Field Evaluation of Salt Stress and Fertilization Effects (Organic and Inorganic) on Seed Yield, Proximate Seed Composition, Seed Elemental Composition and Protein Content in Fenugreek
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Setup
2.2. Harvesting
2.3. Measurements and Analyses
2.4. Data Analysis
3. Results
3.1. Agronomic Traits
3.2. Mineral Content of Seed
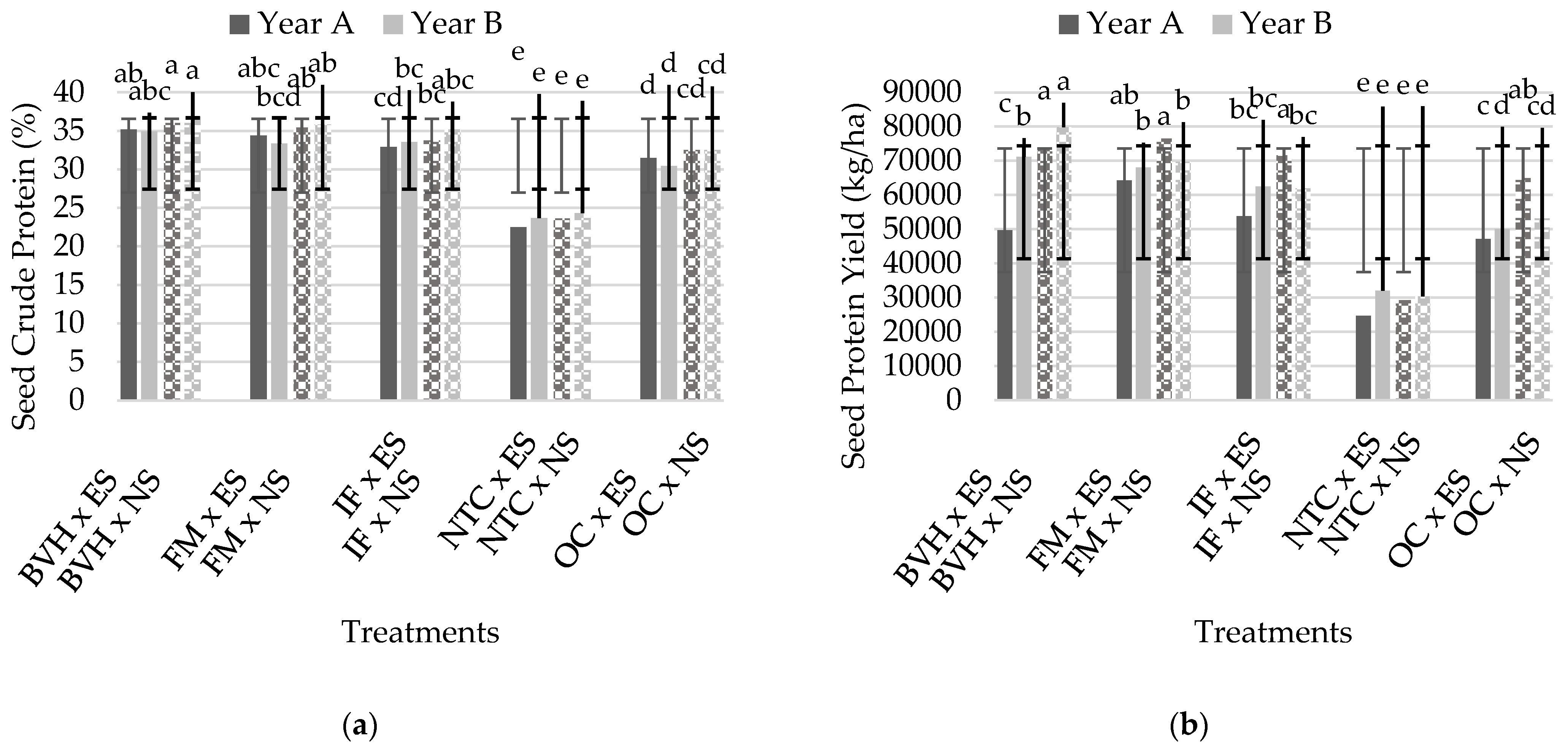
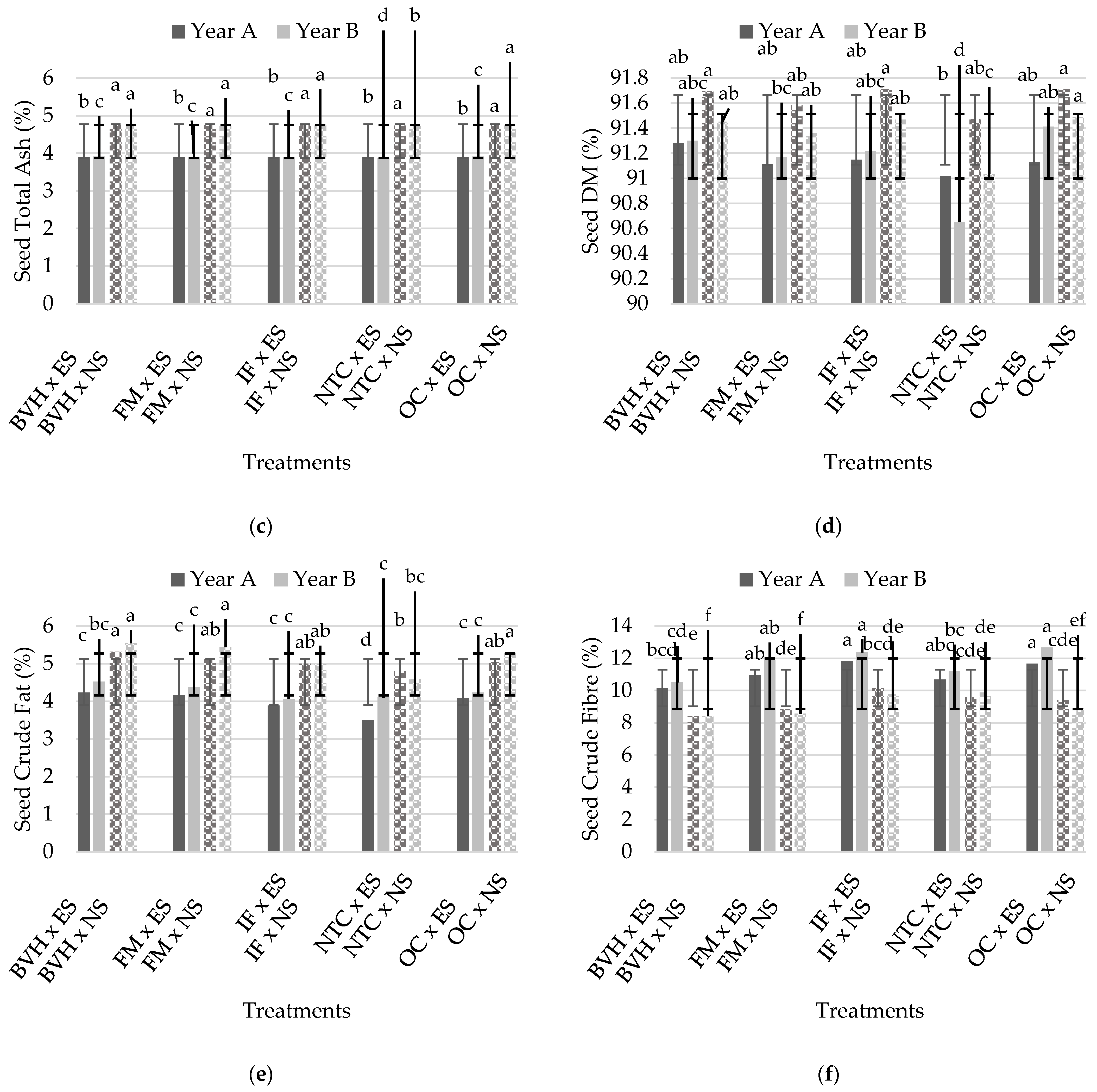
3.3. Proximate Composition of Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilles, A.R.; Mahmood, S. Historical background, origin, distribution, and economic importance of fenugreek. In Fenugreek: Biology and Applications; Springer: Singapore, 2021; pp. 3–11. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Magadlela, A.; Hong, S.; Cheng, Q. Fenugreek cultivation in the middle east and other parts of the world with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. In Fenugreek: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 13–30. [Google Scholar]
- Dhull, S.B.; Chandak, A.; Bamal, P.; Malik, A.; Kidwai, M.K. Fenugreek (Trigonella foenum-graecum): Nutritional, health properties and food uses. In Fenugreek: Biology and Applications; Springer: Singapore, 2021; pp. 219–246. [Google Scholar]
- Narapogu, V.; Swathi, C.; Polsani, M.R.; Premendran, J. Evaluation of anti-diabetic and anti-inflammatory activities of Fenugreek (Trigonella foenum-graecum) seed extracts on Albino Wistar Rats. Natl. J. Physiol. Pharm. Pharmacol. 2021, 11, 1277–1282. [Google Scholar] [CrossRef]
- Pal, D.; Mukherjee, S. Fenugreek (Trigonella foenum) seeds in health and nutrition. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–170. [Google Scholar]
- Salam, S.G.A.; Rashed, M.M.; Ibrahim, N.A.; Rahim, E.A.A.; Aly, T.A.; Al-Farga, A. Phytochemical screening and in-vitro biological properties of unprocessed and household processed fenugreek (Trigonella foenum-graecum Linn.) seeds and leaves. Sci. Rep. 2023, 13, 7032. [Google Scholar] [CrossRef] [PubMed]
- Duke, J. Handbook of Legumes of World Economic Importance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Niknam, R.; Kiani, H.; Mousavi, Z.E.; Mousavi, M. Extraction, detection, and characterization of various chemical components of Trigonella foenum-graecum L.(fenugreek) known as a valuable seed in agriculture. In Fenugreek: Biology and Applications; Springer: Singapore, 2021; pp. 189–217. [Google Scholar]
- Rao, P.U.; Sesikeran, B.; Rao, P.S.; Naidu, A.N.; Rao, V.V.; Ramachandran, E.P. Short term nutritional and safety evaluation of fenugreek. Nutr. Res. 1996, 16, 1495–1505. [Google Scholar] [CrossRef]
- Faisal, Z.; Irfan, R.; Akram, N.; Manzoor, H.M.I.; Aabdi, M.A.; Anwar, M.J.; Khawar, S.; Saif, A.; Shah, Y.A.; Afzaal, M.; et al. The multifaceted potential of fenugreek seeds: From health benefits to food and nanotechnology applications. Food Sci. Nutr. 2024, 12, 2294–2310. [Google Scholar] [CrossRef]
- Aljuhaimi, F.; Şimşek, Ş.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E. Effect of location on chemical properties, amino acid and fatty acid compositions of fenugreek (Trigonella foenum-graecum L.) seed and oils. J. Food Process. Pres. 2018, 42, e13569. [Google Scholar] [CrossRef]
- Srinivasa, U.M.; Naidu, M.M. Fenugreek (Trigonella foenum-graecum L.) seed: Promising source of nutraceutical. Stud. Nat. Prod. Chem. 2021, 71, 141–184. [Google Scholar]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek cultivation with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. Mini Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.; Elkhateeb, F.S.; Ismail, Z.S.; Ghazalah, A.A.; Lohakare, J. Combined effects of fenugreek seeds and probiotics on growth performance, nutrient digestibility, carcass criteria, and serum hormones in growing rabbits. Livest. Sci. 2021, 251, 104616. [Google Scholar] [CrossRef]
- Folina, A.; Tsementzi, K.; Stavropoulos, P.; Mavroeidis, A.; Kakabouki, I.; Bilalis, D. Impact of salinity and fertilization on soil properties, and root development in fenugreek (Trigonella foenum-graecum) cultivation. Not. Bot. Horti Agrobot. 2024, 52, 13868. [Google Scholar] [CrossRef]
- Narayana, P.K.; Bueno, E.; Baur, A.; Ahmed, S.; Von Wettberg, E.J. Fenugreek, a legume spice and multiuse crop adapted to a changing climate. In Developing Climate Resilient Grain and Forage Legumes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 105–123. [Google Scholar]
- Lamsaadi, N.; Ellouzi, H.; Zorrig, W.; El Moukhtari, A.; Abdelly, C.; Savouré, A.; Debez, A.; Farissi, M. Enhancing fenugreek (Trigonella foenum-graecum L.) productivity and seed quality through silicon-based seed priming under salt-stressed conditions. Russ. J. Plant Physiol. 2024, 71, 70. [Google Scholar] [CrossRef]
- Fani, E. The effects of feeding by silica fertilizer on the reduction of stress caused by salinity in fenugreek plants. J. Soil Manag. Sustain. Prod. 2024, 14, 115–129. [Google Scholar]
- Dadresan, M.; Luthe, D.S.; Reddivari, L.; Chaichi, M.R.; Yazdani, D. Effect of salinity stress and surfactant treatment on physiological traits and nutrient absorption of fenugreek plant. Commun. Soil Sci. Plant Anal. 2015, 46, 2807–2820. [Google Scholar] [CrossRef]
- Folina, A.; Mavroeidis, A.; Stavropoulos, P.; Eisenbach, L.; Kakabouki, I.; Bilalis, D. Comparison of Organic and Inorganic Fertilization in Fenugreek Cultivation Using Nitrogen Indicators. Nitrogen 2024, 5, 712–731. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Kovacs, S.; Domokos-Szabolcsy, E.; Bákonyi, N.; Fari, M.; Geilfus, C.M. Sustainable biorefinery and production of alfalfa (Medicago sativa L.): A review. Egypt. J. Bot. 2020, 60, 621–639. [Google Scholar] [CrossRef]
- ISO11261; Soil Quality: Determination of Total Nitrogen: Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995.
- ISO11260; Soil Quality: Determination of Cation Exchange Capacity and Base Saturation Method Using Barium Chloride Solution; International Organization for Standardization: Geneva, Switzerland. ISO: Geneva, Switzerland, 1994.
- ISO14870; Soil Quality–Extraction of Trace Elements by Buffered DTPA Solution. ISO: Geneva, Switzerland, 2001.
- Association of Official Agricultural Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000; pp. 490–510. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 16th ed.; AOCA: Gaithersburg, MD, USA, 1995; p. 870. [Google Scholar]
- Nichiporovich, A.; Stroganova, L.; Vlasova, M. Fotosinteticheskaya deyatel’nost’rasteniy v posevakh [Photosynthetic Activity of Plants in Crops]; AN SSSR: Moscow, Russia, 1961. [Google Scholar]
- Tian, J.; Li, S.; Xing, Z.; Cheng, S.; Guo, B.; Hu, Y.; Wei, H.; Gao, H.; Liao, P.; Wei, H.; et al. Differences in rice yield and biomass accumulation dynamics for different direct seeding methods after wheat straw return. Food Ener. Secur. 2022, 11, e425. [Google Scholar] [CrossRef]
- Papakosta, D.K.; Gagianas, A. Nitrogen and dry matter accumulation, remobilization, and losses for Mediterranean wheat during grain filling. J. Agron. 1991, 83, 864–870. [Google Scholar] [CrossRef]
- Raiyani, V.; Kathiriya, R.; Thummer, V.; Rupareliya, V. Effect of FYM and biofertilizers on growth, yield attributes and yield of fenugreek (Trigonella foenum-graecum L.). Int. J. Chem. Stud. 2018, 6, 746–748. [Google Scholar]
- Tunçtürk, R.; Celen, A.E.; Tunçtürk, M. The effects of nitrogen and sulphur fertilizers on the yield and quality of fenugreek (Trigonella foenum-graecum L.). Turk. J. Field Crops 2011, 16, 69–75. [Google Scholar]
- Tuncturk, R. Salinity exposure modifies nutrient concentrations in fenugreek (Trigonella foenum graecum L.). Afr. J. Agric. Res. 2011, 6, 3685–3690. [Google Scholar]
- Mehta, R.; Anwer, M.; Aishwath, O.; Meena, R. Growth, yield and quality of fenugreek (Trigonella foenum-graecum L.) as influenced by nitrogen, phosphorus and bio-fertilizers. Indian J. Hortic. 2012, 69, 94–97. [Google Scholar]
- Bairva, M.; Meena, S.; Mehta, R. Effect of bio-fertilizers and plant growth regulators on growth and yield of fenugreek (Trigonella foenum-graecum L.). Int. J. Environ. Clim. 2012, 2, 28–33. [Google Scholar]
- Chaichi, M.R.; Dadresan, M.; Hosseini, M.B.; Pourbabaie, A.; Yazdani, D.; Zandvakili, O.R. Effect of bio fertilizers on the growth, productivity and nutrient absorption of fenugreek (Trigonella foenum graecum L.). Int. J. Agric. Innov. Res. 2015, 3, 1628–1633. [Google Scholar]
- Mehta, R.S.; Patel, B.S.; Meena, S.S.; Meena, R.S. Influence of nitrogen, phosphorus and bio-fertilizers on growth characters and yield of fenugreek (Trigonella foenum-graecum L.). J. Spices Arom. Crops. 2010, 19, 23–28. [Google Scholar]
- Bhutia, K.C.; Bhutia, S.O.; Chatterjee, R.; Chattopadhyay, N. Growth, phenology and yield of fenugreek (Trigonella foenum–graecum L.) as influenced by date of sowing. Int. J Curr. Microbiol. App. Sci. 2017, 6, 1810–1817. [Google Scholar] [CrossRef]
- Zandi, P.; Shirani–Rad, A.H.; Daneshian, J.; Bazrkar–Khatibani, L. Agronomic and morphologic analysis of Fenugreek (Trigonella foenum-graecum L.) under nitrogen fertilizer and plant density via factor analysis. Afr. J. Agric. Res. 2011, 6, 1134–1140. [Google Scholar]
- Zandi, P.; Shirani-Rad, A.H.; Bazrkar-Khatibani, L. Agronomic study of fenugreek grown under different in-row spacing and nitrogen levels in a paddy field of Iran. Am.-Eurasian J. Agric. Environ. Sci. 2011, 10, 544–550. [Google Scholar]
- Nair, R.; Pandey, S.K.; Jyothsna, J. Growth and yield of fenugreek (Trigonella foenum graecum L.) in response to different levels of phosphorus and biofertilizer (Rhizobium and PSB) under Kymore Plateau and Satpura hill agro-climatic zone of Madhya Pradesh. Pharma Innov. J. 2021, 10, 419–422. [Google Scholar]
- Husain, N.; Nair, R.; Verma, B.K.; Yadav, B. Growth, Yield and Economics of Fenugreek (Trigonella foenum-graecum L.) as Influenced by Inorganic Fertilizers and Bio-inoculant (Rhizobium, PSB and KSB). Biol. Forum–Int. J. 2022, 14, 80–83. [Google Scholar]
- Biswas, S.; Anusuya, D. Effect of bioinoculants and organic manure (phosphocompost) on growth, yield and nutrient uptake of Trigonella foenum-graecum L. (Fenugreek). Int. J. Sci. Res. 2014, 3, 38–41. [Google Scholar]
- Sahu, P.K.; Naruka, I.S.; Haldar, A.; Chundawat, R.S.; Kumar, L. Studies on the effects of integrated nutrient management on fenugreek (Trigonella foenum-graecum) L. Int. J. Chem. Stud. 2020, 8, 1082–1089. [Google Scholar] [CrossRef]
- Sahu, P.K.; Naruka, I.S.; Haldar, A.; Chundawat, R.S.; Kumar, H. Study the effect of integrated nutrient management on vegetative growth of fenugreek (Trigonella foenum-graecum) L. J. Pharmacogn. Phytochem. 2020, 9, 1389–1394. [Google Scholar]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Israr, D.; Mustafa, G.; Khan, K.S.; Shahzad, M.; Ahmad, N.; Masood, S. Interactive effects of phosphorus and Pseudomonas putida on chickpea (Cicer arietinum L.) growth, nutrient uptake, antioxidant enzymes and organic acids exudation. Plant Physiol. Biochem. 2016, 108, 304–312. [Google Scholar] [CrossRef]
- Godara, A.S.; Gupta, U.S.; Singh, R.; Mehta, R.S. Effect of different combinations of organic and inorganic nutrient sources on productivity and profitability of fenugreek (Trigonella-foenium-graecum). Int. J. Environ. Clim. 2012, 2, 34–37. [Google Scholar]
- Seghatoleslami, M.; Ahmadi Bonakdar, K. The effect of sowing date and plant density on yield and yield components of fenugreek (Trigonella foenum gracum L.). Iran. J. Med. Aromat. Plants 2010, 26, 265–274. [Google Scholar]
- Hedley, C.; Ambrose, M. The effect of plant interaction on crop harvest index. In Efficiency in Plant Breeding: Proceedings of the 10th Congress of the European Association for Research on Plant Breeding, EUCARPIA, Wageningen, The Netherlands, 19–24 June 1983; Bernan Press (PA): Pudoc Wageningen, The Netherlands, 1984. [Google Scholar]
- Zuk-Gołaszewska, K.; Wierzbowska, J.; Bienkowski, T. Effect of potassium fertilization, Rhizobium inoculation and water deficit on the yield and quality of fenugreek seeds. J. Elem. 2015, 20, 513–524. [Google Scholar] [CrossRef]
- Seepaul, R.; Kumar, S.; Boote, K.J.; Small, I.M.; George, S.; Wright, D.L. Physiological analysis of growth and development of winter carinata (Brassica carinata A. Braun). GCB Bioenergy 2021, 13, 1112–1133. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ibrahim, O.M.; Elham, A.B. Effect of bio and mineral phosphorus fertilizer on the growth, productivity and nutritional value of fenugreek (Trigonella foenum graecum L.) in Newly Cultivated Land. Res. J. Agric. Biol. Sci. 2010, 6, 339–348. [Google Scholar]
- Sherif, M.; Awadallah, R.; Mohamed, A.E. Determination of trace elements of egyptian crops by neutron activation analysis. J. Radioanal. Nucl. Chem. 1979, 53, 145–153. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and other regulators assists plants in responding to abiotic stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.E. Organic matter, humus, humate, humic acid, fulvic acid and humin. Wonderful World Humus Carbon 2006. Available online: https://www.semanticscholar.org/paper/ORGANIC-MATTER-%2C-HUMUS-%2C-HUMATE-%2C-HUMIC-ACID-%2C-ACID-Pettit/bd2e61da484c14d9325d16024360a118c4809ba1 (accessed on 26 November 2024).
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Mickky, B.M.; Abbas, M.A.; Sameh, N.M. Morpho-physiological status of fenugreek seedlings under NaCl stress. J. King Saud Univ. Sci. 2019, 31, 1276–1282. [Google Scholar] [CrossRef]
- Zayneb, C.; Bassem, K.; Zeineb, K.; Grubb, C.D.; Noureddine, D.; Hafedh, M.; Amine, E. Physiological responses of fenugreek seedlings and plants treated with cadmium. Environ. Sci. Pollut. Res. 2015, 22, 10679–10689. [Google Scholar] [CrossRef]
- Frossard, E.; Bucher, M.; Mächler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Mnafgui, W.; Rizzo, V.; Muratore, G.; Hajlaoui, H.; De Oliveira Schinoff, B.; Mnafgui, K.; Elleuch, A.; Hussain, S. Trigonella foenum-graecum morphophysiological and phytochemical processes controlling iron uptake and translocation. Crop Pasture Sci. 2022, 73, 957–968. [Google Scholar] [CrossRef]
- Chen, Y. Organic matter reactions involving micronutrients in soils and their effect on plants. In Humic Substances in Terrestrial Ecosystems; Elsevier: Amsterdam, The Netherlands, 1996; pp. 507–529. [Google Scholar]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of humic and fulvic acid under saline soil conditions to improve growth and yield in barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Das, S.K. Role of micronutrient in rice cultivation and management strategy in organic agriculture—A reappraisal. Agric. Sci. 2014, 5, 765–769. [Google Scholar] [CrossRef]
- Akhtar, M.; Gulab, M.; Ghazanfar, M. Assessing Soil Health and Fertility through Microbial Analysis and Nutrient Profiling Implications for Sustainable Agriculture. Innov. Res. Appl. Biol. Chem. Sci. 2023, 1, 29–42. [Google Scholar]
- Tabaxi, I.; Kakabouki, I.; Zisi, C.; Folina, A.; Karydogianni, S.; Kalivas, A.; Bilalis, D. Effect of organic fertilization on soil characteristics, yield and quality of Virginia Tobacco in Mediterranean area. Emir. J. Food Agric. 2020, 32, 610–616. [Google Scholar] [CrossRef]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Fernández, F.G.; Hoeft, R.G. Managing soil pH and crop nutrients. Ill. Agron. Handb. 2009, 24, 91–112. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Ho, T.T.K.; Le, T.H.; Tran, C.S.; Nguyen, P.T.; Thai, V.N.; Bui, X.T. Compost to improve sustainable soil cultivation and crop productivity. Case Stud. Chem. Environ. Eng. 2022, 6, 100211. [Google Scholar] [CrossRef]
- Bengough, A. Root growth and function in relation to soil structure, composition, and strength. In Root Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 151–171. [Google Scholar]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; CABI: Wallingford, UK, 2017; pp. 24–63. [Google Scholar]
- Olaiya, C.O.; Soetan, K.O. A review of the health benefits of fenugreek (Trigonella foenum-graecum L.): Nutritional, Biochemical and pharmaceutical perspectives. Am. J. Soc. Issues Humanit. 2014, 4, 3–12. [Google Scholar]
- Rao, P.U.; Sharma, R. An evaluation of protein quality of fenugreek seeds (Trigonella foenumgraecum) and their supplementary effects. Food Chem. 1987, 24, 1–9. [Google Scholar] [CrossRef]
- El Nasri, N.A.; El Tinay, A.H. Functional properties of fenugreek (Trigonella foenum graecum) protein concentrate. Food Chem. 2007, 103, 582–589. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, P.; Wani, S.A. Effect of extraction parameters on the isolation of fenugreek seed protein and characterization of fenugreek protein concentrate. Qual. Assur. Saf. Crops Foods 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Zuk-Golaszewska, K. The impact of nitrogen fertilization and Rhizobium inoculation on the yield and quality of Trigonella foenum-graecum L. J. Elem. 2014, 19, 1109–1118. [Google Scholar] [CrossRef]
- Meena, S.S.; Mehta, R.S.; Bairwa, M.; Meena, R.D. Productivity and profitability of fenugreek (Trigonella foenum-graecum L.) as influenced by bio-fertilizers and plant growth regulators. Legume Res. 2014, 37, 646–650. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, S.X.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Okon, O.G. Effect of salinity on physiological processes in plants. In Microorganisms in Saline Environments: Strategies and Functions; Springer: Cham, Switzerland, 2019; pp. 237–262. [Google Scholar]
- Bilalis, D.; Tabaxi, I.; Zervas, G.; Tsiplakou, E.; Travlos, I.S.; Kakabouki, I.; Tsioros, S. Chia (Salvia hispanica) fodder yield and quality as affected by sowing rates and organic fertilization. Commun. Soil Sci. Plant Anal. 2016, 47, 1764–1770. [Google Scholar] [CrossRef]
- Muhammed, S.R. Response of Two Fenugreek Trigonella foenum-graecum Varieties to Different Cutting Dates and Nitrogen Fertilizer for Growth and Forage Yield Traits under Rainfed Condition. J. Tikrit Univ. Agric. Sci. 2017, 17, 134–142. [Google Scholar]

| Measurements | Measurement Unit | Analysis Method/Equation | Reference |
|---|---|---|---|
| Nitrogen (N) | (%) | ISO, 1995 (11,261) | [23] |
| Calcium (Ca) | (g/100 g) | ISO, 1994 (11,260) | [24] |
| Potassium (K) | (g/100 g) | ISO, 1994 (11,260) | [24] |
| Iron (Fe) | (ppm) | ISO, 2001 (14,870) | [25] |
| Phosphorus (P) | (%) | ISO, 1994 (11,263) | [24] |
| Magnesium (Mg) | (g/100 g) | ISO, 1994 (11,260) | [24] |
| Seed Crude Ash | (%) | 924.05 | [26,27] |
| Seed DM | (%) | 943.01 | [26,27] |
| Seed Crude Fat | (%) | 920.39 | [26,27] |
| Seed Crude Fiber | (%) | 978.10 | [26,27] |
| Seed Crude Protein | (%) | Multiplying N concentration by 6.25 | [26,27] |
| Seed Protein Yield | Kg N/ha | Seed Crude Protein (%) × Seed Yield (kg/ha) | [28] |
| Harvest Index (HI) | - | (Seed yield (kg/ha)/Biological yield (kg/ha)) × 100 | [28] |
| Translocation Percentage (TP) | - | Straw weight at flowering—straw weight at harvest/Panicle weight at flowering—panicle weight at harvest | [29,30] |
| Treatments | Plant Height | Νο Pods/Plant | No Double Pods/Plant | Pod Length | No Seed/Pod | |
|---|---|---|---|---|---|---|
| YEAR A | ||||||
| BVH × ES | 73.67 a | 32.66 a | 4.17 a | 17.33 a | 20.80 a | |
| BVH × NS | 74.00 a | 31.97 a | 3.67 a | 18.00 a | 21.60 a | |
| FM × ES | 73.00 a | 32.66 a | 4.00 a | 17.33 a | 20.80 a | |
| FM × NS | 72.27 a | 31.28 a | 2.33 b | 19.00 a | 22.80 a | |
| IF × ES | 70.33 a | 32.67 a | 3.33 a | 18.00 a | 21.60 a | |
| IF × NS | 70.10 a | 31.28 a | 3.00 ab | 18.67 a | 22.40 a | |
| NTC × ES | 50.37 c | 18.94 c | 1.00 c | 10.00 b | 12.00 b | |
| NTC × NS | 51.00 c | 21.00 bc | 1.00 c | 11.00 b | 13.20 b | |
| OC × ES | 60.35 b | 28.54 ab | 3.33 a | 17.00 a | 20.40 a | |
| OC × NS | 60.55 b | 20.31 bc | 3.00 ab | 16.67 a | 20.00 a | |
| Std Dev. | ±9.37 | ±6.64 | ±1.12 | ±4.41 | ±4.09 | |
| FFertilization | 4 | 48.53 *** | 8.41 ** | 22.87 *** | 24.19 *** | 24.19 *** |
| FSalinity | 1 | ns | ns | 8.76 * | ns | ns |
| FFertiliz×Salin | 4 | ns | ns | ns | ns | ns |
| YEAR B | ||||||
| BVH × ES | 74.67 ab | 38.83 a | 5.33 a | 25.00 a | 30.00 a | |
| BVH × NS | 75.67 a | 38.83 a | 5.00 ab | 24.67 a | 29.60 a | |
| FM × ES | 70.33 bc | 33.34 ab | 4.00 abc | 23.00 ab | 27.60 ab | |
| FM × NS | 71.83 abc | 36.09 a | 4.00 abc | 24.33 a | 29.20 a | |
| IF × ES | 67.50 c | 32.66 ab | 3.00 cde | 21.67 bc | 26.00 bc | |
| IF × NS | 67.33 c | 36.09 a | 3.67 bcd | 23.00 ab | 27.60 ab | |
| NTC × ES | 49.93 e | 23.06 c | 1.00 fg | 15.33 d | 18.40 d | |
| NTC × NS | 52.50 e | 27.17 bc | 0.67 g | 17.33 d | 20.80 d | |
| OC × ES | 58.72 d | 27.17 bc | 2.33 def | 21.00 bc | 25.20 bc | |
| OC × NS | 59.92 d | 35.40 a | 2.00 efg | 20.33 c | 2.40 c | |
| Std Dev. | ±9.09 | ±6.53 | ±1.64 | ±4.11 | ±4.93 | |
| Df | ||||||
| Rep | 2 | |||||
| FFertilization | 4 | 135.03 *** | 24.13 *** | 16.44 *** | 62.67 *** | 62.67 *** |
| FSalinity | 1 | ns | 5.32 * | ns | ns | ns |
| FFertiliz×Salin | 4 | ns | ns | ns | ns | ns |
| Error | 16 | |||||
| Total | 29 |
| Treatments | PAI 130 | Seed Yield | TSW | HI | TP | |
|---|---|---|---|---|---|---|
| YEAR A | ||||||
| BVH × ES | 45.83 a | 1403.30 de | 16.41 a | 31.54 c | 51.06 a | |
| BVH × NS | 45.83 a | 2040.20 a | 17.60 a | 43.44 abc | 49.44 ab | |
| FM × ES | 42.78 ab | 1866.70 abc | 15.53 a | 42.76 abc | 49.55 ab | |
| FM × NS | 42.80 ab | 2160.20 a | 16.47 a | 46.54 ab | 47.99 abc | |
| IF × ES | 37.43 b | 1613.40 bcd | 14.40 a | 38.37 abc | 50.24 a | |
| IF × NS | 41.25 ab | 2120.20 a | 15.78 a | 47.41 ab | 46.55 abc | |
| NTC × ES | 26.74 c | 1096.70 e | 9.26 b | 37.29 bc | 36.64 de | |
| NTC × NS | 28.26 c | 1240.10 de | 10.48 b | 37.99 abc | 35.10 e | |
| OC × ES | 38.19 ab | 1500.70 cde | 13.20 a | 41.56 abc | 42.13 cd | |
| OC × NS | 37.43 b | 2000.20 ab | 13.61 a | 50.59 a | 42.81 bcd | |
| Std Dev. | ±7.45 | ±445.02 | ±2.94 | ±8.86 | ±6.39 | |
| F Fertilization | 59.95 *** | 16.86 *** | 526.28 *** | ns | 22.21 *** | |
| F Salinity | ns | 20.43 ** | 14.18 ** | 7.98 ** | ns | |
| F Fertiliz×Salin | ns | ns | ns | ns | ns | |
| YEAR B | ||||||
| BVH × ES | 53.59 a | 2161.10 a | 18.33 a | 47.64 a | 49.68 a | |
| BVH × NS | 52.71 a | 2042.10 ab | 17.57 ab | 45.97 ab | 47.30 ab | |
| FM × ES | 49.19 ab | 2041.80 ab | 17.10 abc | 45.96 ab | 47.24 ab | |
| FM × NS | 48.66 ab | 1947.10 abc | 16.27 bcd | 45.85 ab | 45.42 ab | |
| IF × ES | 46.02 ab | 1871.10 bcd | 15.90 cd | 45.09 ab | 45.11 ab | |
| IF × NS | 43.05 b | 1756.10 cde | 15.53 cd | 44.51 ab | 42.10 bc | |
| NTC × ES | 43.05 b | 1682.70 de | 15.37 d | 43.54 ab | 38.57 cd | |
| NTC × NS | 41.98 b | 1628.40 e | 15.00 d | 41.83 ab | 36.35 de | |
| OC × ES | 32.50 c | 1346.70 f | 12.07 e | 41.46 ab | 33.19 e | |
| OC × NS | 30.75 c | 1255.10 f | 11.63 e | 40.79 b | 33.02 e | |
| Std Dev. | ±8.63 | ±418.21 | ±2.29 | ±9.00 | ±6.52 | |
| Df | ||||||
| Rep | 2 | |||||
| FFertilization | 4 | 80.28 *** | 29.92 *** | 34.63 *** | ns | 69.44 *** |
| FSalinity | 1 | ns | ns | ns | ns | ns |
| FFertiliz×Salin | 4 | ns | ns | ns | ns | ns |
| Error | 16 | |||||
| Total | 29 |
| Df | N Seed (%) | Ca Seed (g/100 g) | K Seed (g/100 g) | Fe Seed (ppm) | P Seed (%) | Mg Seed (g/100 g) | |
|---|---|---|---|---|---|---|---|
| Year A | |||||||
| FFertilization | 4 | 175.98 *** | 8.47 ** | 41.08 *** | 114.34 *** | 71.59 *** | 81.68 *** |
| FSalinity | 1 | 10.14 ** | 6.4 * | 70.95 *** | ns | 45.52 *** | ns |
| FFertiliz×Salin | 4 | ns | ns | 7.95 ** | ns | 8.99 ** | ns |
| Year B | |||||||
| Rep | 2 | ||||||
| FFertilization | 4 | 175.98 *** | 11.31 ** | 41.08 *** | 109.37 *** | 70.04 *** | 51.30 *** |
| FSalinity | 1 | 10.14 ** | 33.8 *** | 70.95 *** | 13.82 ** | 25.82 *** | ns |
| FFertiliz×Salin | 4 | ns | 4.05 * | 7.95 ** | ns | 4.15 * | ns |
| Total | 29 |
| Df | Seed Crude Protein (%) | Seed Protein Yield (kg/ha) | Seed Total Ash | Seed DM | Seed Crude Fat | Seed Crude Fiber | |
|---|---|---|---|---|---|---|---|
| Year A | |||||||
| FFertilization | 4 | 83.66 *** | 39.40 *** | ns | ns | 14.85 *** | 5.59 * |
| FSalinity | 1 | 6.97 * | 23.02 *** | 10,824.98 *** | 9.97 * | 159.78 *** | 44.68 *** |
| FFertiliz×Salin | 4 | ns | ns | ns | ns | ns | ns |
| Year B | |||||||
| Rep | 2 | ||||||
| FFertilization | 4 | 107.07 *** | 39.41 *** | 16.86 *** | 16.86 *** | 11.80 ** | 10.46 ** |
| FSalinity | 1 | 5.89 * | ns | 59,495.62 *** | 10.20 *** | 34.30 *** | 136.39 *** |
| FFertiliz×Salin | 4 | ns | ns | ns | ns | ns | 3.8 * |
| Total | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folina, A.; Efthimiadou, A.; Stavropoulos, P.; Mavroeidis, A.; Kakabouki, I.; Tsiplakou, E.; Bilalis, D. Field Evaluation of Salt Stress and Fertilization Effects (Organic and Inorganic) on Seed Yield, Proximate Seed Composition, Seed Elemental Composition and Protein Content in Fenugreek. Seeds 2025, 4, 9. https://doi.org/10.3390/seeds4010009
Folina A, Efthimiadou A, Stavropoulos P, Mavroeidis A, Kakabouki I, Tsiplakou E, Bilalis D. Field Evaluation of Salt Stress and Fertilization Effects (Organic and Inorganic) on Seed Yield, Proximate Seed Composition, Seed Elemental Composition and Protein Content in Fenugreek. Seeds. 2025; 4(1):9. https://doi.org/10.3390/seeds4010009
Chicago/Turabian StyleFolina, Antigolena, Aspasia Efthimiadou, Panteleimon Stavropoulos, Antonios Mavroeidis, Ioanna Kakabouki, Eleni Tsiplakou, and Dimitrios Bilalis. 2025. "Field Evaluation of Salt Stress and Fertilization Effects (Organic and Inorganic) on Seed Yield, Proximate Seed Composition, Seed Elemental Composition and Protein Content in Fenugreek" Seeds 4, no. 1: 9. https://doi.org/10.3390/seeds4010009
APA StyleFolina, A., Efthimiadou, A., Stavropoulos, P., Mavroeidis, A., Kakabouki, I., Tsiplakou, E., & Bilalis, D. (2025). Field Evaluation of Salt Stress and Fertilization Effects (Organic and Inorganic) on Seed Yield, Proximate Seed Composition, Seed Elemental Composition and Protein Content in Fenugreek. Seeds, 4(1), 9. https://doi.org/10.3390/seeds4010009









