role of Chitosan Priming in Induction of GABA Shunt Pathway during Wheat Seed Germination under Salt Stress. Biol. Plant. 2023, 67, 234–248. [Google Scholar] [CrossRef]
- Cecchetti, D.; Pawełek, A.; Wyszkowska, J.; Antoszewski, M.; Szmidt-Jaworska, A. Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size. Agronomy 2022, 12, 1423. [Google Scholar] [CrossRef]
- Mouradi, M.; Bouizgaren, A.; Farissi, M.; Makoudi, B.; Kabbadj, A.; Very, A.-A.; Sentenac, H.; Qaddoury, A.; Ghoulam, C. Osmopriming Improves Seeds Germination, Growth, Antioxidant Responses and Membrane Stability during Early Stage of Moroccan Alfalfa Populations under Water Deficit. Chil. J. Agric. Res. 2016, 76, 265–272. [Google Scholar] [CrossRef][Green Version]
- Chattha, M.U.; Hassan, M.U.U.; Khan, I.; Nawaz, M.; Shah, A.N.; Sattar, A.; Hashem, M.; Alamri, S.; Aslam, M.T.; Alhaithloul, H.A.S.; et al. Hydrogen Peroxide Priming Alleviates Salinity Induced Toxic Effect in Maize by Improving Antioxidant Defense System, Ionic Homeostasis, Photosynthetic Efficiency and Hormonal Crosstalk. Mol. Biol. Rep. 2022, 49, 5611–5624. [Google Scholar] [CrossRef] [PubMed]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming Technology for Enhancing Germination and Starch Metabolism of Aged Rice Seeds Using Phytosynthesized Silver Nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Ben Youssef, R.; Jelali, N.; Martínez-Andújar, C.; Abdelly, C.; Hernández, J.A. Salicylic Acid and Calcium Chloride Seed Priming: A Prominent Frontier in Inducing Mineral Nutrition Balance and Antioxidant System Capacity to Enhance the Tolerance of Barley Plants to Salinity. Plants 2024, 13, 1268. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhital, P.R.; Ranabhat, S.; Poudel, H. Effect of Seed Hydro-Priming Durations on Germination and Seedling Growth of Bitter Gourd (Momordica charantia). PLoS ONE 2021, 16, e0255258. [Google Scholar] [CrossRef]
- Yan, M. Seed Priming Stimulate Germination and Early Seedling Growth of Chinese Cabbage under Drought Stress. S. Afr. J. Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of Broccoli Sprouts (Brassica oleracea L. Var. Italica) Growth and Quality by KCl Seed Priming and Methyl Jasmonate under Salinity Stress. Sci. Hortic. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Seed Priming with Iron Oxide Nanoparticles Modulate Antioxidant Potential and Defense-Linked Hormones in Watermelon Seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Karim, M.R.; Sultana, S.; Altaf-Un-Nahar, M.; Islam, M.R.; Rahman, F.; Pretha, S.J.; Azam, M.G.; Hussain, S.; Yang, X.; Ibrahimova, U.; et al. Integrated Molecular Defense Mitigating Salt Stress in Tomatoes Using Synergistic Signaling Molecules. Physiol. Plant. 2025, 177, e70344. [Google Scholar] [CrossRef] [PubMed]
- Shelar, A.; Singh, A.V.; Chaure, N.; Jagtap, P.; Chaudhari, P.; Shinde, M.; Nile, S.H.; Chaskar, M.; Patil, R. Nanoprimers in Sustainable Seed Treatment: Molecular Insights into Abiotic-Biotic Stress Tolerance Mechanisms for Enhancing Germination and Improved Crop Productivity. Sci. Total Environ. 2024, 951, 175118. [Google Scholar] [CrossRef]
- Imtiaz, H.; Shiraz, M.; Mir, A.R.; Siddiqui, H.; Hayat, S. Nano-Priming Techniques for Plant Physio-Biochemistry and Stress Tolerance. J. Plant Growth Regul. 2023, 42, 6870–6890. [Google Scholar] [CrossRef]
- Faizan, M.; Sharma, P.; Sultan, H.; Alam, P.; Sehar, S.; Rajput, V.D.; Hayat, S. Nano-Priming: Improving Plant Nutrition to Support the Establishment of Sustainable Agriculture under Heavy Metal Stress. Plant Nano Biol. 2024, 10, 100096. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Mahra, S.; Tripathi, S.; Tiwari, K.; Sharma, S.; Mathew, S.; Kumar, V.; Sharma, S. Harnessing Nanotechnology for Sustainable Agriculture: From Seed Priming to Encapsulation. Plant Nano Biol. 2025, 11, 100124. [Google Scholar] [CrossRef]
- Sanchez-Lucas, R.; Bosanquet, J.L.; Henderson, J.; Catoni, M.; Pastor, V.; Luna, E. Elicitor Specific Mechanisms of Defence Priming in Oak Seedlings Against Powdery Mildew. Plant Cell Environ. 2025, 48, 4455–4474. [Google Scholar] [CrossRef]
- Guru, A.; Dwivedi, P.; Kaur, P.; Pandey, D.K. Exploring the Role of Elicitors in Enhancing Medicinal Values of Plants under in Vitro Condition. S. Afr. J. Bot. 2022, 149, 1029–1043. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Murali, M.; Shilpa, N.; Amruthesh, K.N.; Gafur, A.; Antonius, S.; Sayyed, R.Z. Harnessing Abiotic Elicitors to Bolster Plant’s Resistance against Bacterial Pathogens. Plant Stress. 2024, 11, 100371. [Google Scholar] [CrossRef]
- Tania, S.S.; Rahaman, M.M.; Rauf, F.; Suborna, M.A.; Humayun Kabir, M.; Hoque, M.A.; Rhaman, M.S. Seed Priming with Salicylic Acid (SA) and Hydrogen Peroxide (H2O2) Improve Germination and Seedling Growth of Wheat (Triticum aestivum) under Salt Stress. Asian J. Res. Crop Sci. 2021, 6, 60–69. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; Ren, B.; Liu, P.; Zhao, B.; Zhang, J. Effects of Hydrogen Peroxide Priming on Yield, Photosynthetic Capacity and Chlorophyll Fluorescence of Waterlogged Summer Maize. Front. Plant Sci. 2022, 13, 1042920. [Google Scholar] [CrossRef]
- Silva, P.C.C.; Gheyi, H.R.; Jesus, M.J.D.S.D.; Correia, M.R.S.; Azevedo Neto, A.D.D. Seed Priming with Hydrogen Peroxide Enhances Tolerance to Salt Stress of Hydroponic Lettuce. Rev. Bras. Eng. Agríc. Ambient. 2023, 27, 704–711. [Google Scholar] [CrossRef]
- Gammoudi, N.; Nagaz, K.; Ferchichi, A. Hydrotime Analysis to Explore the Effect of H2O2−priming in the Relationship between Water Potential (Ψ) and Germination Rate of Capsicum annuum L. Seed under NaCl− and PEG−induced Stress. Plant Physiol. Biochem. 2021, 167, 990–998. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Karami, S.; Ghanaei, S. Hydrogen Peroxide Priming Promotes Salinity Tolerance in Plants—A Comprehensive Review. Agron. J. 2024, 116, 612–629. [Google Scholar] [CrossRef]
- Faraz Ali, M.; Sajid Aqeel Ahmad, M.; Gaafar, A.-R.Z.; Shakoor, A. Seed Pre-Treatment with Electromagnetic Field (EMF) Differentially Enhances Germination Kinetics and Seedling Growth of Maize (Zea mays L.). J. King Saud. Univ. Sci. 2024, 36, 103184. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Peng, Q.; Sun, X.; Yang, Q.; Song, Z.; Tian, F.; Yan, Y.; Liu, M. Enhancing Maize Seed Resistance to Chilling Stress through Seed Germination and Surface Morphological Changes Using High Voltage Electrostatic Field. Sci. Rep. 2025, 15, 3972. [Google Scholar] [CrossRef]
- Kaur, S.; Vian, A.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Sensitivity of Plants to High Frequency Electromagnetic Radiation: Cellular Mechanisms and Morphological Changes. Rev. Environ. Sci. Biotechnol. 2021, 20, 55–74. [Google Scholar] [CrossRef]
- Mshenskaya, N.S.; Grinberg, M.A.; Kalyasova, E.A.; Vodeneev, V.A.; Ilin, N.V.; Slyunyaev, N.N.; Mareev, E.A.; Sinitsyna, Y.V. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants 2023, 12, 826. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold Plasma Seed Priming Modulates Growth, Redox Homeostasis and Stress Response by Inducing Reactive Species in Tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Dong, Y. Seed Priming with Cold Plasma Mitigated the Negative Influence of Drought Stress on Growth and Yield of Rapeseed (Brassica napus L.). Ind. Crops Prod. 2025, 228, 120899. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).