The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity
Abstract
1. Introduction
2. Biological Categories of Seeds
3. The Lifespan of Seeds
4. Loss of Seed Viability
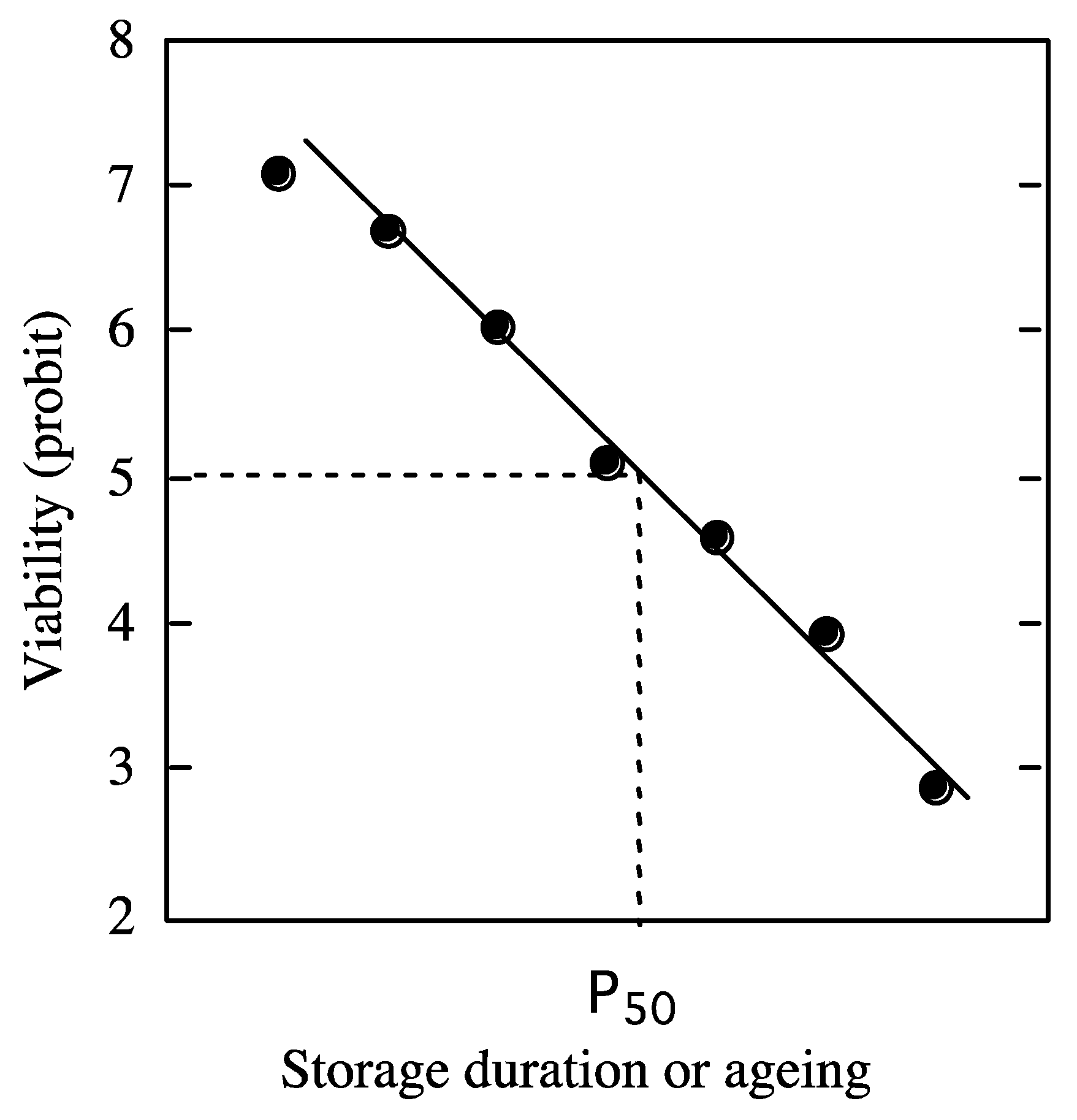
4.1. Change in Viability during Storage: Viability Equations
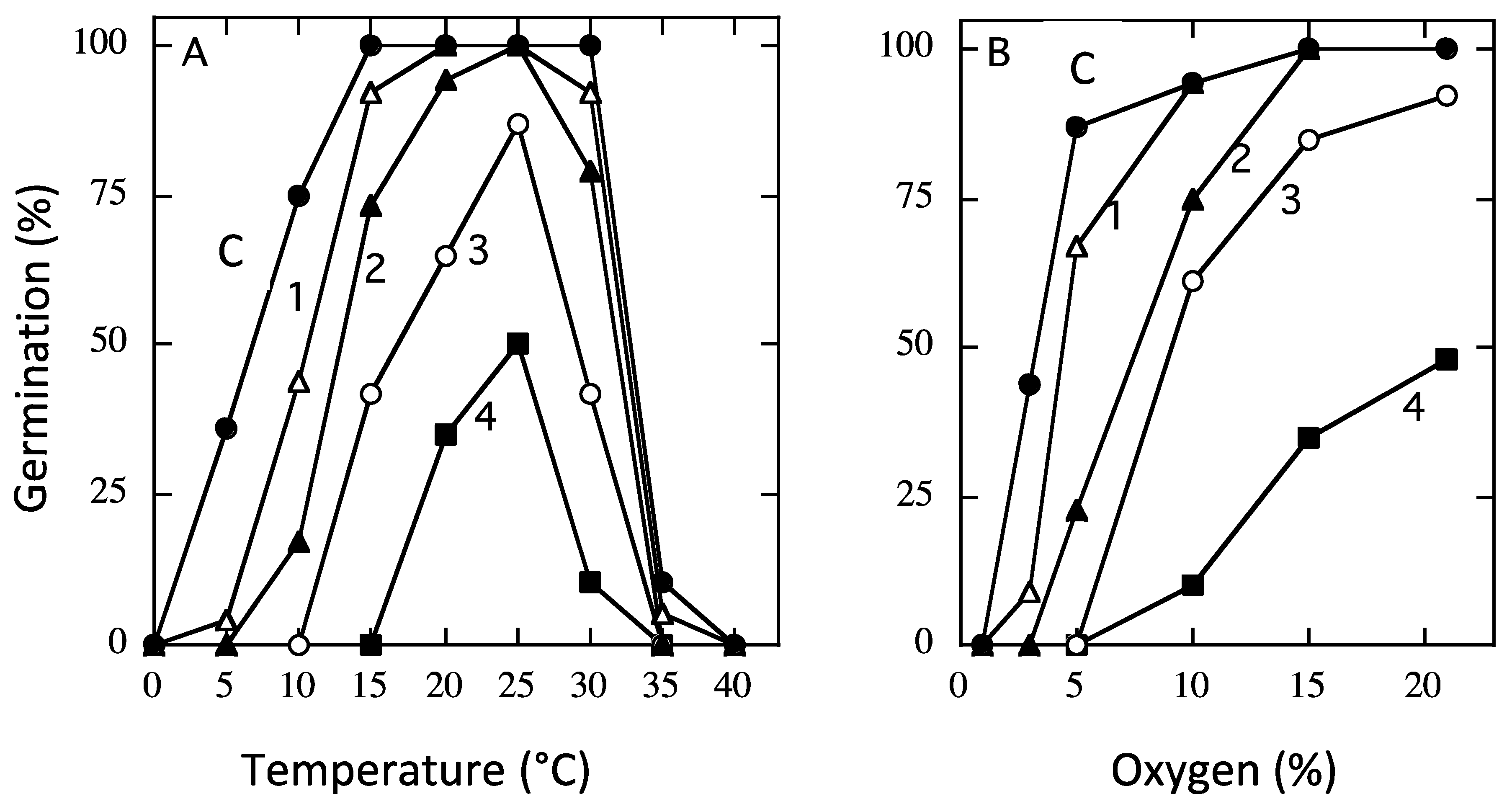
4.2. Modulation of Viability by Storage Conditions
- -
- For each 1–2% decrease in seed moisture content (when the MC ranges between 5 and 14%), the seed storage life is doubled;
- -
- For each 10 °F (5.6 °C) decrease in seed storage temperature (between 0 °C and 50 °C), the seed storage life is doubled.
4.3. Procedures for Long-Term Storage in Genebanks
5. Damage Occurring during the Dehydration of Recalcitrant Seeds
6. Physiological and Biochemical Events Associated with the Ageing of Orthodox Seeds
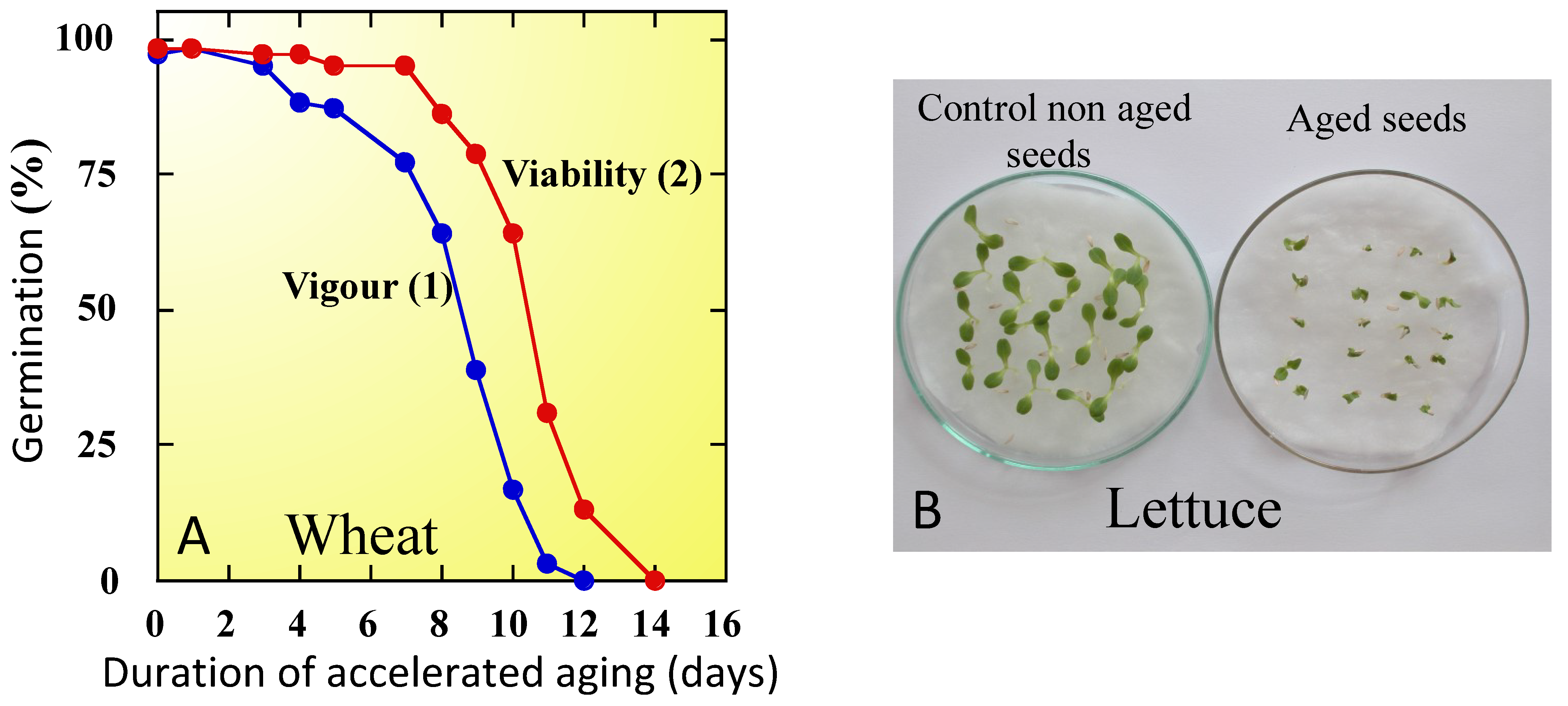
6.1. Loss of Vigor during Ageing
6.2. Cellular and Metabolic Deterioration during the Ageing of Orthodox Seeds
6.3. Repair
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priestley, D.A. Seed aging. In Implications for Seed Storage and Persistence in the Soil; Cornell University Press: Ithaca, NY, USA, 1986; p. 304. [Google Scholar]
- Murdoch, A.J.; Ellis, R.H. Dormancy, Viability and Longevity. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK; CAB International: Oxon, UK, 2000; pp. 183–214. [Google Scholar]
- McDonald, M.B. Orthodox seed deterioration and its repair. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; The Haworth Reference Press: New York, NY, USA; London, UK; Oxford, UK, 2004; pp. 273–304. [Google Scholar]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Solberg, S.O.; Yndgaard, F.; Andreasen, C.; Von Bothmer, R.; Loskutov, I.G.; Asdal, A. Long-term storage and longevity of orthodox seeds: A systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Hay, F.R.; Rezaei, S.; Buitink, J. Seed moisture isotherms, sorption models and longevity. Front. Plant Sci. 2022, 13, 891913. [Google Scholar] [CrossRef]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed longevity—The evolution of knowledge and a conceptual framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.H.; Ellis, R.H. Water and seed survival. Ann. Bot. 1989, 63, 39–52. [Google Scholar] [CrossRef]
- Leopold, A.C.; Vertucci, C.W. Moisture as a regulator of physiological reaction in seeds. In Seed moisture; Stanwood, P.C., McDonald, M.B., Eds.; CSSA: Madison, WI, USA, 1989; Volume 14, pp. 51–67. [Google Scholar]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of Seeds in a Gene-Bank: Species Characteristics; Cambridge University Press: Cambridge, UK, 2005; Volume 15. [Google Scholar]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural mechanics of seed deterioration: Standing the test of time. Plant Sci. 2010, 179, 565–573. [Google Scholar] [CrossRef]
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Hong, T.D.; Ellis, R.H. A Protocol to Determine Seed Storage Behaviour; International Plant Genetic Resources Institute: Rome, Italy, 1996; p. 64. [Google Scholar]
- Pritchard, H.W.; Dickie, J.B. Predicting seed longevity: The use and abuse of seed viability equations. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., Eds.; Kew, Royal Botanic Gardens: London, UK, 2003; pp. 653–722. [Google Scholar]
- Wyse, S.V.; Dickie, J.B. Predicting the global incidence of seed desiccation sensitivity. J. Ecol. 2017, 105, 1082–1093. [Google Scholar] [CrossRef]
- Hong, T.D.; Linington, S.; Ellis, R.H. Seed storage behavior: A compendium. In Handbooks for Genebanks: No 4; International Plant Genetic Resources Institute: Rome, Italy, 1996; p. 105. [Google Scholar]
- Smith, M.T.; Berjak, P. Deteriorative changes associated with the loss of viability of stored desiccation-tolerant and desiccation-sensitive seeds. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 701–746. [Google Scholar]
- Walters, C. Understanding the mechanisms and kinetics of seed ageing. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Mazuy, C.; Corbineau, F.; Bailly, C. DNA alteration and programmed cell death during ageing of sunflower seed. J. Exp. Bot. 2011, 62, 5003–5011. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Chen, H.; Osuna, D.; Colville, L.; Lorenzo, O.; Graeber, K.; Dennis, E.S.; Peacock, W.J. Transcriptome-wide mapping of pea seed ageing reveals a pivotal role for genes related to oxidative stress and programmed cell death. PLoS ONE 2013, 10, e78471. [Google Scholar] [CrossRef]
- Fleming, M.; Hill, L.M.; Walters, C. The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann. Bot. 2019, 123, 1133–1146. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Farrant, J.M. Acquisition and loss of desiccation tolerance. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 237–271. [Google Scholar]
- King, M.W.; Roberts, E.H. The Storage of Recalcitrant Seeds—Achievements and Possible Approaches; IBPGR: Rome, Italy, 1979; p. 96. [Google Scholar]
- Pammenter, N.W.; Berjak, P. A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Sci. Res. 1999, 9, 13–37. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Recalcitrant seeds. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; The Haworth Reference Press: New York, NY, USA; London, UK; Oxford, UK, 2004; pp. 305–345. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour? I. Coffee. J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour. II. Effects of provenance, immaturity and imbibition on desiccation tolerance in coffee. J. Exp. Bot. 1991, 42, 653–657. [Google Scholar] [CrossRef]
- Hong, T.D.; Linington, S.H.; Ellis, R.H. Compendium of Information on Seed Storage Behaviour; Kew, Royal Botanic Gardens: London, UK, 1998; Volumes 1–2, p. 901. [Google Scholar]
- Kermode, A.R.; Finch-Savage, B.E. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 149–184. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1994; p. 445. [Google Scholar]
- Kermode, A.R. Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Sci. Res. 1997, 7, 75–95. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef]
- Obendorf, R.L. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci. Res. 1997, 7, 63–74. [Google Scholar] [CrossRef]
- Buitink, J.; Hoekstra, F.A.; Leprince, O. Biochemistry and biophysics of tolerance systems. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 293–318. [Google Scholar]
- Chin, H.F.; Krishnapillay, B.; Stanwood, P.C. Seed moisture: Recalcitrant vs. orthodox seeds. In Seed Moisture; Stanwood, P.C., McDonald, M.B., Eds.; CSSA: Madison, WI, USA, 1989; Volume 14, pp. 15–22. [Google Scholar]
- Chin, H.F. Production and storage of recalcitrant seeds in the tropics: Seed problems. Acta Hort. 1978, 83, 17–21. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds. In Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: London, UK, 1998; p. 666. [Google Scholar]
- Berjak, P.; Pammenter, N.W. Aspects of our understanding of the biology and responses of non-orthodox seeds. In Progress in Seed Research; Taylor, A.G., Huang, X.L., Eds.; Cornell University Press: Ithaca, NY, USA, 1997; pp. 81–100. [Google Scholar]
- Dickie, J.B.; May, K.; Morris, S.V.A.; Titley, S.E. The effects of desiccation on seed survival in Acer platanoides L. and Acer pseudoplatanus L. Seed Sci. Res. 1991, 1, 149–162. [Google Scholar] [CrossRef]
- Pukacka, S.; Wojkiewicz, A. Carbohydrate metabolism in Norway maple and sycomore seeds in relation to desiccation tolerance. J. Plant Physiol. 2002, 159, 273–279. [Google Scholar] [CrossRef]
- Kozeko, L.E.; Troyan, V.M. The relationship between the mitotic activity and moisture content of recalcitrant seeds of Acer saccharinum (L.) during maturation, post maturation drying and germination. Seed Sci. Res. 2000, 10, 225–232. [Google Scholar] [CrossRef]
- Tompsett, P.B. Desiccation studies in relation to the storage of Araucaria seed. Ann. Appl. Biol. 1984, 105, 581–586. [Google Scholar] [CrossRef]
- Salmen-Espindola, L.; Noin, M.; Corbineau, F.; Côme, D. Some cellular and metabolic damage induced by desiccation in recalcitrant Araucaria angustifolia embryos. Seed Sci. Res. 1994, 4, 193–201. [Google Scholar] [CrossRef]
- Fu, J.R.; Jin, J.P.; Peng, Y.F.; Xia, Q.H. Desiccation tolerance in two species with recalcitrant seeds: Clausena lansium (Lour.) and Litchi chinensis (Sonn.). Seed Sci. Res. 1994, 4, 257–261. [Google Scholar] [CrossRef]
- Fu, J.R.; Huang, X.M.; Song, S.Q. Manipulation of desiccation-sensitive axes of wampee (Clausena lansium) to facilitate increaded dehydration tolerance. Seed Sci. Res. 2000, 10, 397–400. [Google Scholar] [CrossRef]
- Fu, J.R.; Zhang, B.Z.; Wang, X.F.; Qiao, Y.Z.; Huang, X.L. Physiological studies in desiccation, wet storage and cryopreservation of recalcitrant seeds of three fruit species and their excised embryonic axes. Seed Sci. Tecnol. 1990, 18, 743–754. [Google Scholar]
- Chin, H.F.; Aziz, M.; Ang, B.B.; Hamzah, S. The effect of moisture and temperature on the ultrastructure and viability of seeds of Hevea brasiliensis. Seed Sci. Technol. 1981, 9, 411–422. [Google Scholar]
- Corbineau, F.; Côme, D. Storage of recalcitrant seeds of four tropical species. Seed Sci. Technol. 1988, 16, 97–103. [Google Scholar]
- Ray, P.K.; Sharma, S.B. Growth, maturity, germination and storage of lichi seeds. Sci. Hort. 1987, 33, 213–221. [Google Scholar] [CrossRef]
- Corbineau, F.; Kanté, M.; Côme, D. Seed germination and seedling development in the mango (Mangifera indica L.). Tree Physiol. 1986, 1, 151–160. [Google Scholar] [CrossRef]
- Suszka, B.; Muller, C.; Bonnet-Masimbert, M. Seeds of Forest Broadleaves from Harvest to Sowing; INRA Editions: Paris, France, 1996; p. 294. [Google Scholar]
- Finch-Savage, W.E. Seed water status and survival in the recalcitrant species Quercus robur L.: Evidence for a critical moisture content. J. Exp. Bot. 1992, 43, 671–679. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Blake, P.S. Indeterminate development in desiccation-sensitive seeds of Quercus robur L. Seed Sci. Res. 1994, 4, 127–133. [Google Scholar] [CrossRef]
- Corbineau, F.; Côme, D. Experiments on the storage of seeds and seedlings of Symphonia globulifera L.f. (Guttiferae). Seed Sci. Technol. 1986, 14, 585–591. [Google Scholar]
- Li, C.; Sun, W.Q. Desiccation sensitivity and activities of free radical-scavenging enzymes in recalcitrant Theobroma cacao seeds. Seed Sci. Res. 1999, 9, 209–218. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.Q. Desiccation tolerance of recalcitrant Theobroma cacao embryonic axes: The optimal drying rate and its physiological basis. J. Exp. Bot. 2000, 51, 1911–1919. [Google Scholar] [CrossRef]
- Chin, H.F.; Roberts, E.H. Recalcitrant Crop Seeds; Tropical Press: Kuala Lumpur, Malaysia, 1980; p. 152. [Google Scholar]
- Côme, D.; Corbineau, F. Storage of seeds. In Improving Postharvest Technologies of Fruits, Vegetables and Ornamentals; Artés, F., Gil, M.I., Conesa, M.A., Eds.; IIR-IIF: Paris, France, 2000; pp. 755–770. [Google Scholar]
- Côme, D.; Corbineau, F. Les semences et le froid. In Les Végétaux et le Froid; Côme, D., Ed.; Hermann: Paris, France, 1992; pp. 401–461. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Soetisna, U. Seed storage behaviour in Elaeis guineensis. Seed Sci. Res. 1991, 1, 99–104. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Effect of storage temperature and moisture on the germination of papaya seeds. Seed Sci. Res. 1991, 1, 69–72. [Google Scholar] [CrossRef]
- Sacande, M. Stress, Storage & Survival of Neem Seed. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2000; p. 124. [Google Scholar]
- Dussert, S.; Chabrillance, N.; Engelmann, F.; Hamon, S. Quantitative estimation of seed desiccation sensitivity using a quantal response model: Application to nine species of the genus Coffea L. Seed Sci. Res. 1999, 9, 135–144. [Google Scholar] [CrossRef]
- Dussert, S.; Chabrillance, N.; Engelmann, F.; Anthony, F.; Louarn, J.; Hamon, S. Relationship between seed desiccation sensitivity, seed water content at maturity and climatic characteristics of native environments of nine Coffea L. species. Seed Sci. Res. 2000, 10, 293–300. [Google Scholar] [CrossRef]
- Dickie, J.B.; Pritchard, H.W. Systematic and evolutionary aspects of desiccation tolerance in seeds. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 239–259. [Google Scholar]
- Ewart, A.J. On the longevity of seeds; Kessinger Publishing: Whitefish, MT, USA, 1908; Volume 21. [Google Scholar]
- Becquerel, C. La longévité des graines macrobiotiques. C. R. Acad. Sci. Paris 1934, 199, 1662–1664. [Google Scholar]
- Dum, S. Germination of ancient seeds. Floristical observations and experiments with archaelogically dated soil samples. Dank Bot. Arkiv. 1965, 24, 1–70. [Google Scholar]
- Posild, A.E.; Harrington, C.R.; Mulligan, G.A. Lupinus arcticus Wats. Grown from seeds of the pleistocene age. Science 1967, 158, 113–114. [Google Scholar] [CrossRef]
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M. Germination, genetics, and growth of an ancient date seeds. Science 2008, 320, 1464. [Google Scholar] [CrossRef]
- Shen-Miller, J. Sacred lotus, the long-living fruits of China Antique. Seed Sci. Res. 2002, 12, 131–143. [Google Scholar] [CrossRef]
- Lerman, J.C.; Cigliano, E.M. New carbon-14 evidence for six hundred years old Canna compacta seed. Nature 1971, 232, 568–570. [Google Scholar] [CrossRef]
- Côme, D.; Corbineau, F. Dictionnaire de la Biologie des Semences et des Plantules; Lavoisier: Paris, France, 2006; p. 226. [Google Scholar]
- Bewley, J.D.; Black, M. Physiology and biochemistry of seeds in relation to germination. In Viability, Dormancy and Environmental Control; Spinger: Berlin/Heidelberg, Germany; New York, NY, USA, 1982; Volume 2, p. 375. [Google Scholar]
- Roberts, E.H. Storage environment and the control of viability. In Viability of Seeds; Roberts, E.H., Ed.; Chapman and Hall: London, UK, 1972; pp. 14–58. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Dickie, J.B.; Ellis, R.H.; Kraak, H.L.; Ryder, K.; Tompsett, P.B. Temperature and seed storage longevity. Ann. Bot. 1990, 55, 147–151. [Google Scholar] [CrossRef]
- Ellis, R.H.; Osei-Bonsu, K.; Roberts, E.H. The influence of genotype, temperature and moisture on seed longevity in chickpea, cowpea and soya bean. Ann. Bot. 1982, 50, 69–82. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. A comparison of low moisture content limit to the logarithmic relation between seed moisture and longevity in 12 species. Ann. Bot. 1989, 63, 601–611. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Tao, K.L. Low moisture-content limits to relations between seed longevity and moisture. Ann. Bot. 1990, 65, 493–504. [Google Scholar] [CrossRef]
- Walters, C. Deterioration and longevity. In The Encyclopedia of Seeds. Science, Technology and Uses; Black, M., Bewley, J.D., Hamer, P., Eds.; CAB International: Oxfordshire, UK; Cambridge, MA, USA, 2006; pp. 137–138. [Google Scholar]
- Hay, F.R.; Valdez, R.; Lee, J.-S.; Sta. Cruz, P.C. Seed longevity phenotyping: Recommendations on research methodology. J. Exp. Bot. 2019, 70, 425–434. [Google Scholar] [CrossRef]
- Hay, F.R.; Davies, R.M.; Dickie, J.B.; Merritt, D.J.; Wolkis, D.M. More on seed longevity phenotyping. Seed Sci. Res. 2022, 32, 144–149. [Google Scholar] [CrossRef]
- Newton, R.; Hay, F.; Probert, R. Protocol for comparative seed longevity testing. In Technical Information Sheet 01; Kew, Royal Botanic Gardens: London, UK, 2009. [Google Scholar]
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. Plant Physiol. 1990, 94, 1019–1023. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Roos, E.E.; Crane, J. Theoretical basis of protocols for seed storage. III. Optimum moisture contents for pea seeds stored at different temperatures. Ann. Bot. 1994, 74, 531–540. [Google Scholar] [CrossRef]
- Chai, J.; Ma, R.; Li, L.; Du, Y. Optimum moisture contents of seeds stored at ambient temperatures. Seed Sci. Res. 1998, 8, 23–28. [Google Scholar]
- Walters, C.; Engels, J. The effects of storing seeds under extremely dry conditions. Seed Sci. Res. 1998, 8, 3–8. [Google Scholar]
- Harrington, J.F. Seed storage and longevity. In Seed Biology; Kozlowski, T.T., Ed.; Academic Press: London, UK; New York, NY, USA, 1972; Volume III, pp. 145–245. [Google Scholar]
- Ellis, R.H.; Hong, T.D. Seed longevity—Moisture content relationships in hermetic and open storage. Seed Sci. Technol. 2007, 35, 423–431. [Google Scholar] [CrossRef]
- Schwember, A.R.; Bradford, K.J. Oxygen interacts with priming, moisture content and temperature to affect the longevity of lettuce and onion seeds. Seed Sci. Res. 2011, 21, 175–185. [Google Scholar] [CrossRef]
- Roberts, E.H.; Abdalla, F.H. The influence of temperature, moisture, and oxygen on period of seed viability in barley, broad beans, and peas. Ann. Bot. 1968, 32, 97–117. [Google Scholar] [CrossRef]
- Bass, L.N. Controlled atmosphere and seed storage. Seed Sci. Technol. 1973, 1, 463–492. [Google Scholar]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture, Revised ed.; Food and Agricultural Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Ballesteros, D.; Pence, V.C. Survival and death of seeds during liquid nitrogen storage: A case study on seeds with short lifespans. CryoLetters 2017, 38, 278–289. [Google Scholar]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. II. The influence of temperature on optimal moisture levels. Seed Sci. Res. 1993, 3, 201–213. [Google Scholar] [CrossRef]
- Salmen Espindola, L. Caractéristiques de la Germination des Graines Récalcitrantes d’Araucaria Angustifolia et Événements Cellulaires et Métaboliques Associés à la Perte de Viabilitévde L’embryon au Cours de la Déshdratation. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 1995; p. 134. [Google Scholar]
- Odawara, S.A.; Watanabe, H.; Imaseki, H. Involvement of cellular membrane in regulation of ethylene production. Plant Physiol. 1977, 18, 569–575. [Google Scholar]
- Poulsen, K.M.; Ericksen, E.N. Physiological aspects of recalcitrance in embryonic axes of Quercus robur L. Seed Sci. Res. 1992, 2, 215–221. [Google Scholar] [CrossRef]
- Becwar, M.R.; Stanwood, P.C.; Roos, E.E. Dehydration effects on imbibitional leakage from desiccation sensitive seeds. Plant Physiol. 1982, 69, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Pammenter, N.W.; Vertucci, C.; Berjak, P. Homeohydrous (recalcitrant) seeds: Dehydration, the state of water and viability characteristics in Landolphia kirkii. Plant Physiol. 1991, 96, 1093–1098. [Google Scholar] [CrossRef]
- Hendry, G.A.F. Oxygen, free radical processes and seed longevity. Seed Sci. Res. 1993, 3, 141–153. [Google Scholar] [CrossRef]
- Hendry, G.A.F.; Finch-Savage, W.E.; Thorpe, P.C.; Atherton, N.M.; Buckland, S.M.; Nilsson, K.A.; Seel, W.E. Free radical processes and loss of seed viability during desiccation in the recalcitrant species Quercus robur L. New Phytol. 1992, 122, 273–279. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Grange, R.I.; Hendry, G.A.F.; Atherton, N.M. Embryo water status and loss of viability during desiccation in the recalcitrant species Quercus robur L. In Proceedings of the Fourth International Workshop on Seeds: Basic and Applied Aspects of Seed Biology, Angers, France, 20–24 July 1992; Côme, D., Corbineau, F., Eds.; ASFIS: Paris, France, 1993; pp. 723–730. [Google Scholar]
- Finch-Savage, W.E.; Hendry, G.A.F.; Atherton, N.M. Free radical activity and loss of viability during drying of desiccation-sensitive tree seeds. Proc. R. Soc. Edinb. 1994, 102B, 257–260. [Google Scholar] [CrossRef]
- Chaitanya, K.S.K.; Naithani, S.C. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane pertubation during loss of viability in seeds of Shorea robusta Gaertn. New Phytol. 1994, 126, 623–627. [Google Scholar] [CrossRef]
- Leprince, O.; Deltour, R.; Thorpe, P.C.; Atherton, N.M.; Hendry, G.A.F. The role of free radicals and radical processing systems in loss of desiccation tolerance in germinating maize. New Phytol. 1990, 116, 573–580. [Google Scholar] [CrossRef]
- Farrant, J.M.; Bailly, C.; Leymarie, J.; Hamman, B.; Côme, D.; Corbineau, F. Wheat seedlings as a model to understand desiccation-tolerance and -sensitivity. Physiol. Plant. 2004, 120, 563–574. [Google Scholar] [CrossRef]
- Lah, N.H.C.; El Enshasy, H.A.; Mediani, A.; Azizan, K.A.; Aizat, W.M.; Tan, K.J.; Afzan, A.; Noor, N.M.; Rohani, E.R. An insight into the behaviour of recalcitrant seeds by understanding their molecular changes upon desiccation and low temperature. Agronomy 2023, 13, 2099. [Google Scholar] [CrossRef]
- Osborne, D.J. Senescence in seeds. In Senescence in Plants; Thimann, K.V., Ed.; CRC Press: Boca Raton, FL, USA, 1980; pp. 13–37. [Google Scholar]
- Roberts, E.H. Seed aging: The genome and its expression. In Senescence and Aging in Plants; Noodén, L.D., Leopold, A.C., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 465–498. [Google Scholar]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Advances in the understanding of reactive oxygen species-dependent regulation on seed dormancy, germination, and deterioration in crops. Front. Pant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Ramos, J.M.; Lopez, S.; Vazquez, E.; Murillo, E. DNA integrity and DNA polymerase activity in deteriorated maize embryo axes. J. Plant Physiol. 1988, 133, 600–604. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.B.; Richards, C.M.; Walters, C. Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. J. Exp. Bot. 2017, 68, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the fate of mRNA in aging seeds: Protection, destruction, or slow decay ? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: New York, NY, USA, 1999; p. 980. [Google Scholar]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and non-enzymatic mechanisms contribute to lipid oxidation during seed aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plant. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Mira, S.; Estrelles, E.; Gonzalez-Benito, M.E.; Corbineau, F. Biochemical changes induced in seeds of Brassicaceae wild species during ageing. Acta Physiol. Plant. 2011, 33, 1803–1809. [Google Scholar] [CrossRef]
- Sung, J.M. Lipid peroxidation and peroxide-scavenging in soybean seeds during aging. Physiol. Plant. 1996, 97, 85–89. [Google Scholar] [CrossRef]
- Pukacka, S. Changes in membrane lipid components and anti-oxidant levels during natural ageing of Acer platanoides seeds. Physiol. Plant. 1991, 82, 306–310. [Google Scholar] [CrossRef]
- Gidrol, X.; Serghini, H.; Noubhani, A.; Mocquot, B.; Mazliak, P. Biochemical changes induced by accelerated aging in sunflower seeds. I. lipid peroxidation and membrane damage. Physiol. Plant. 1989, 76, 591–597. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Girard, J.; Le Meste, M. Absence de relation entre le taux de radicaux libres mesuré par RPE et la viabilité des semences de blé. Comptes Rendus Acad. Sci. Paris 1992, 111, 417–422. [Google Scholar]
- Lehner, A.; Mamadou, N.; Poels, P.; Côme, D.; Bailly, C.; Corbineau, F. Changes in soluble carbohydrates, lipid peroxidation and antioxidant enzyme activities in the embryo during ageing in wheat grains. J. Cereal Sci. 2008, 47, 555–565. [Google Scholar] [CrossRef]
- Linn, S.S.; Pearce, R.S. Changes in lipids in bean seeds (Phaseolus vulgaris) and corn caryopses (Zea mays) aged in contrasting environments. Ann. Bot. 1990, 65, 452–456. [Google Scholar] [CrossRef]
- Kalpana, R.; Madhava Rao, K.V. Lipid changes during accelerated ageing of seeds of pigeonpea (Cajanus cajan (L.) Millsp) cultivars. Seed Sci. Technol. 1996, 24, 475–483. [Google Scholar]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Morscher, F.; Kranner, I.; Arc, E.; Bailly, C.; Roach, T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann. Bot. 2015, 116, 669–678. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Free radical scavenging as affected by accelerated ageing and susequent priming in sunflower seeds. Physiol. Plant. 1998, 104, 646–652. [Google Scholar] [CrossRef]
- Boucelha, L.; Abrous-Belbachir, O.; Djebbar, R. Is protein carbonylation a biomarker of seed priming and ageing? Funct. Plant Biol. 2021, 48, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Cheah, K.S.E.; Osborne, D.J. DNA lesions occur with loss of viability in embryos of ageing rye seeds. Nature 1978, 272, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.M.; Lin, C.L.G. Oxidative damage to RNA: Mechanisms, consequences, and diseases. Cell. Mol. Life Sci. 2010, 67, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Bazin, J.; Batla, D.; Dussert, S.; El-Maarouf-Bouteau, H.; Bailly, C. Role of relative humidity, temperature, and water status in dormancy alleviation of sunflower seeds during dry after-ripening. J. Exp. Bot. 2011, 62, 627–640. [Google Scholar] [CrossRef]
- Corbineau, F.; Taskiran-Ôzbingöl, N.; El-Maarouf-Bouteau, H. Improvement of seed quality by priming: Concept and biologicl basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- Dell’Aquila, A.; Taranto, G. Cell division and DNA-synthesis during osmopriming treatment and following germination in aged wheat embryos. Seed Sci. Technol. 1986, 14, 333–341. [Google Scholar]
- Fujikura, Y.; Karssen, C.M. Effects of controlled deterioration and osmopriming on protein synthesis of cauliflower during early germination. Seed Sci. Res. 1992, 2, 23–31. [Google Scholar] [CrossRef]
- Van Pijlen, J.G.; Kraak, H.L.; Bino, R.J.; De Vos, C.H.R. Effects of ageing and osmopriming on germination characteristics and chromosome aberrations of tomato. Seed Sci. Technol. 1995, 29, 823–830. [Google Scholar]
- Georghiou, K.; Thanos, C.A.; Passam, H.C. Osmoconditioning as a means of counteracting the ageing of pepper seeds during high temperature storage. Ann. Bot. 1987, 60, 279–285. [Google Scholar] [CrossRef]
- Kibinza, S.; Bazin, J.; Bailly, C.; Farrant, J.M.; Corbineau, F.; El-Maarouf-Bouteau, H. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 2011, 181, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A plant DNA ligase is an important determinant of seed longevivty. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Ogé, L.; Bourdais, G.; Bove, J.; Collet, B.; Godin, B.; Granier, F.; Boutin, J.P.; Job, D.; Jullien, M.; Grappin, P. Protein repair L-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis. Plant Cell 2008, 20, 3022–3037. [Google Scholar] [CrossRef]
- Verma, P.; Kaur, H.; Petla, B.P.; Rao, V.; Saxena, S.C.; Majee, M. PROTEIN L-ISASPARTYL METHYLTRANSFERASE2 gene is differentially expressed in chickpea and enhances seed vigor and longevity by reducing abnormal isoaspartyl accumulation predominantly in seed nuclear proteins. Plant Physiol. 2013, 16, 1141–1157. [Google Scholar] [CrossRef]
- Corbineau, F. Markers of seed quality: From present to future. Seed Sci. Res. 2012, 22, S61–S68. [Google Scholar] [CrossRef]

| Origine | Species | Family |
|---|---|---|
| Temperate | Acer saccharinum | Sapindaceae |
| Acer pseudoplatanus | Sapindaceae | |
| Aesculus hippocastanum | Hippocastanaceae | |
| Castanea spp. | Fagaceae | |
| Corylus avellana | Corylaceae | |
| Juglans spp. | Juglandaceae | |
| Quercus sp. | Fagaceae | |
| Populus spp. | Salicaceae | |
| Salix spp. | Salicaceae | |
| Tropical | Araucaria spp. | Araucariaceae |
| Avicenia marina | Avicenniaceae | |
| Camellia sinensis | Theaceae | |
| Cocos nucifera | Arecaceae/Palmaceae | |
| Euphorbia longan | Euphorbiaceae/Sapindaceae | |
| Garcinia mangostana | Clusiaceae/Guttiferae | |
| Hevea brasiliensis | Euphorbiaceae | |
| Hopea odorata | Dipterocarpaceae | |
| Litchi chinensis | Sapindaceae | |
| Mangifera indica | Anacardiaceae | |
| Nephelium lappaceum | Sapindaceae | |
| Persea americana | Lauraceae | |
| Shorea roxburghii | Dipterocarpaceae | |
| Shorea talura | Dipterocarpaceae | |
| Symphonia globulefera | Guttifereae | |
| Theobroma cacao | Steruliaceae |
| Species | Minimum Water Content (% of Dry Matter) | References |
|---|---|---|
| Acer pseudoplatanus (European sycamore) | 30–45 | [43,44] |
| Acer saccharinum (silver maple) | 30–35 | [45] |
| Araucaria angustifolia (Parana pine) | 25–35 | [46,47] |
| Clausena lansium | 33–35 | [48,49] |
| Euphorbia longan (longan) | 25–30 | [50] |
| Hevea brasiliensis (hevea) | 20–25 | [51] |
| Hopea odorata | 20–25 | [52] |
| Litchi chinensis (litchi) | 20–30 | [48,50,53] |
| Mangifera indica (mango tree) | 30–35 | [50,52,54] |
| Quercus petraea (sessile oak) | 30–60 | [55] |
| Quercus robur (pedunculate oak) | 30–48 | [56,57] |
| Quercus rubra (Red oak) | 60–75 | [55] |
| Shorea roxburghii | 17–30 | [52] |
| Symphonia globulifera | 37–40 | [52,58] |
| Theobroma cacao (cocoa tree) | 45–50 | [59,60] |
| Species | Temperature Limit (°C) |
|---|---|
| Cedrela odorata (Spanish cedar) | 10 |
| Dryobalanops aromatica (Bornean camphol tree) | 5 |
| Hevea brasiiensis (rubber) | 15–16 |
| Hopea odorata (Chengal pasir) | 12 |
| Mangifera indica (mango tree) | 12 |
| Shorea roxburghii (Lac tree) | 12–15 |
| Shorea talura (Jalari tree) | 4 |
| Symphonia globulifera (Buckwax tree) | 15 |
| Type of Species | Species | P50 (Years) |
|---|---|---|
| Cereals | Avena sativa (oat) | 12.9 |
| Hordeum vulgare (barley) | 7.2 | |
| Triticum aestivum (wheat) | 7.6 | |
| Secale cereale (rye) | 4.5 | |
| Zea mays (corn) | 9.6 | |
| Legumes | Glycine max (soybean) | 3.4 |
| Medicago sativa (lucerne) | 10.5 | |
| Phaseolus vulgaris (French bean) | 15.9 | |
| Pisum sativum (garden pea) | 15.8 | |
| Vicia faba (broad bean) | 15.6 | |
| Other crops | Beta vulgaris (beet) | 16.5 |
| Brassica napus (rape) | 13.9 | |
| Helianthus annuus (sunflower) | 5.4 | |
| Nicotiana tabacum (tobacco) | 10.3 | |
| Allium cepa (onion) | 5.4 | |
| Vegetables | Apium graveolens (celery) | 4.1 |
| Cichorium intybus (endive) | 5.4 | |
| Cucumis sativus (cucumber) | 4.9 | |
| Daucus carota (carrot) | 6.6 | |
| Lactuca sativa (lettuce) | 6.4 | |
| Lycopersicon esculentum (tomato) | 24.5 | |
| Pastinaca sativa (parsnip) | 4.1 | |
| Petroselinum crispum (parsley) | 3.4 |
| Species | Germination (%) after Storage for | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (Harvest) | 4 Years | 8 Years | 12 Years | 20 Years | ||||||
| C | FD | C | FD | C | FD | C | FD | C | FD | |
| Allium cepa | 92 | 92 | 8 | 72 | 2 | 74 | 2 | 76 | 0 | 68 |
| (onion) | ||||||||||
| Asparagus officinale | 93 | 96 | 0 | 62 | 0 | 60 | 2 | 35 | 0 | 25 |
| (asparagus) | ||||||||||
| Cichorium intybus | 96 | 90 | 4 | 100 | 0 | 68 | 0 | 65 | 0 | 85 |
| (endive) | ||||||||||
| Foeniculum officinale | 100 | 83 | 4 | 72 | 0 | 50 | 0 | 56 | 0 | 50 |
| (fennel) | ||||||||||
| Lonicera caprifolium | 90 | 92 | 60 | 100 | 50 | 78 | 38 | 71 | 32 | 96 |
| (honeysuckle) | ||||||||||
| Papaver somnifera | 92 | 90 | 80 | 100 | 16 | 72 | 0 | 60 | 0 | 80 |
| (opium poppy) | ||||||||||
| Portulaca oleracea | 84 | 100 | 100 | 100 | 69 | 96 | 7 | 95 | 0 | 80 |
| (purslane) | ||||||||||
| Trifolium repens | 100 | 100 | 100 | 100 | 90 | 88 | 82 | 81 | 100 | 100 |
| (white clover) | ||||||||||
| Valerianella olitoria | 88 | 96 | 4 | 100 | 0 | 80 | 0 | 84 | 0 | 72 |
| (lamb’s lettuce) | ||||||||||
| Duration of Desiccation (h) | Moisture Content (% Dry Weight) | Cellular and Metabolic Events |
|---|---|---|
| 0–0.5 | 90–95 |
|
| 0.5–1 | 70–75 |
|
| 1–1.5 | 55–60 |
|
| 1–2 | 50–55 |
|
| 1.5–2 | 40–45 |
|
| 2–114 | 30–45 * |
|
| 4–6 | 20–27 * |
|
| 6–7 | 20–22 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbineau, F. The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds 2024, 3, 56-75. https://doi.org/10.3390/seeds3010005
Corbineau F. The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds. 2024; 3(1):56-75. https://doi.org/10.3390/seeds3010005
Chicago/Turabian StyleCorbineau, Françoise. 2024. "The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity" Seeds 3, no. 1: 56-75. https://doi.org/10.3390/seeds3010005
APA StyleCorbineau, F. (2024). The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds, 3(1), 56-75. https://doi.org/10.3390/seeds3010005






