Geometric Analysis of Seed Shape Diversity in the Cucurbitaceae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Photographs
2.3. Model Design by Elliptic Fourier Transform (EFT)
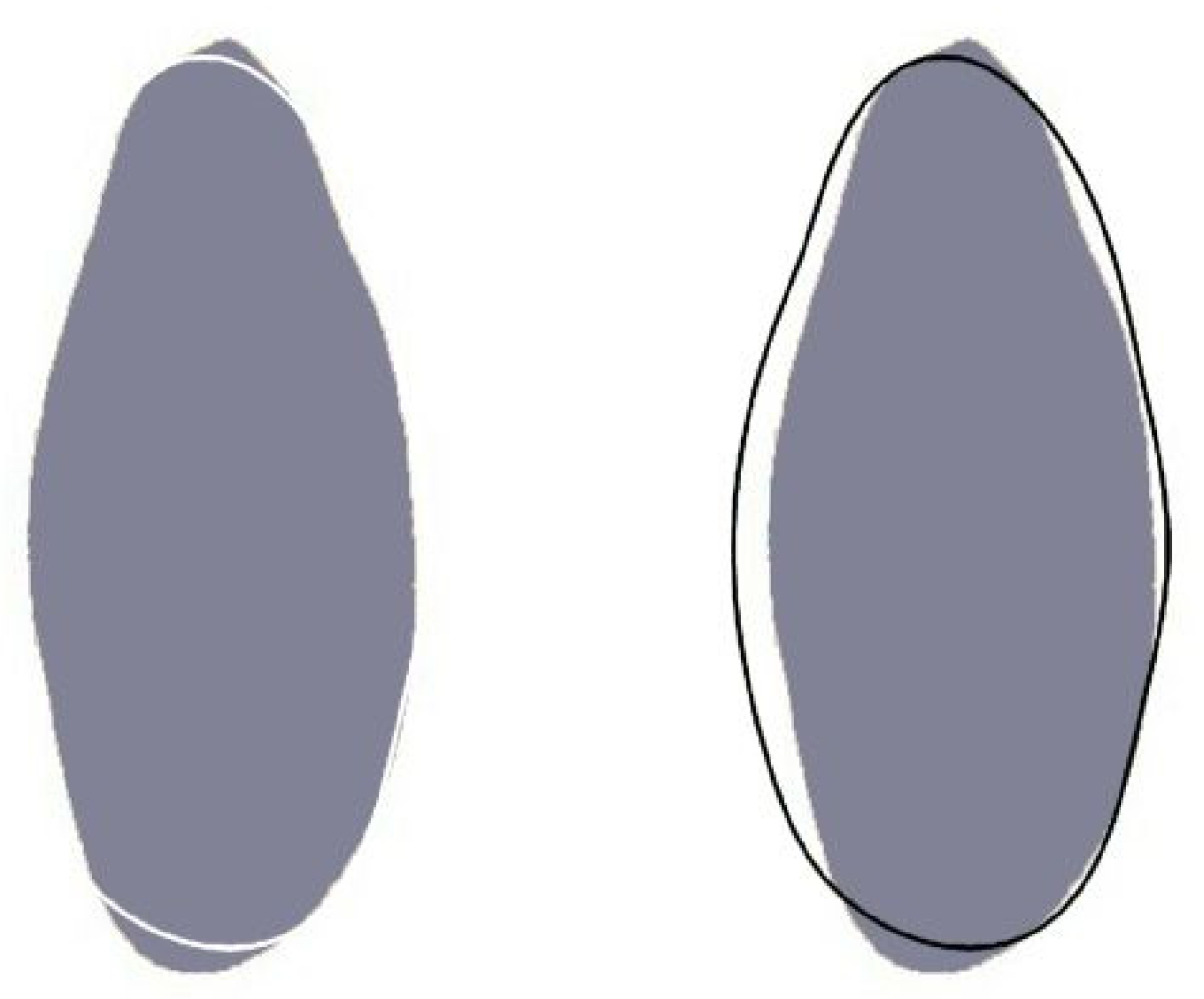
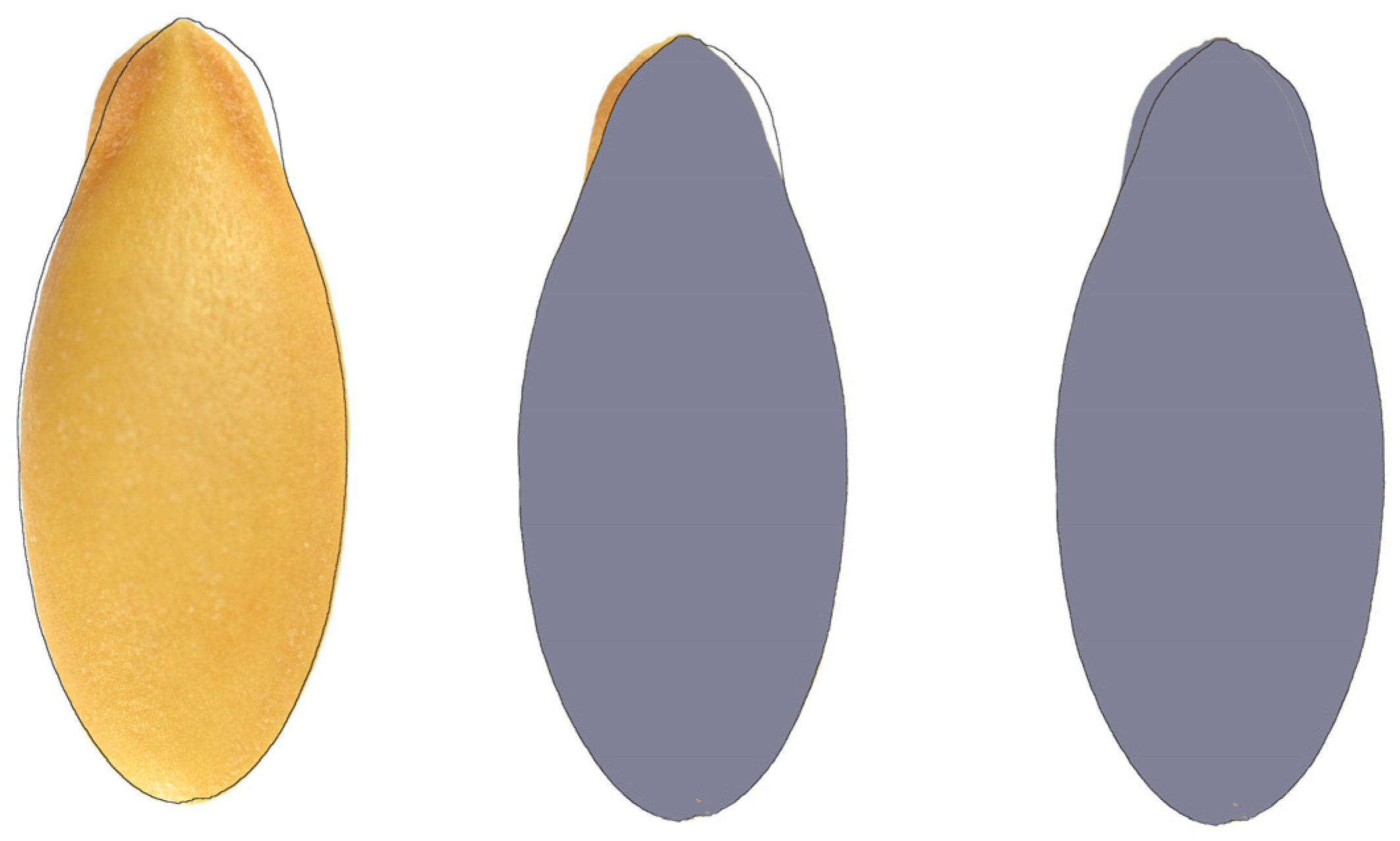
2.4. Seed Shape Quantification by Comparison with a Model
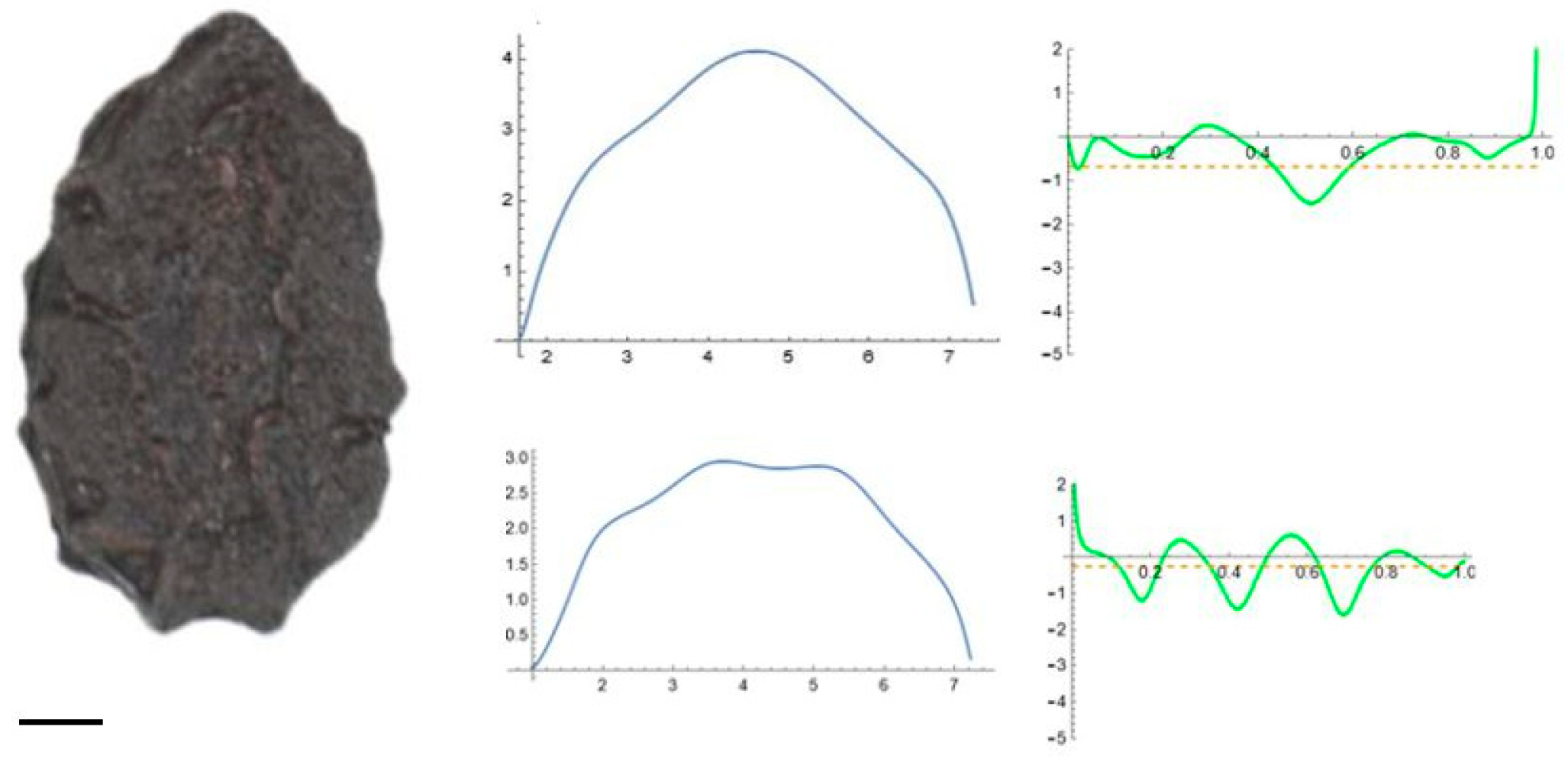
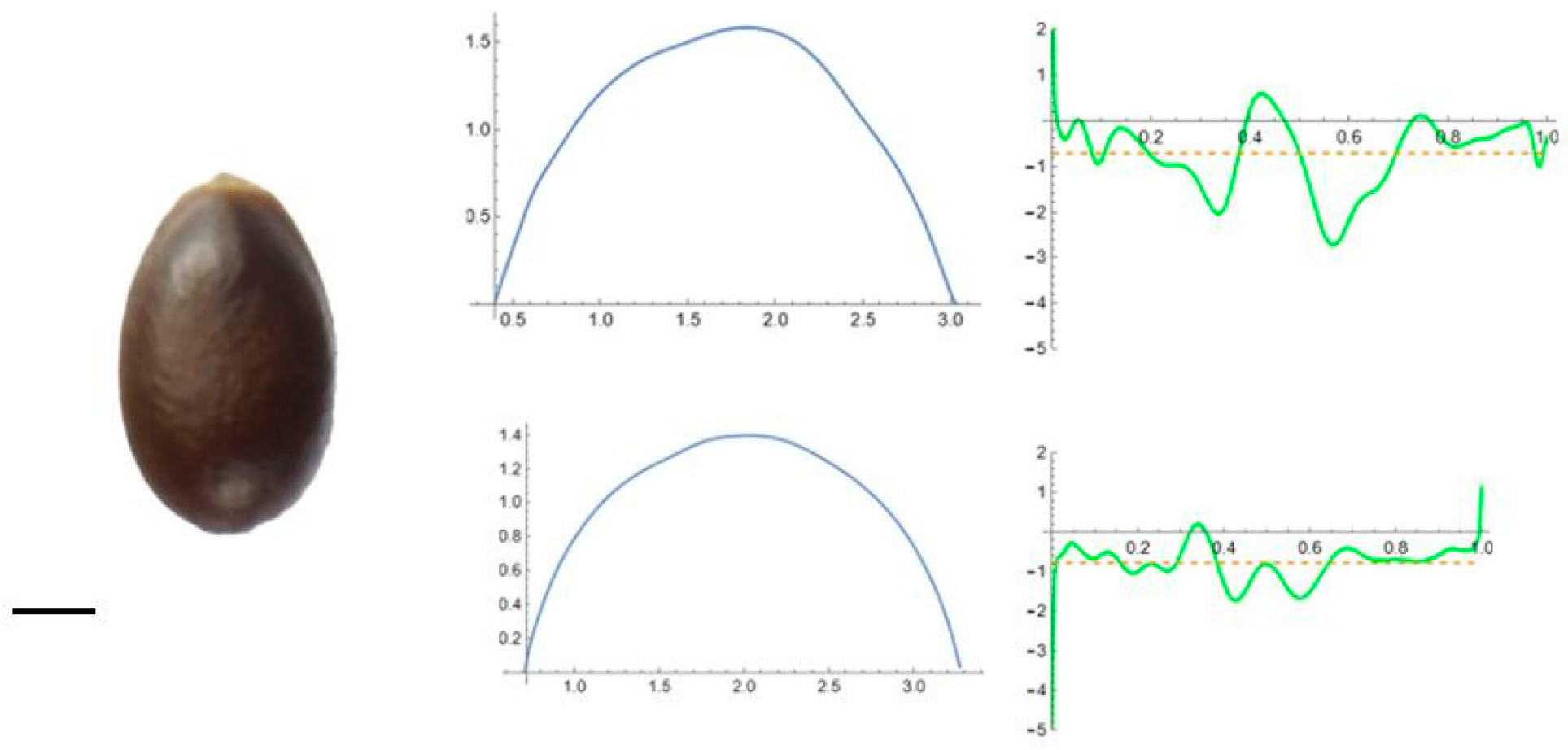
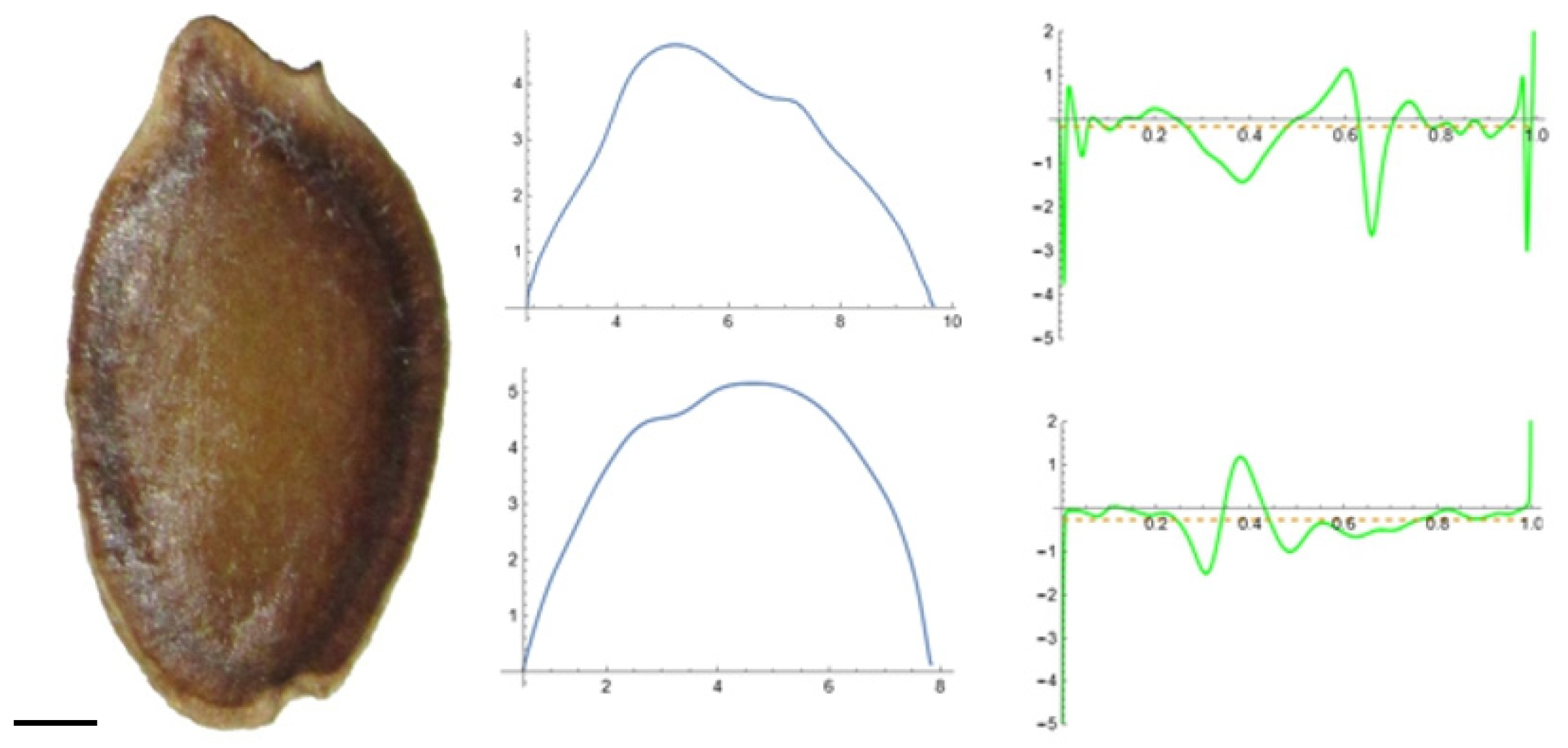
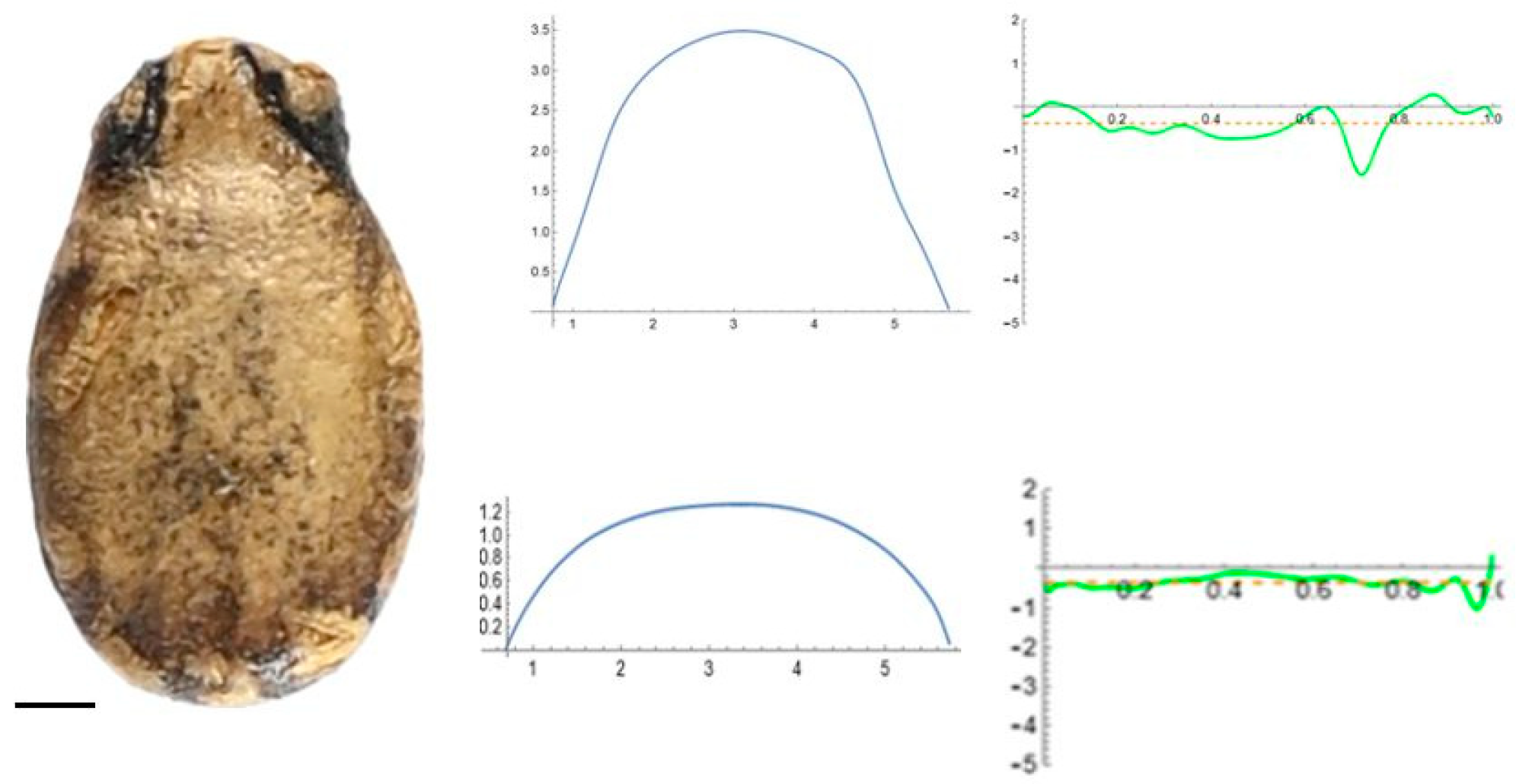
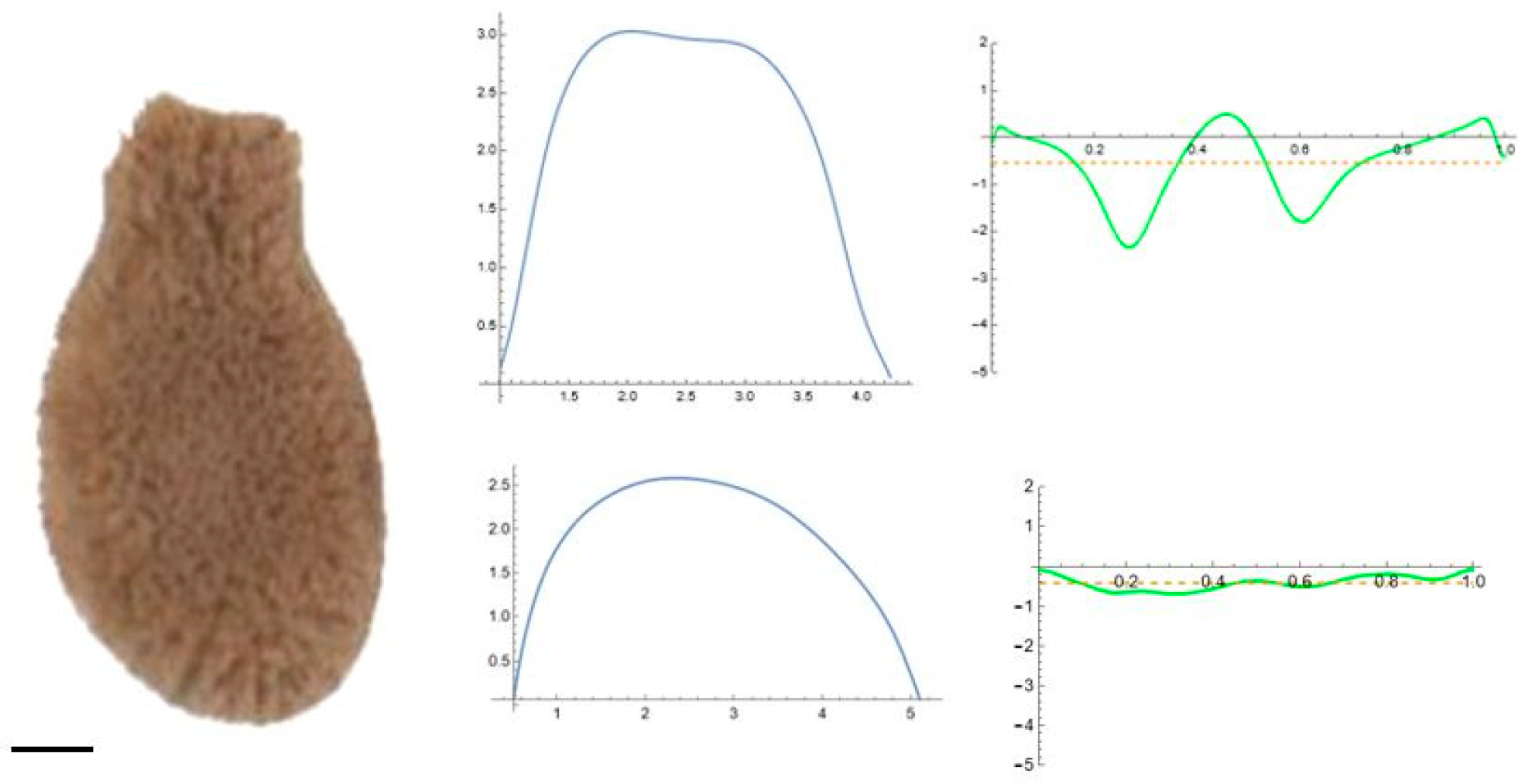
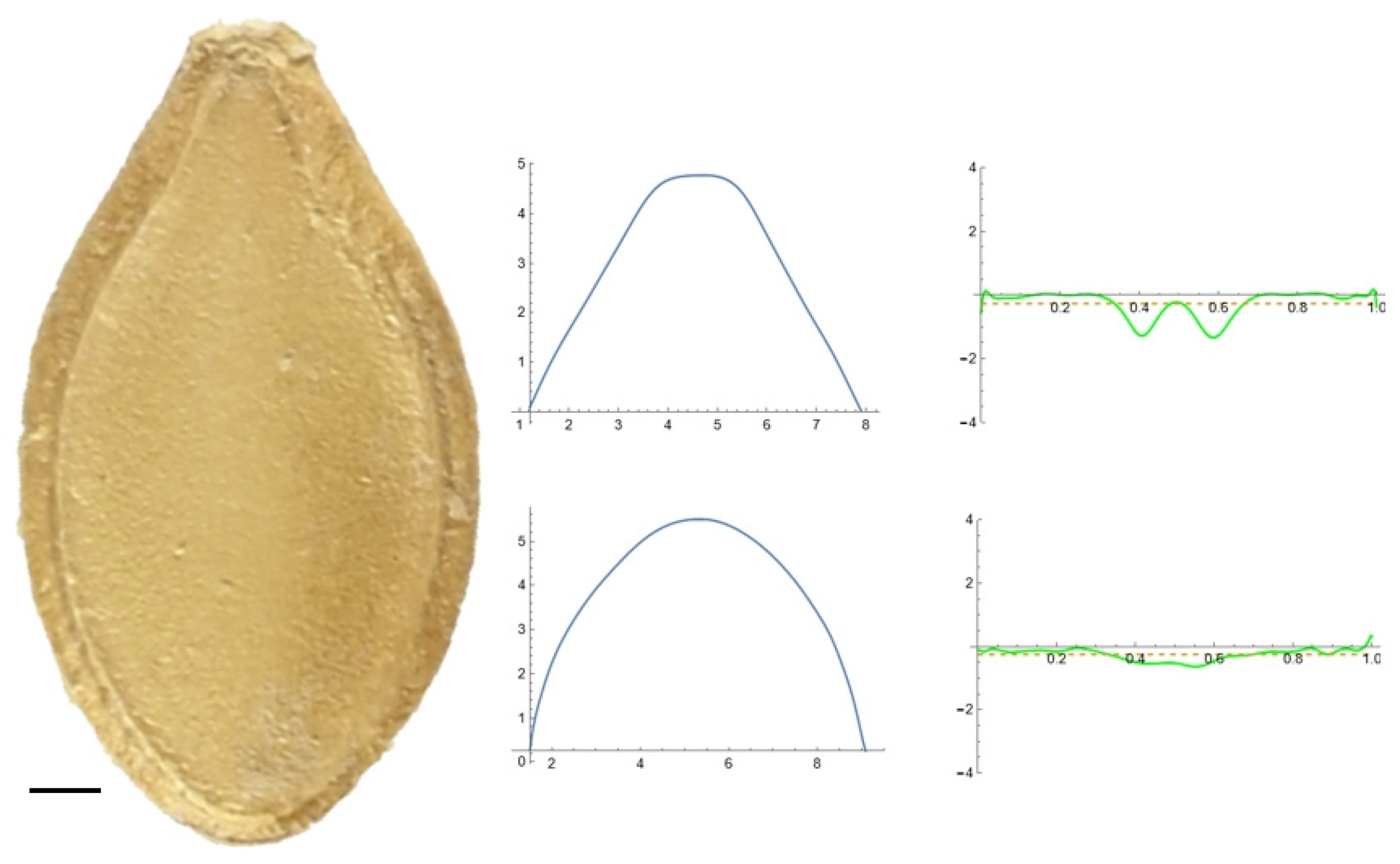
2.5. Curvature Analysis
2.6. Symmetry Calculation
2.7. Statistical Analysis
3. Results
3.1. General Morphological Measurements
3.2. Curvature Analysis
3.3. Symmetry Analysis
3.4. Morphological Analysis in Cucumis
3.4.1. Curvature Analysis
3.4.2. Morphological Comparison by Models Based on EFT Curves Reproducing the Seed Silhouettes
3.4.3. Symmetry Analysis
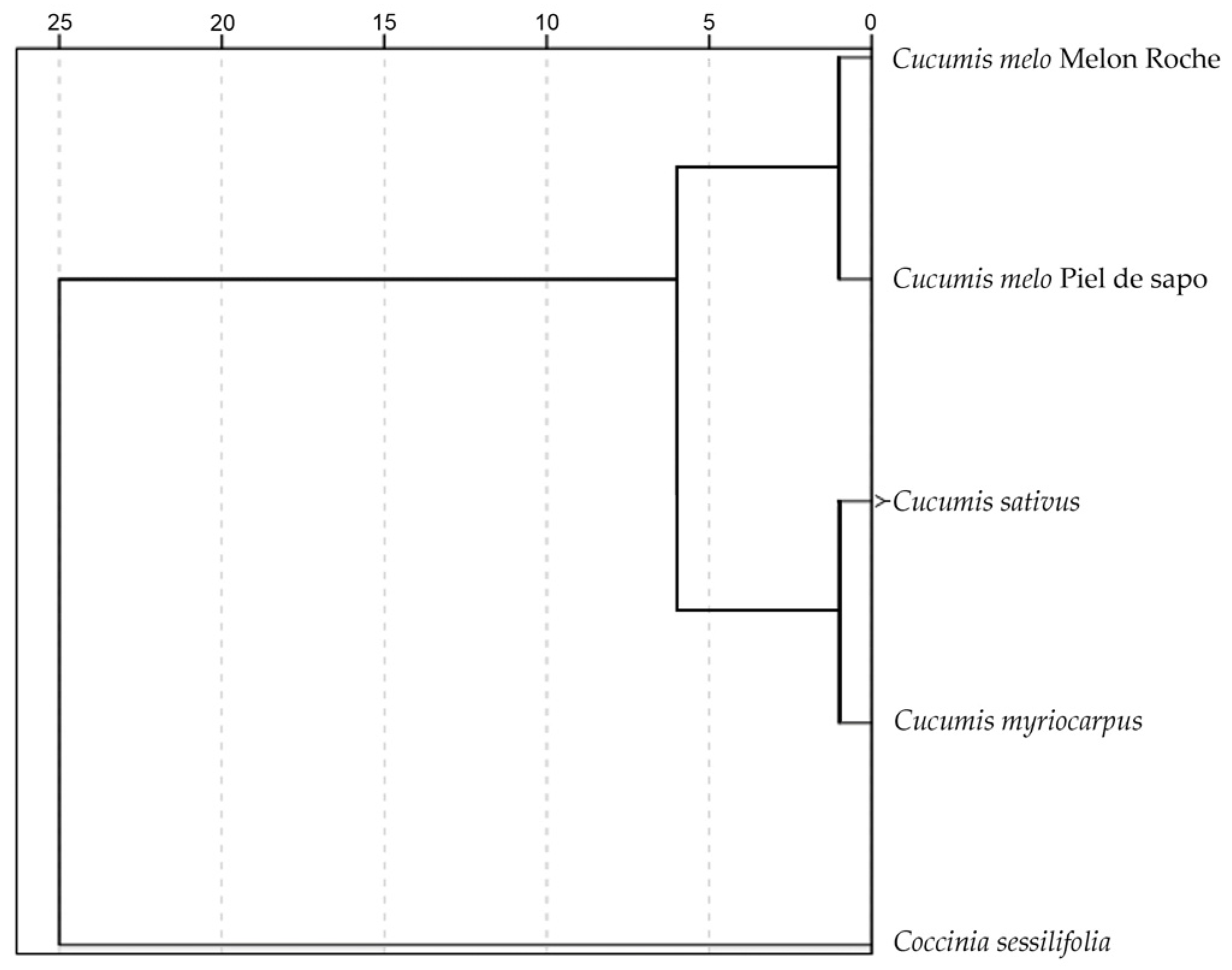
3.4.4. Morphological Measurements in Relation with the Taxonomy of Cucumis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; Volume XIV, 666p. [Google Scholar]
- Machado, M. Apodanthera—Two caduciform cucumbers from Bahia, Brazil. J. Cact. Succ. Soc. Amer. 2009, 81, 147–149. [Google Scholar] [CrossRef]
- Pitrat, M.; Chauvet, M.; Foury, C. Diversity, history and production of cultivated cucurbits. Acta Hortic. 1999, 492, 21–28. [Google Scholar] [CrossRef]
- Ng, T.J. New Opportunities in the Cucurbitaceae. In New Crops; Janick, J., Simon, J.E., Eds.; Wiley: New York, NY, USA, 1993; pp. 538–546. Available online: https://hort.purdue.edu/newcrop/proceedings1993/V2-538.html#Old%20World%20Cucurbits (accessed on 29 December 2023).
- Shaik, R.S.; Lepschi, B.J.; Gopurenko, D.; Urwin, N.; Burrows, G.E. An integrative morphological and molecular approach to identification of three Australian cucurbitaceous invasive weeds: Citrullus colocynthis, C. lanatus and Cucumis myriocarpus. Aust. Syst. Bot. 2016, 29, 247–264. [Google Scholar] [CrossRef]
- Portela, J.L.C.; Pérez, J.J.P.; Pérez, R.P. Sicyos angulatus L. (Cucurbitaceae), nueva adventicia para la flora de Galicia. Boletín Biga 2022, 20, 91–97. [Google Scholar] [CrossRef]
- Schaefer, H.; Heibl, C.; Renner, S.S. Gourds afloat: A dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proc. R. Soc. B 2009, 276, 843–851. [Google Scholar] [CrossRef]
- Schaefer, H.; Renner, S.S. Cucurbitaceae. In Flowering Plants. Eudicots; Kubitzki, K., Ed.; The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2010; Volume 10. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.; Hu, Y.; Huang, J.; Zhao, Y.; Zhang, L.; Huang, C.-H.; Ma, H. Phylotranscriptomics in Cucurbitaceae reveal multiple whole-genome duplications and key morphological and molecular innovations. Mol. Plant 2020, 13, 1117–1133. [Google Scholar] [CrossRef]
- Flora Malesiana. Available online: https://portal.cybertaxonomy.org/flora-malesiana/cdm_dataportal/taxon/6c2340ef-b044-4b06-b0d0-9b6dfc900e83 (accessed on 6 October 2023).
- Shamrov, I.I. Diversity and typification of ovules in flowering plants. Wulfenia 2018, 25, 81–109. [Google Scholar]
- Johri, B.M.; Ambegaokar, K.B.; Srivastava, P.S. Comparative Embryology of Angiosperms; Springer: Berlin/Heidelberg, Germany, 1992; Volume 1. [Google Scholar]
- Singh, B. Studies on the structure and development of seeds of Cucurbitaceae 1. Seed of Echinocystis wrightii Cogn. Phytomorphology 1952, 2, 201–209. [Google Scholar]
- Singh, B. Studies on the structure and development of seeds of Cucurbitaceae. Phytomorphology 1953, 3, 224–239. [Google Scholar]
- Singh, B. The structure and development of Abelmoschus moschatus Medic. seed. Phytomorphology 1967, 17, 282–290. [Google Scholar]
- Singh, D. Cucurbitaceae. In: Symp Comparative embryology of angiosperms. Indian Natl. Sci. Acad. Bull. 1970, 41, 212–219. [Google Scholar]
- Singh, D.; Dathan, A.S.R. Structure and development of the seed coat in Cucurbitaceae 6. Seeds of Cucurbita. Phytomorphology 1972, 22, 29–45. [Google Scholar]
- Singh, D.; Dathan, A.S.R. Structure and development of the seed coat in Cucurbitaceae 7. Seeds of Marah KelL. Bull. Torrey Bot. Club 1972, 99, 239–242. [Google Scholar] [CrossRef]
- Singh, D.; Dathan, A.S.R. Structure and development of the seed coat in Cucurbitaceae 9. Seeds of Corallocarpus, Kedrostis and Ibervillea. Bull. Torrey Bot. Club 1974, 101, 78–82. [Google Scholar] [CrossRef]
- Heneidak, S.; Khalik, K.A. Seed coat diversity in some tribes of Cucurbitaceae: Implications for taxonomy and species identification. Acta Bot. Bras. 2015, 29, 129–142. [Google Scholar] [CrossRef]
- Murovec, J.; Draslar, K.; Bohanec, B. Detailed analysis of Cucurbita pepo seed coat types and structures with scanning electronmicroscopy. Botany 2012, 90, 1161–1169. [Google Scholar] [CrossRef]
- Juan, R.; Pastor, J.; Fernández, I. SEM and light microscope observations on fruit and seeds in Scrophulariaceae from southwest Spain and their systematic significance. Ann. Bot. 2000, 86, 323–338. [Google Scholar] [CrossRef]
- Segarra, J.G.; Mateu, I. Seed morphology of Linaria species from eastern Spain: Identification of species and taxonomic implications. Bot J Linn. Soc. 2001, 135, 375–389. [Google Scholar] [CrossRef]
- Plaza, L.; Fernández, I.; Juan, R.; Pastor, J.; Pujadas, A. Micromorphological studies on seeds of Orobanche species from the Iberian Peninsula and the Balearic Islands, and their systematic significance. Ann. Bot. 2004, 94, 167–178. [Google Scholar] [CrossRef]
- Adams, C.A.; Baskin, J.M.; Baskin, C.C. Comparative morphology of seeds of four closely related species of Aristolochia subgenus Siphisia (Aristolochiaceae, Piperales). Bot. J. Linn. Soc. 2005, 148, 433–436. [Google Scholar] [CrossRef]
- Amini, E.; Zarre, S.; Assadi, M. Seed micro-morphology and its systematic significance in Gypsophila (Caryophyllaceae) and allied genera. Nord. J. Bot. 2011, 29, 660–669. [Google Scholar] [CrossRef]
- Crow, G.E. The systematic significance of seed morphology in Sagina (Caryophyllaceae) Under Scanning Electron Microscopy. Brittonia 1979, 31, 52–63. [Google Scholar] [CrossRef]
- Ullah, F.; Papini, A.; Shah, S.N.; Zaman, W.; Sohail, A.; Iqbal, M. Seed micromorphology and its taxonomic evidence in subfamily Alsinoideae (Caryophyllaceae). Microsc. Res. Tech. 2019, 82, 250–259. [Google Scholar] [CrossRef]
- Wyatt, R. Intraspecific variation in seed morphology of Arenaria uniflora (Caryophyllaceae). Syst. Bot. 1984, 9, 423. [Google Scholar] [CrossRef]
- Minuto, L.; Fior, S.; Roccotiello, E.; Casazza, G. Seed morphology in Moehringia L. and its taxonomic significance in comparative studies within the Caryophyllaceae. Plant Syst. Evol. 2006, 262, 189–208. [Google Scholar] [CrossRef]
- Poyraz, I.E.; Ataşlar, E. Pollen and seed morphology of Velezia L. (Caryophyllaceae) genus in Turkey. Turk. J. Bot. 2010, 34, 179–190. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín, J.J.; Ardanuy, R.; de Diego, J.G.; Tocino, Á. Modeling the Arabidopsis seed shape by a cardioid: Efficacy of the adjustment with a scale change with factor equal to the Golden Ratio and analysis of seed shape in ethylene mutants. J. Plant Physiol. 2010, 67, 408–410. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín-Gómez, J.J. Seed Shape Description and Quantification by Comparison with Geometric Models. Horticulturae 2019, 5, 60. [Google Scholar] [CrossRef]
- Martín-Gómez, J.J.; Rewicz, A.; Rodríguez-Lorenzo, J.L.; Janoušek, B.; Cervantes, E. Seed morphology in Silene based on geometric models. Plants 2020, 9, 1787. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín-Gómez, J.J. Seed shape quantification in the order Cucurbitales. Mod. Phytomorphol. 2018, 12, 1–13. [Google Scholar]
- Cervantes, E.; Rodríguez-Lorenzo, J.L.; Gutiérrez del Pozo, D.; Martín-Gómez, J.J.; Janousek, B.; Tocino, Á.; Juan, A. Seed Silhouettes as Geometric Objects: New applications of Elliptic Fourier Transform to seed morphology. Horticulturae 2022, 8, 974. [Google Scholar] [CrossRef]
- Cervantes, E.; Tocino, A. Geometric analysis of Arabidopsis root apex reveals a new aspect of the ethylene signal transduction pathway in development. J. Plant Physiol. 2005, 162, 1038–1045. [Google Scholar] [CrossRef]
- Noriega, A.; Tocino, A.; Cervantes, E. Hydrogen peroxide treatment results in reduced curvature values in the Arabidopsis root apex. J. Plant Physiol. 2009, 166, 554–558. [Google Scholar] [CrossRef]
- Cervantes, E.; Rodríguez-Lorenzo, J.L.; Martín-Gómez, J.J.; Tocino, Á. Curvature analysis of seed silhouettes in Silene L. Plants 2023, 12, 2439. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, J.L.; Martín-Gómez, J.J.; Juan, A.; Tocino, Á.; Cervantes, E. Quantitative analysis of seed surface tubercles in Silene Species. Plants 2023, 12, 3444. [Google Scholar] [CrossRef]
- Sokal, R.R.; Braumann, C.A. Significance tests for coefficients of variation and variability profiles. Syst. Zool. 1980, 29, 50. [Google Scholar] [CrossRef]
- Schaefer, H. Version 1, January 2020. Cucurbit Website. 2020. Available online: www.cucurbit.de (accessed on 29 December 2023).
- Oyama, R.K.; Volz, S.M.; Renner, S.S. A sex-linked SCAR marker in Bryonia dioica (Cucurbitaceae), a dioecious species with XY sex-determination and homomorphic sex chromosomes. J. Evol. Biol 2009, 22, 214–224. [Google Scholar] [CrossRef]
- Holstein, N. Monograph of Coccinia (Cucurbitaceae). PhytoKeys 2015, 3, 1–166. [Google Scholar] [CrossRef]
- Kouonon, L.C.; Jacquemart, A.L.; Bi, A.I.; Bertin, P.; Baudoin, J.P.; Dje, Y. Reproductive biology of the andromonoecious Cucumis melo subsp. Agrestis (Cucurbitaceae). Ann. Bot. 2009, 104, 1129–1139. [Google Scholar] [CrossRef]
- Shaik, R.S.; Burrows, G.E.; Urwin, N.A.R.; Gopurenko, D.; Lepschi, B.J.; Weston, L.A. The biology and management of prickly paddy melon (Cucumis myriocarpus L.), an important summer annual weed in Australia. Crop. Prot. 2017, 92, 29–40. [Google Scholar] [CrossRef]
- Gao, J.; Huang, W.; Gielis, J.; Shi, P. Plant morphology and function, geometric morphometrics, and modelling: Decoding the mathematical secrets of plants. Plants 2023, 12, 3724. [Google Scholar] [CrossRef]
- Koyama, T. Regulatory mechanisms of transcription factors in plant morphology and function. Int. J. Mol. Sci. 2023, 24, 7039. [Google Scholar] [CrossRef]
- Ganhão, E.; Dias, L.S. Seed Volume Dataset—An Ongoing inventory of seed size expressed by volume. Data 2019, 4, 61. [Google Scholar] [CrossRef]
- Martín-Gómez, J.J.; del Pozo, D.G.; Tocino, Á.; Cervantes, E. Geometric models for seed shape description and quantification in the Cactaceae. Plants 2021, 10, 2546. [Google Scholar] [CrossRef]
- Martín-Gómez, J.J.; Rodríguez-Lorenzo, J.L.; Tocino, Á.; Janoušek, B.; Juan, A.; Cervantes, E. The outline of seed silhouettes: A morphological approach to Silene (Caryophyllaceae). Plants 2022, 11, 3383. [Google Scholar] [CrossRef]
- Gray, A. Modern Differential Geometry of Curves and Surfaces with Mathematica; CRC Press: Boca Raton, FL, USA, 1998; pp. 163–165. [Google Scholar]
- Zheng, L.; Zhang, T.; Xie, L.; Karrar, E.; Shi, L.; Jin, J.; Wang, X.; Jin, Q. Physicochemical characteristics of Actinostemma lobatum Maxim. kernel oil by supercritical fluid extraction and conventional methods. Ind. Crops Prod. 2020, 152, 112516. [Google Scholar] [CrossRef]
- Szkudlarz, P. Variation in seed morphology in the genus Erica L. (Ericaceae). Biodivers. Res. Conserv. 2009, 16, 1–106. [Google Scholar] [CrossRef]
- Ahedor, A.R.; Elisens, W. Morphological analyses of the Mecardonia acuminata (Plantaginaceae) Species Complex in the Southeastern USA. Southeast. Nat. 2015, 14, 173–196. [Google Scholar] [CrossRef]
- Muñoz-Centeno, L.M.; Albach, D.C.; Sánchez-Agudo, J.A.; Martínez-Ortega, M.M. Systematic significance of seed morphology in Veronica (Plantaginaceae): A phylogenetic perspective. Ann. Bot. 2006, 98, 335–350. [Google Scholar] [CrossRef]
- Hall, B.K. Homology and homoplasy. In Handbook of the Philosophy of Science, Philosophy of Biology; Matthen, M., Stephens, C., Eds.; Elsevier: Amstercam, The Netherlands, 2007; pp. 429–453. [Google Scholar] [CrossRef]
- Ocampo, G.; Michelangeli, F.A.; Almeda, F. Seed Diversity in the Tribe Miconieae (Melastomataceae): Taxonomic, Systematic, and Evolutionary Implications. PLoS ONE 2014, 9, e100561. [Google Scholar] [CrossRef]
- Jafari, F.; Zarre, S.; Gholipour, A.; Eggens, F.; Rabeler, R.K.; Oxelman, B. A new taxonomic backbone for the infrageneric classification of the species-rich genus Silene (Caryophyllaceae). Taxon 2020, 69, 337–368. [Google Scholar] [CrossRef]
- Belgrano, M.J.; Pozner, R. Sinopsis del género Apodanthera (Cucurbitaceae, Coniandreae). Darwiniana 2017, 5, 5–50. [Google Scholar] [CrossRef]
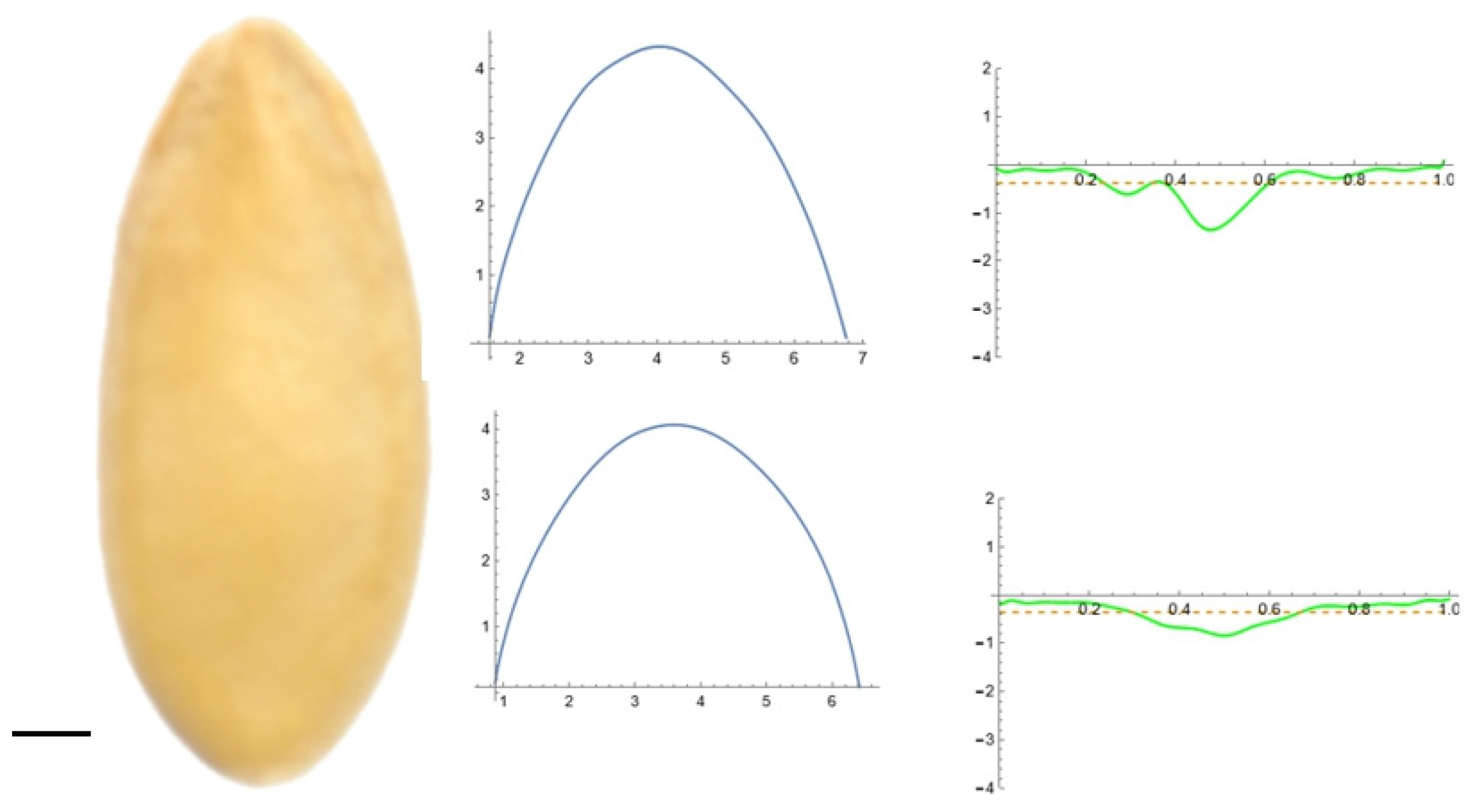
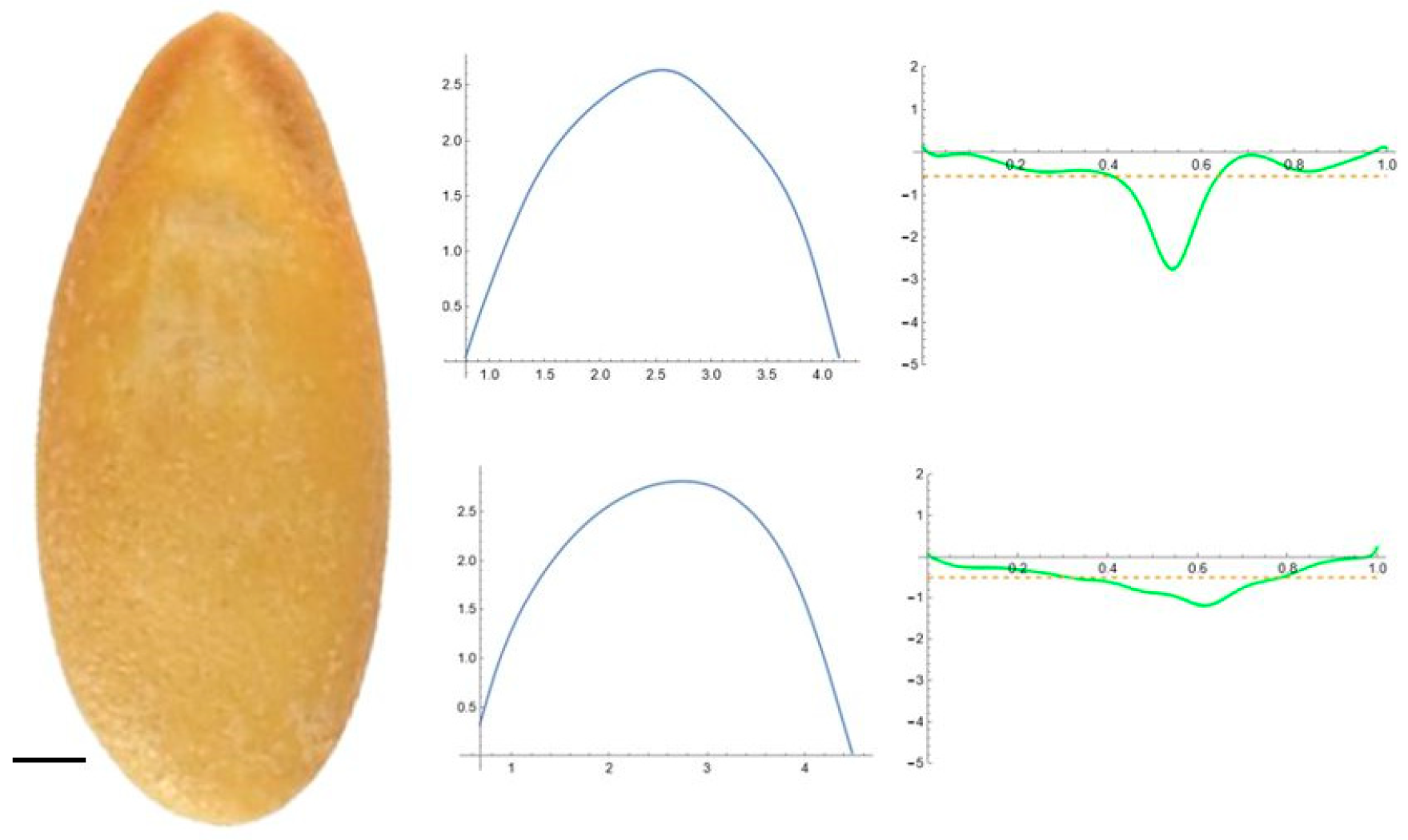
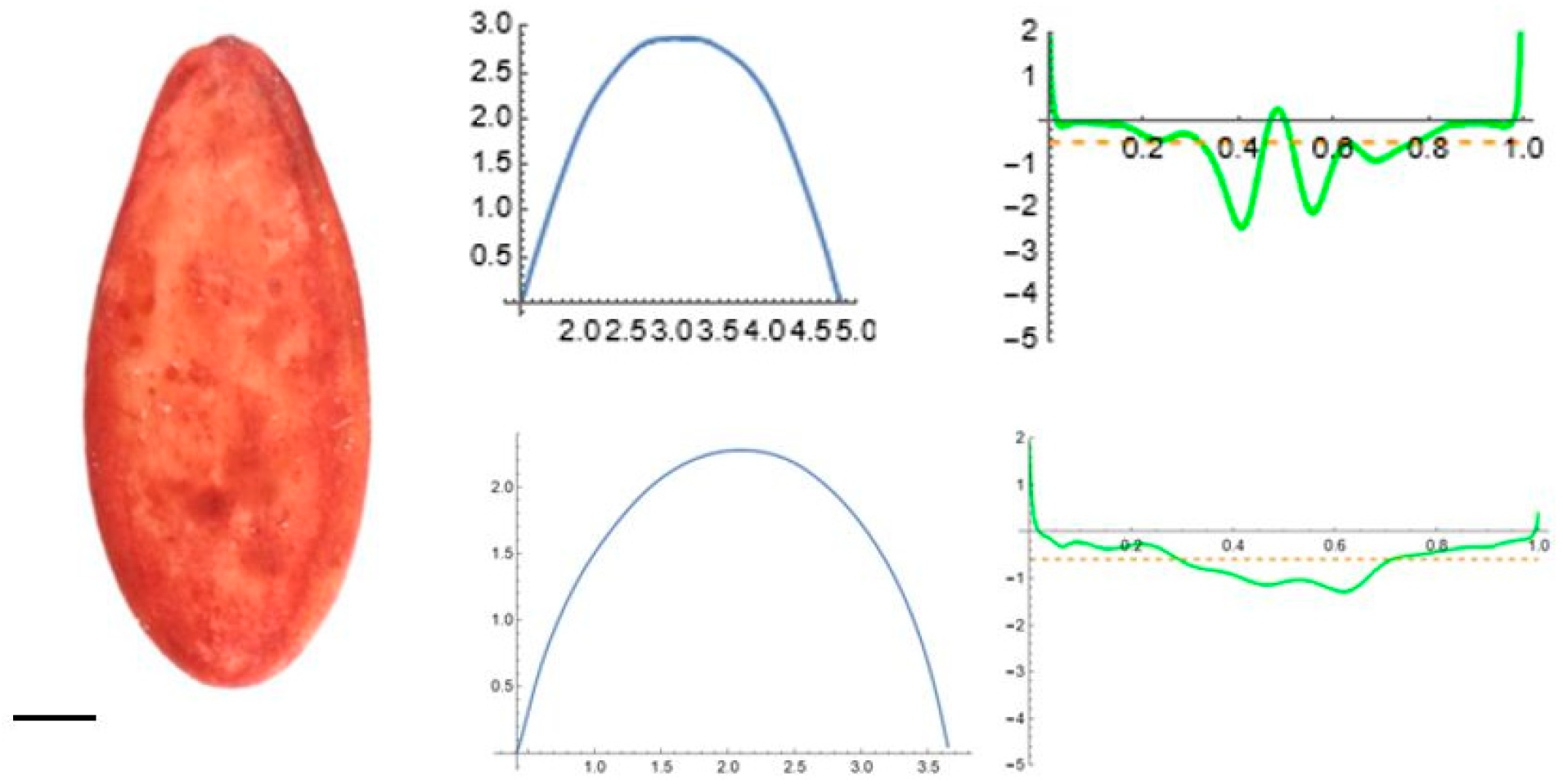
| Species | N | A | P | L | W | C | AR | R | S |
|---|---|---|---|---|---|---|---|---|---|
| Bryonia dioica | 16 | 13.05 b (12.2) | 14.68 b (7.3) | 4.87 b (6.0) | 3.41 c (7.7) | 0.76 e (8.8) | 1.43 a (6.3) | 0.70 g (6.3) | 0.988 d (0.5) |
| Citrullus lanatus | 30 | 34.22 e (9.0) | 23.47 e (4.8) | 8.43 d (5.0) | 5.16 f (4.9) | 0.78 e (2.0) | 1.63 b (4.0) | 0.61 f (4.0) | 0.986 d (0.2) |
| Momordica sp. | 5 | 50.47 f (7.8) | 29.96 g (4.6) | 10.55 e (4.8) | 6.10 g (6.7) | 0.71 abc (4.7) | 1.74 bc (8.4) | 0.58 ef (8.5) | 0.961 a (0.4) |
| Coccinia sessilifolia | 6 | 26.15 d (12.4) | 21.71 d (9.2) | 8.07 cd (11.7) | 4.13 e (4.5) | 0.70 abc (6.6) | 1.96 e (12.9) | 0.52 c (14.0) | 0.972 a (0.8) |
| Cucumis melo Melon Rochet | 25 | 48.66 f (11.6) | 29.98 g (6.3) | 12.17 g (6.9) | 5.08 f (6.7) | 0.68 a (4.3) | 2.40 g (7.4) | 0.42 a (7.3) | 0.992 f (0.1) |
| Cucumis melo Piel de sapo | 25 | 35.02 e (15.7) | 25.62 f (7.6) | 10.28 e (8.2) | 4.32 e (8.8) | 0.67 a (4.6) | 2.39 g (7.5) | 0.42 a (7.8) | 0.990 e (0.1) |
| Cucumis myriocarpus | 13 | 9.68 a (4.3) | 13.28 a (3.3) | 4.89 b (3.9) | 2.52 a (2.9) | 0.69 ab (3.7) | 1.94 e (5.3) | 0.52 c (5.3) | 0.990 e (0.3) |
| Cucumis sativus | 30 | 22.88 c (10.2) | 20.13 c (6.1) | 7.93 c (7.1) | 3.67 d (4.9) | 0.71 bc (3.7) | 2.16 f (6.8) | 0.46 b (6.9) | 0.993 g (0.1) |
| Cucurbita pepo | 40 | 61.48 g (6.7) | 32.87 h (3.7) | 12.00 f (3.2) | 6.52 h (3.6) | 0.72 c (4.3) | 1.84 d (2.7) | 0.54 d (2.7) | 0.986 c (0.9) |
| Ecbalium elaterium | 38 | 10.04 a (10.6) | 12.99 a (5.4) | 4.72 a (4.2) | 2.70 b (7.7) | 0.75 d (4.0) | 1.75 c (6.8) | 0.57 e (6.5) | 0.991 e (0.4) |
| Sicana odorifera | 9 | 78.33 h (6.0) | 37.77 i (2.9) | 13.75 h (4.5) | 7.25 i (4.2) | 0.69 ab (2.3) | 1.90 de (6.2) | 0.53 cd (6.3) | 0.979 b (0.2) |
| Species | Upper | Lower | |||||
|---|---|---|---|---|---|---|---|
| Maximum Curvature | Mean Curvature | Maximum/Mean | Maximum Curvature | Mean Curvature | Maximum/Mean | Maximum Upper/Maximum Lower | |
| Citrullus lanatus (6) | 1.65 a | 0.39 b | 4.23 ab | 0.69 a | 0.46 bc | 1.50 a | 2.39 c |
| Momordica sp. | 1.22 a | 0.29 a | 4.21 ab | 1.55 d | 0.34 ab | 4.56 de | 0.79 a |
| Coccinia sessilifolia (6) | 2.40 b | 0.54 c | 4.44 ab | 1.00 bc | 0.45 bc | 2.22 ab | 2.40 c |
| Cucumis melo cv. Melon Rochet (14) | 1.55 a | 0.43 b | 3.52 a | 0.85 b | 0.40 bc | 2.13 ab | 1.82 bc |
| Cucumis melo cv. Piel de sapo (13) | 2.48 b | 0.52 c | 4.77 b | 1.10 bc | 0.46 bc | 2.39 bc | 2.25 c |
| Cucumis sativus (17) | 1.57 a | 0.42 b | 3.74 ab | 1.05 bc | 0.41 abc | 2.56 cd | 1.49 b |
| Cucurbita pepo (17) | 1.49 a | 0.31 a | 4.81 b | 0.65 a | 0.30 a | 2.16 ab | 2.29 c |
| Ecbalium elaterium (5) | 2.91 b | 0.62 c | 4.69 b | 1.89 d | 0.66 c | 2.86 cde | 1.54 b |
| Sicana odorifera (3) | 2.13 ab | 0.30 ab | 7.11 b | 1.44 cd | 0.27 a | 5.33 e | 1.48 b |
| Mean values for all species (coefficient of variation) | 1.93 (22.6) | 0.42 (19.38) | 4.84 (33.4) | 1.13 (19.2) | 0.42 (24.3) | 3.05 (33.7) | 1.87 (26.3) |
| Species or Variety | N | Symmetry |
|---|---|---|
| Bryonia dioica (Dioecious) [42] | 10 | 91.97 bcd (2.80) |
| Citrullus lanatus (Monoecious) [43] | 10 | 93.89 de (1.13) |
| Momordica sp. (Dioecious) [43,44] | 5 | 89.5 b (1.39) |
| Coccinia sessilifolia (Dioecious) [43,44] | 6 | 85.39 a (2.31) |
| Cucumis melo Melon Rochet (Andromonoecious) [43] | 20 | 90.10 b (3.58) |
| Cucumis melo Piel de sapo (Andromonoecious) [43] | 20 | 91.04 b (2.54) |
| Cucumis myriocarpus [45,46] (Monoecious) | 10 | 93.16 cde (1.98) |
| Cucumis sativus (Monoecious) [43] | 20 | 94.27 ef (2.23) |
| Cucurbita pepo (Monoecious) [43] | 10 | 93.11 cd (0.97) |
| Ecbalium elaterium (Dioecious) [43] | 10 | 95.32 f (1.22) |
| Sicana odorifera (Monoecious) [43] | 8 | 91.88 bc (0.74) |
| Species (or Variety) | N | J Index |
|---|---|---|
| Cucumis melo Melon Rochet | 23 | 87.7 a (3.8) |
| Cucumis melo Piel de sapo | 24 | 86.1 a (4.1) |
| Cucumis myriocarpus | 10 | 90.4 b (1.2) |
| Cucumis sativus | 32 | 92.1 c (2.0) |
| Species | N | Symmetry |
|---|---|---|
| Cucumis melo Melon Rochet | 20 | 90.10 a (3.58) |
| Cucumis melo Piel de sapo | 20 | 91.04 a (2.54) |
| Cucumis myriocarpus | 10 | 93.16 b (1.98) |
| Cucumis sativus | 20 | 94.27 c (2.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Gómez, J.J.; Gutiérrez del Pozo, D.; Rodríguez-Lorenzo, J.L.; Tocino, Á.; Cervantes, E. Geometric Analysis of Seed Shape Diversity in the Cucurbitaceae. Seeds 2024, 3, 40-55. https://doi.org/10.3390/seeds3010004
Martín-Gómez JJ, Gutiérrez del Pozo D, Rodríguez-Lorenzo JL, Tocino Á, Cervantes E. Geometric Analysis of Seed Shape Diversity in the Cucurbitaceae. Seeds. 2024; 3(1):40-55. https://doi.org/10.3390/seeds3010004
Chicago/Turabian StyleMartín-Gómez, José Javier, Diego Gutiérrez del Pozo, José Luis Rodríguez-Lorenzo, Ángel Tocino, and Emilio Cervantes. 2024. "Geometric Analysis of Seed Shape Diversity in the Cucurbitaceae" Seeds 3, no. 1: 40-55. https://doi.org/10.3390/seeds3010004
APA StyleMartín-Gómez, J. J., Gutiérrez del Pozo, D., Rodríguez-Lorenzo, J. L., Tocino, Á., & Cervantes, E. (2024). Geometric Analysis of Seed Shape Diversity in the Cucurbitaceae. Seeds, 3(1), 40-55. https://doi.org/10.3390/seeds3010004







