Abstract
Cicer arietinum L. (chickpea, or garbanzo bean) is one of the most consumed legumes worldwide. It is a rich source of carbohydrates, proteins, fibers, minerals and vitamins with very low cholesterol. From a nutritional point of view, despite the low content of fats, the seeds contain various unsaturated acids, such as linoleic and oleic acids, as well as bioactive compounds, like antioxidants, with reactive oxygen species-scavenging activities. It is known that long periods of storage can drastically affect the preservation of these compounds in seeds. For this reason, in the last few years, different methods have been tested with the aim of increasing the shelf life of economically relevant beans, seeds and cereals. A promising and eco-friendly alternative to traditional storage is the use of a controlled atmosphere, represented by N2-pressurized silos. The present study aims at evaluating the content of different compounds, e.g., fatty acids, proteins, vitamins, and molecules of nutraceutical interest, in chickpeas stored at ambient temperature in N2-pressurized silos (98.5 ± 0.5% (v/v)) and control ones (standard storage) in long-term kinetics (1 year). The results show the stable content of most compounds during the kinetics. However, vitamin E decreased in samples stored under both standard and controlled atmosphere conditions, with a more pronounced and significant decrease under standard conditions as compared to the controlled atmosphere. Additionally, samples stored under a controlled atmosphere show a total higher content of quinic, indole butyric and benzoic acid, as well as their derivates.
1. Introduction
Chickpea (Cicer arietinum L.) is the third most important legume in the world [1], produced in over 50 countries, particularly Afro-Asian countries. In 2020, 14.84 million hectares were cultivated for a total of over 15 million tons produced according to FAOSTAT. The consumption of chickpeas is increasing not only in developing countries; this is due to the growing demand for both gluten-free products and healthy food [2]. Chickpeas are pulse crops rich in proteins and carbohydrates (starch, sucrose) with important nutritional values: they are cholesterol-free and an inexpensive source of essential amino acids (except sulfur-containing ones), as well as of dietary fibers, vitamins (riboflavin, niacin, thiamin, folate and vitamin A) and minerals (Ca, Mg, P, K) [3]. Although lipids are present in low quantities, chickpeas have a substantial amount of nutritionally important unsaturated fatty acids, such as linoleic and oleic acids [3]. All these characteristics show that chickpeas have potential beneficial effects as healthy food in the human diet [4,5,6,7]. The seeds also contain bioactive compounds, such as α-tocopherol (vitamin E), which play an important role in the prevention of degenerative diseases, due to their antioxidant activity [8,9,10].
Chickpeas are chiefly consumed as seeds, although they are also consumed in the form of hummus dip or spread and as leaves [11].
The consumption of chickpeas has been linked to a reduction in chronic diseases and to several health benefits. The presence of bioactive molecules such as phytosterols, phytates and phenolic compounds lowers the risk of cardiovascular diseases; dietary fibers with β-sitosterol help reduce the level of LDL-cholesterol; and butyrate (the main short-chain fatty acid) could contribute to reduce the risk of developing colorectal cancer [3].
Unfortunately, these compounds are susceptible to degradation during long-term storage, and this is mostly caused by the presence of oxygen inside the silos [12]. To solve this problem, interest in the use of controlled atmospheres (CAs) has grown in the last few years. The method uses gases such as carbon dioxide (CO2) or nitrogen (N2) to replace the oxygen in the storage atmosphere and thus pressurize the silos. CAs were also shown to prevent the attack of insects and mycotoxigenic molds and to constitute a low-environmental-impact alternative to the use of fumigants [13,14,15,16,17,18,19,20]. From an environmental point of view, the use of N2 in CAs stands out positively compared to CO2, since it is not considered a greenhouse gas and it is easy to find (78% of it is present in the air).
This work aims to compare and evaluate physicochemical characteristics (peroxides, proteins, fatty acids, moisture content, acidity and bacterial, yeast/mold count) together with vitamin E and secondary metabolite content in chickpeas stored in a CA and standard conditions for 12 months. The CA system used generates an atmosphere enriched with N2 (98.5 ± 0.5% (v/v))—resulting from an in situ separation of N2 from air using a membrane nitrogen separator (MNS). The same system was used in a previous study, and it was validated as successful in the preservation of the quality, especially the content of vitamin E, in stored wheat [21]. Moreover, chickpeas stored under the CA showed complete pest mortality and a reduction in mycotoxin contamination [22].
The results obtained here showed the stable content of most compounds during the period of storage. However, vitamin E decreased in samples stored under both control and CA conditions, with a stronger and more significant decrease under standard conditions as compared to the controlled atmosphere. Samples stored under the CA also showed a higher content of quinic, indole butyric and benzoic acid, as well as their derivates.
2. Materials and Methods
2.1. Storage Conditions
Chickpeas (Cicer arietinum L., cv. Sultano) were provided by Del Colle srl (Bientina, Pisa, Italy). The samples were sown in March 2020 and harvested in August 2020 in the locality of Rosignano Marittimo (GPS coordinates: 43.427955, 10.484438). The seeds were then stored in 6 laboratory-scale prototype silos at the CRISBA-ISIS Research Center “Leopoldo II di Lorena” (Grosseto, Italy), as previously described [21]. Six silos of 60 L volume were filled with 40 kg of chickpeas. Three silos were pressurized with 98.5 ± 0.5% (v/v) N2 (CA). N2 was separated from the air through an MNS filter (Membrane Nitrogen Separator), and the silos were pressurized at 0.07 bar. The 3 remaining silos were kept under traditional storage and used as controls (Cntrl). The seeds were stored in the systems for 12 months and sampled every 3 months by pooling aliquots from the top, middle and bottom part of the silos for a total of 1 kg. These 3 aliquots were pooled to obtain a homogeneous representative sample for each of the silos. Three biological replicates were collected from the Cntrl and CA silos.
2.2. Determination of Moisture Content and Germination Rate
The samples (5 g) were harvested and dried in an oven at 105 °C for 4–6 h and then weighed with an analytic balance. The final moisture content was expressed as g per 100 g of FW. The method used for the moisture content determination is according to the American Society of Agricultural and Biological Engineers (2008) ASAE S352.2. Moisture Measurement—Unground Grain and Seeds [23].
Two hundred seeds per replicate for each treatment were used to conduct the germination test. The germination test was carried out according to ISTA (2019) [24]; the seeds were planted in sterilized sand media in the germination room and the temperature was maintained at 20 °C. After 8 days, the final counts were taken; the following formula was used to calculate the percentage of germination:
2.3. Determination of Acidity
The samples were homogenized using a Burr grinder mixer. Five grams of samples was dissolved in 30 mL of EtOH 95–96% (v/v) and stirred for 60 min. The solution was then stored for 24 h in the dark. Two drops of phenolphthalein were added to 20 mL of the supernatant. The acidity of the samples was determined by adding a few drops of 10 g/L phenolphthalein diluted in EtOH 95–96% (v/v) and titrated with a solution of NaOH 0.05 N. The results are expressed as mL of H2SO4.
2.4. Quantification of Peroxides
The samples were homogenized using a Burr grinder mixer. A solution of 10 mL of EtOH, 30 mL 95–96% (v/v), petroleum ether and 30 mL of ethyl ether was added to 15 g of samples and stirred for 5 min. The extraction was repeated two times with a solution of 15 mL of ethyl ether and 15 mL of petrol ether. The supernatant was collected in a single flask and evaporated through nitrogen flux. The pellet was dissolved in 10 mL of trichloromethane and 15 mL of acetic acid and stirred. Then, 1 mL of a saturated potassium chloride solution was added and incubated for 5 min in the dark. Finally, 75 mL of water was added, and the solution was titrated with a solution of thiosulfate 0.1 N. The results are expressed as mEq of O2 per kg of FW.
2.5. Determination of Protein Content
The content of proteins was determined following the Kjeldahl method with a mineralizer and VELP distiller. One gram of homogenized sample was added to 15 g of K2SO4, 0.35 g of cupric oxide catalyst or 1 g of copper sulphate pentahydrate catalyst and 25 mL of concentrated sulfuric acid. The samples were heated at 200 °C for 30 min and at 420 °C for 60 min. Then, the samples were cooled down, and the nitrogen content was determined as follows: 10 mL of sulfuric acid and a few drops of indicator were mixed with the samples and about 70 mL of 40% NaOH solution (w/v) was slowly poured into the tube. The reaction was basified with a few drops of phenolphthalein. At the end of the distillation, the excess of sulfuric acid was titrated in the collecting flask by means of NaOH until the final point was reached, as indicated by the color change of the indicator. The results are expressed as g per 100 g of FW.
2.6. Bacteria and Yeast/Mold Count
Bacteria counts were carried out following the ISO 4833-1:2013 method [25] for the enumeration of microorganisms at 30 °C; the count of yeasts and molds was determined following the ISO 21527-2:2008-07 method [26].
2.7. Determination of Fatty Acids (FAs)
The quantification of FAs was performed following the Association of Official Analytical Chemists (AOAC) official 996.06 method [27]. The AOAC method describes the standard procedure to determine the saturated and unsaturated fatty acids in foods using capillary GC-FID (Flame Ionization Detection) (7890 A, Agilent Technologies Palo Alto, Palo Alto, CA, USA). The procedure involves hydrolytic extraction, methylation and capillary GC-FID analysis of the resulting fatty acid methyl esters (Supelco 37 Component FAME Mix, CRM47885). Triundecanoin, the C11:0 triglyceride, is used as an internal standard for quantification, as reported in the AOAC 996.06 method. The method uses a 100 mm × 0.25 mm × 0.20 µm column with a highly polar bis(cyanopropyl)polysiloxane stationary phase, such as the Rt-2560.
2.8. Determination of α-Tocopherol
Ten grams of homogenized sample was dissolved in 50 mL of heptane. After shaking for a few seconds, the sample was placed inside an ultrasonic bath for 30 min. Then, 1 ml of solution was filtered and placed in an HPLC vial. The samples were injected in an HPLC instrument with an FLD (fluorescence) detector (G1321A, Agilent 1200 series, Palo Alto, CA, USA) and equipped with a LiChrosorb Si 250 mm × 4.60 mm i.d. 5 µm column. The mobile phase was composed of hexane/THF (25:1, v/v), the time of the run was 35 min, and the flux was maintained at 1 mL/min in isocratic elution. The FLD detector was set as follows: exposure 295 nm, emission 330 nm, response time >0.2 min and PTM-gain 10.
2.9. HPLC-MS Analyses of Quinic Acid, Indoleacetic, Benzoic Acid and Biochanin Derivatives
A total of 2 g of samples was put in 50 mL of EtOH 50% (v/v), formic acid pH 3.2, o/n. The supernatant was collected, dried, and resuspended in 4 mL of hydroalcoholic solution. The samples were injected in an HPLC instrument with a DAD (Diode-Array Detector) detector (HP-1260, Agilent-Technologies, Palo Alto, Santa Clara, CA, USA) and equipped with a Luna C18 250 × 4.6 mm i.d. 5 µm column (Phenomenex, CA, USA). The mobile phase was composed of H2O-formic acid pH 3.2 and CH3CN in a linear gradient (from 95% of H2O to 100% CH3CN), the time of the run was 50 min and the flux was maintained at 0.8 mL/min. The UV spectrum was acquired between 190 and 600 nm. The HPLC system was coupled with an ESI-MS (Agilent Corp, Santa Clara, CA, USA). The spectra were acquired in full-scan mode, with the mass range set as 100–1500 m/z in the positive and negative mode. The identification of various molecules was carried out by comparing the retention time, UV-Vis spectrum and mass spectra with those of reference standards when available and with bibliographic data. Quantitative analysis was performed using HPLC/DAD with the aid of 4-point calibration curves, constructed with standards representing different subclasses of present compounds (r2 = 0.998). Specifically, derivatives of indoleacetic acid were calibrated at 280 nm using indoleacetic acid (IAA) as the reference standard, and isoflavones (derivatives of biochanin A) were calibrated at 260 nm using biochanin A as the standard, while benzoic acid and derivatives were calibrated using benzoic acid as the reference standard.
2.10. Statistics and Principal Component Analysis
Statistics was performed with IBM SPSS statistics v26 (IBM SPSS, Chicago, IL, USA) on log10-transformed data. Normality and homogeneity were checked with a Shapiro–Wilk and Levene’s test. A univariate analysis with a Tukey’s post hoc test or an independent-samples t-test was conducted for homogeneous data following normal distribution. Otherwise, a non-parametric test for independent samples was carried out with a Kruskal–Wallis and Dunn’s post hoc test. The principal component analysis—PCA—was obtained with ClustVis [28] (https://biit.cs.ut.ee/clustvis/ accessed on 1 October 2023).
3. Results
3.1. Physico-Chemical Parameters
The values of acidity, peroxides, proteins, fatty acids, as well as moisture content, bacterial and yeast/mold colonies were measured on chickpeas stored for 1 year in silos under standard (control) and CA conditions. The results are shown in Table 1 for samples taken after 0, 3, 6, 9 and 12 months.

Table 1.
Physico-chemical parameters of chickpeas stored under standard (Cntrl) and controlled N2 atmosphere (CA). The values are expressed as means of 3 independent biological replicates ± standard deviation (SD). Asterisks denote statistically significant values (p < 0.05) with the Student’s t-test.
The acidity, proteins, fatty acids, and yeast/mold count of the samples did not show any significant changes, regardless of the storage method. The microbiological data do not indicate any significant differences between the samples stored under control and CA conditions at the different sampling time points; over time and along the storage period, the values decreased following the same trend as moisture content. The dry mass and peroxide content, instead, increased over time compared to the samples at time 0. More specifically, the peroxides showed a peak after 3 months (from 0.10 ± 0.001 mEq O2/kg at time 0 to 0.60 ± 0.17 and 0.67 ± 0.29 mEq O2/kg in the control and CA conditions) to reach stable values.
Conversely, the fatty acids and moisture content decreased over time. The former decreased from 3.16 ± 0.18 g/100 g at time 0 to 2.94 ± 0.22 and 2.84 ± 0.18 g/100 g under the control and CA conditions after 12 months; moisture content decreased from values of 12.84 ± 0.30 g/100 g at time 0 to 8.31 ± 0.08 and 9.62 ± 0.33 g/100 g in the control and CA conditions after 12 months. The impurity content of the seeds was 2.35%.
The germination rate revealed no statistical differences at the different time points between the control and CA conditions (Table S1).
3.2. Fatty Acid Content
The fatty acids (FAs) with values above the detection limit are shown in Table 2. These correspond to unsaturated (UFAs), saturated (SFAs), polyunsaturated (PUFAs), monounsaturated fatty acids (MFAs), ω-6 and ω-9 FAs, as well as palmitic (PA), oleic (OA), linoleic (LA), stearic (SA) and α-linolenic acid (α-LA). The complete profile of FAs is shown in Table S2.

Table 2.
FA content in chickpeas stored under standard (Cntrl) and controlled N2 atmosphere (CA) conditions. The values are expressed as means of 3 independent biological replicates (expressed as g/100 g) ± standard deviation (SD). Asterisks denote statistically significant values (p ≤ 0.05) with the Student’s t-test.
UFAs, SFAs, PUFAs, MFAs, ω-6 and ω-9 FAs decreased during storage both under standard and CA conditions, whereas the content of PA, OA, LA, SA and α-LA remained stable over time, regardless of the storage conditions.
3.3. α-Tocopherol Content
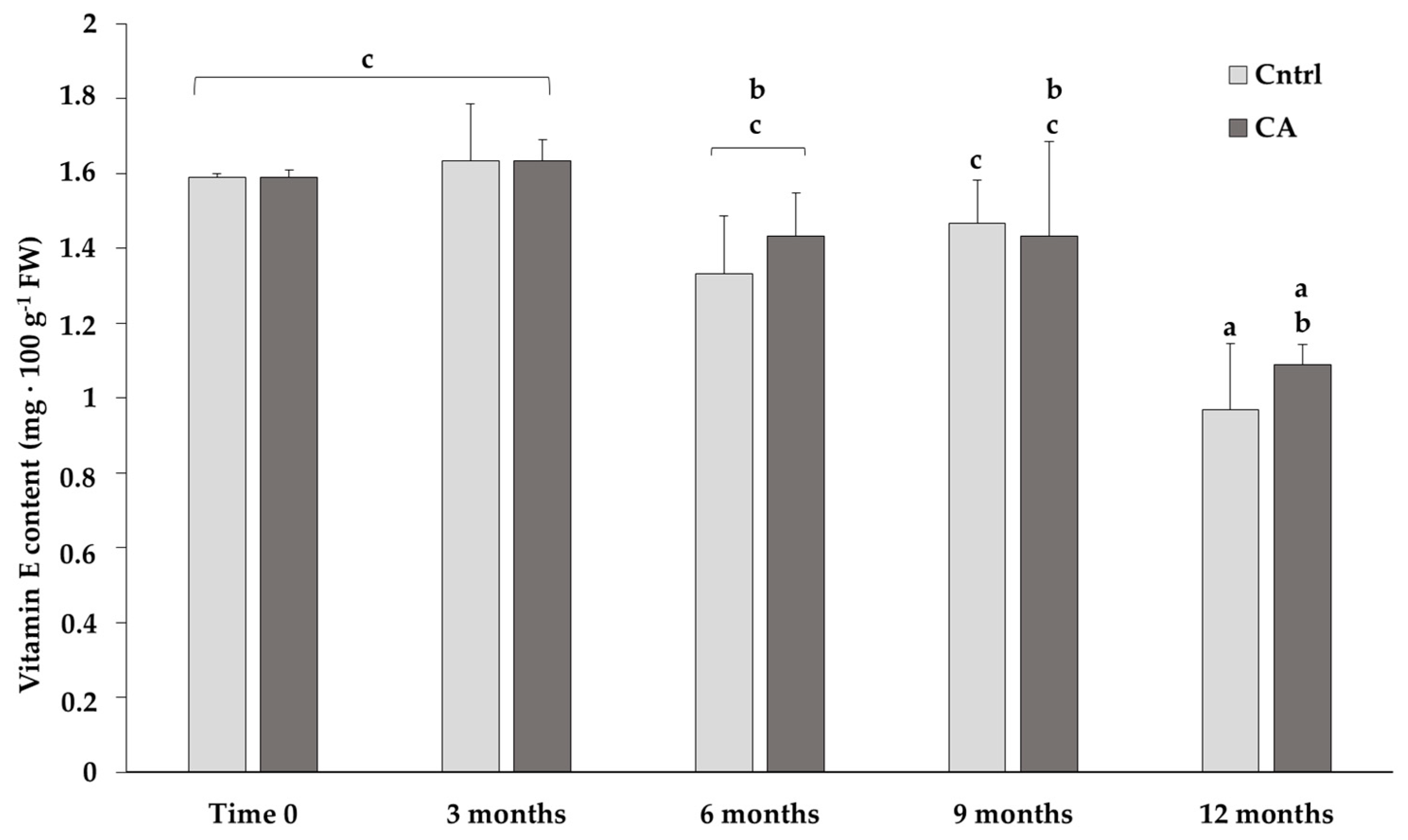
The content of α-tocopherol (vitamin E) progressively decreased, regardless of the storage conditions, with a significant decline at 12 months under both standard and CA conditions (Figure 1). Although the content of vitamin E was lower at the last time point, statistics showed no significant difference between 9 and 12 months in samples stored under CA: 1.43 ± 0.25 and 1.06 ± 0.05 mg/100 g of FW, respectively. Conversely, the samples stored using the traditional standard methods showed a more pronounced and significant decrease between 9 and 12 months of storage: 1.47 ± 0.12 and 0.97 ± 0.08 mg/100 g of FW, respectively.
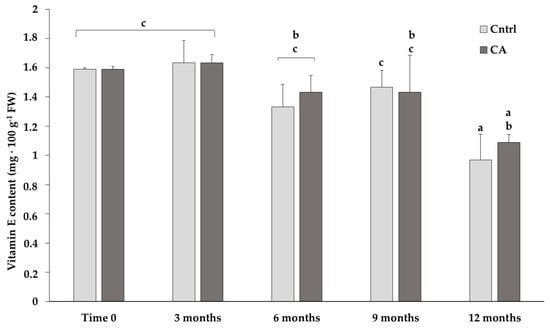
Figure 1.
Content of vitamin E in chickpeas stored under standard (Cntrl) and controlled N2 atmosphere (CA). The bar-chart shows the means of 3 independent biological replicates (expressed as mg/100 g of fresh weight-FW) ± standard deviation (SD). Different letters indicate statistically significant differences (p ≤ 0.05) among the groups calculated with a one-way ANOVA followed by Tukey’s post hoc test. If a letter is shared, then the difference is not significant.
3.4. Targeted Metabolite Quantification
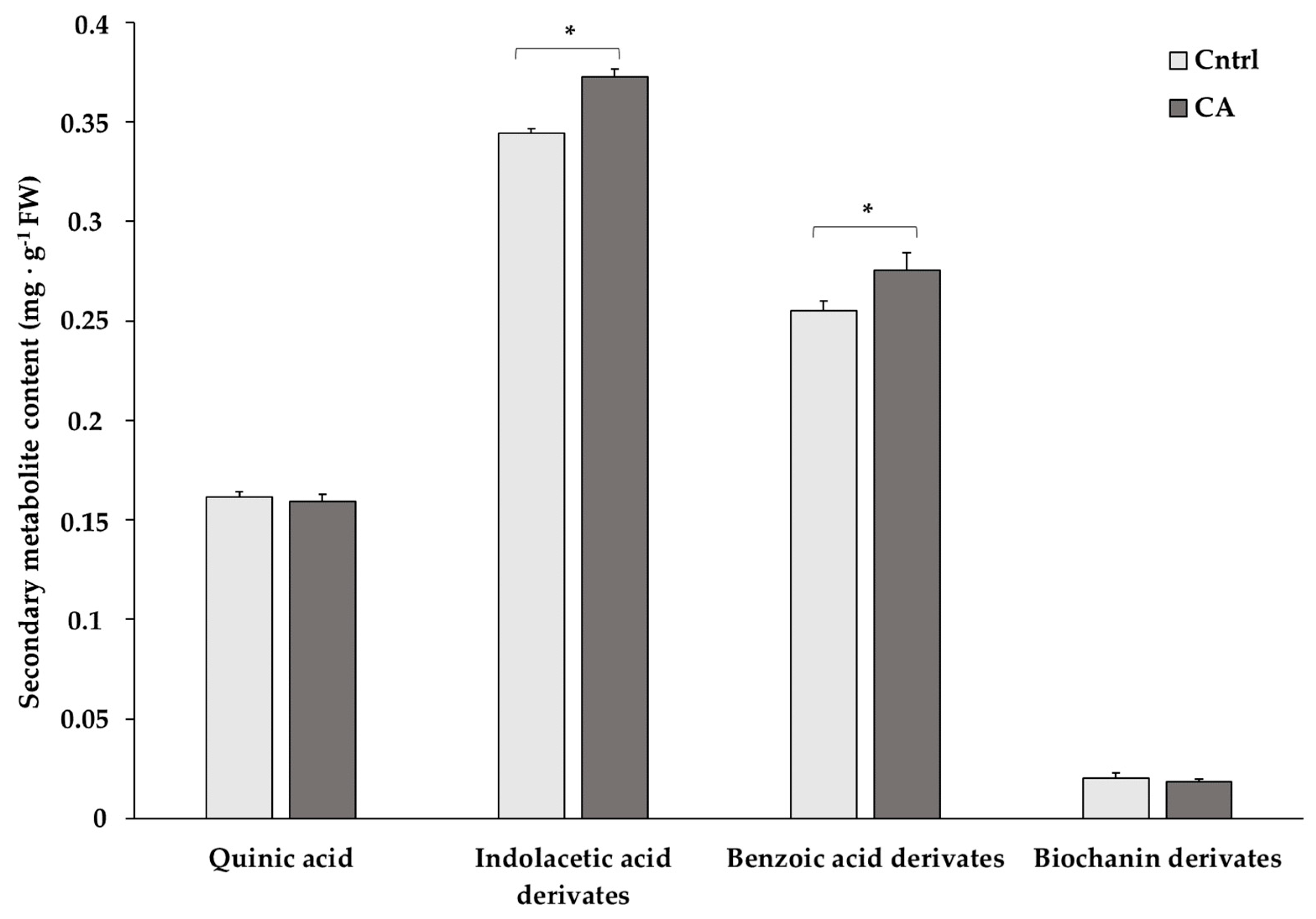
The content of a set of compounds found in higher abundance in chickpeas was determined at 12 months to evaluate the suitability of the storage methods in preserving them (Figure 2 and Table S3). Statistics revealed significantly higher contents of indoleacetic and benzoic acid derivatives under the CA storage condition.
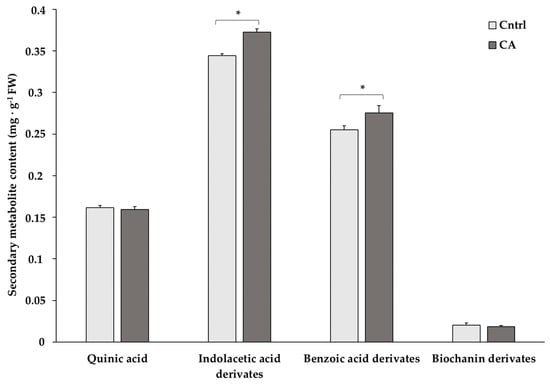
Figure 2.
Content of the most abundant secondary metabolites in chickpeas stored for 12 months under standard (Cntrl) and controlled N2 atmosphere (CA) conditions. The bar-chart shows the means of 3 independent biological replicates (expressed as mg/g FW) ± standard deviation (SD). Asterisks denote statistically significant values (p < 0.05) in Student’s t-test.
4. Discussion
The long-term storage of legumes can lead to a loss in the quality and nutraceutical features; therefore, it is important to find effective methods that can maintain their quality and preserve them against post-harvest losses. Concerning chickpeas, storage in triple-layered bags (two 80 μm inner high-density polyethylene bags enveloped by a third woven nylon layer [29]) was shown to be effective against bruchid infestation and prevented germination loss. Indeed, in the long-term storage of chickpeas, they are prone to attack by pulse beetles.
In this study, a comparison of the quality and nutraceutical features was carried out on chickpeas stored under standard (control) conditions (in a silo with the lid partially open) and under a CA. Previously, it was demonstrated that the use of a CA was effective on chickpeas for complete pest mortality and the reduction in mycotoxin contamination [22] and also in the reduction in the loss of bioactive molecules in old wheat varieties [21]; the same set-up was studied here with the goal of verifying whether it can be used for a wide range of agricultural commodities.
While no major changes were observed in the FA content (Table 2), noteworthy differences were detected in the vitamin E content (Figure 1): although a gradual loss in its content was observed after 12 months regardless of the storage conditions, no significant differences were obtained when comparing the values measured after 3, 6 or 9 months under the CA. Instead, under the control conditions, the decline in vitamin E content after 12 months was higher and more significant compared to the values at 3, 6 and 9 months.
Vitamin E is known to be prone to degradation during storage, and the suitability of CAs in mitigating the loss in molecules of nutraceutical interest has already been proven in wheat [21].
Germination percentages did not differ significantly between the CA and control conditions (Table S1), which may have been due to the temperature used (ambient): the use of higher temperatures may have led to more evident and significant differences, as already reported for lentils [30].
CA proved to be effective in preventing the loss of other types of molecules, namely indole acetic acid and benzoic acid derivatives, which are interesting due to their antioxidant power [31]. Indole compounds have also relevance in the modulation of plant growth [32], with IAA (indole-3-acetic acid a phytohormone of the auxin class) being an emblematic example: auxin is crucial for root development, and the presence of an auxin gradient is important in root development and in the establishment of a root stem cell niche [33]. Benzoic acid confers tolerance to multiple stress in plants [34] and is thus important post-germination to have more resilient plants to environmental constraints. Additionally, benzoic acid derivatives belong to phenolic acids: storage under a CA may prevent their complexation with the seed coat cell wall, a phenomenon known to prevent their extractability. For example, lower phenolic acid losses were observed in lentil [35], cowpea [36], Carioca bean [37] and faba bean [38] under CAs. Preserving these classes of molecules in the seeds is thus physiologically important to ensure, on the one hand, an optimal development of the plantlets and, on the other, their subsequent higher capacity to withstand exogenous stresses.
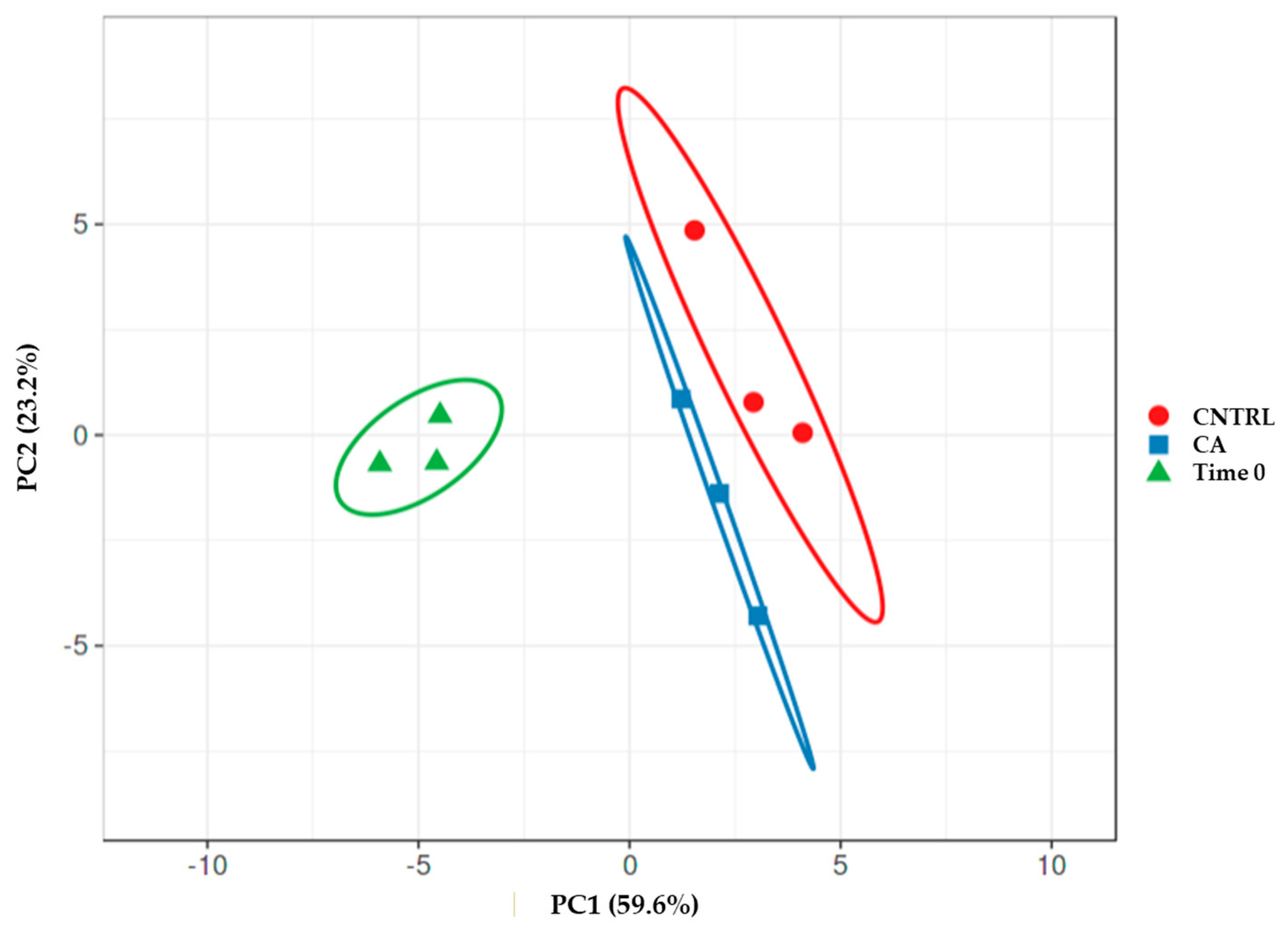
A principal component analysis (PCA) realized by merging the data of FA and secondary metabolite content after 12 months and at time 0 (Figure 3) revealed the existence of well-separated groups. The time 0 group was clearly separated from the 12 months group; however, among the samples of the last time points, it was possible to discern two distinct groups according to the storage method.
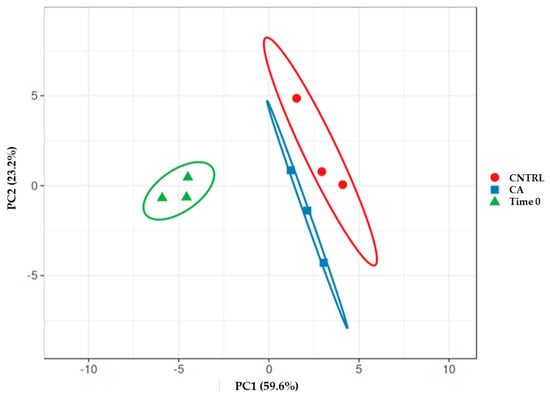
Figure 3.
Principal component analysis-PCA obtained by using the data of FA and secondary metabolite content of the samples at time 0 and after 12 months under control or CA.
This study therefore confirms the suitability of CAs for the long-term storage of agricultural commodities and validates their use for the preservation of functional molecules in legumes such as chickpeas.
To validate the suitability of CAs in the long-term storage of dried products, it will be interesting, in the future, to extend the analysis of the quality and nutraceutical features to other types of commodities, such as herbs, spices, nuts and beans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/seeds3010002/s1, Table S1: Germination rate of chickpeas stored under standard (Cntrl) and controlled N2 atmosphere (CA) conditions. Asterisks denote statistically significant values (p < 0.05) in Student’s t-test; Table S2: FA content in chickpeas stored under standard (Cntrl) and controlled N2 atmosphere (CA) conditions. The values are expressed as means of 3 independent biological replicates (expressed as g/100 g of FW); Table S3: Content of the most abundant secondary metabolites in chickpeas stored under standard (Cntrl) and controlled N2 atmosphere (CA) conditions, expressed as mg/100 g ± standard deviation (SD). Asterisks denote statistically significant values (p < 0.05) in Student’s t-test.
Author Contributions
Conceptualization, L.M. and R.B.; methodology L.M., G.S., C.V. and R.B.; software, L.M., G.S., C.V. and R.B.; validation, L.M., G.G., G.S., C.V. and R.B.; formal analysis, L.M., G.S., C.V. and R.B.; investigation, L.M., G.S., C.V. and R.B.; resources, L.M., G.S., C.V. and R.B.; data curation, L.M., G.S., C.V. and R.B.; writing—original draft preparation, L.M. and R.B.; writing—review and editing, L.M., G.S., C.V. and R.B.; project administration, L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Foundation Cassa Di Risparmio di Firenze (Fondazione CR Firenze).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data presented have been made available as tables, figures and supplementary tables.
Conflicts of Interest
Author Roberto Berni was employed by the company Log2Go. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Segev, A.; Badani, H.; Kapulnik, Y.; Shomer, I.; Oren-Shamir, M.; Galili, S. Determination of Polyphenols, Flavonoids, and Antioxidant Capacity in Colored Chickpea (Cicer arietinum L.). J. Food Sci. 2010, 75, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.L.; Cendoya, E.; Nichea, M.J.; Zachetti, V.G.L.; Chulze, S.N. Impact of Toxigenic Fungi and Mycotoxins in Chickpea: A Review. Curr. Opin. Food Sci. 2018, 23, 32–37. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional Quality and Health Benefits of Chickpea (Cicer arietinum L.): A Review. Br. J. Nutr. 2012, 108 (Suppl. S1), S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira Raphaelli, C.; de Oliveira, M. Changes in the Chemical Composition and Bioactive Compounds of Chickpea (Cicer arietinum L.) Fortified by Germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- He, Y.; Shim, Y.Y.; Mustafa, R.; Meda, V.; Reaney, M.J. Chickpea Cultivar Selection to Produce Aquafaba with Superior Emulsion Properties. Foods 2019, 8, 685. [Google Scholar] [CrossRef]
- Summo, C.; De Angelis, D.; Ricciardi, L.; Caponio, F.; Lotti, C.; Pavan, S.; Pasqualone, A. Data on the Chemical Composition, Bioactive Compounds, Fatty Acid Composition, Physico-Chemical and Functional Properties of a Global Chickpea Collection. Data Brief 2019, 27, 104612. [Google Scholar] [CrossRef]
- Flores^1, T.S.H.; Alvarado, A.D.; Carrasco, P.R.; de Anda, E.M.L.; Contreras, M.G.M.; Chávez, C.P.M. Estudio de la composición proximal de variedades de garbanzo (Cicer arietinum L.) COSTA 2004 Y BLANORO. Cienc. Tecnol. Agropecu. México 2023, 2, 9–15. [Google Scholar]
- Nino-Medina, G.; Muy-Rangel, D.; de Jesus Garza-Juarez, A.; Alberto Vazquez-Rodriguez, J.; Mendez-Zamora, G.; Urias-Orona, V. Nutritional Composition, Phenolic Compounds and Antioxidant Capacity of Chickpea (Cicer arietinum) Husk. Arch. Latinoam. Nutr. 2017, 67, 68–73. [Google Scholar]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Arora, M.; Singh, S.; Kaur, P. Pharmacognostic & Phytochemical Evaluation of Selected Seeds of ‘Cicer arietinum’ Linn. Seeds from Roopnagar Punab. Int. J. Pharm. Sci. Invent 2013, 2, 18–29. [Google Scholar]
- Ibrikci, H.; Knewtson, S.J.; Grusak, M.A. Chickpea Leaves as a Vegetable Green for Humans: Evaluation of Mineral Composition. J. Sci. Food Agric. 2003, 83, 945–950. [Google Scholar] [CrossRef]
- Perez-Perez, L.M.; Huerta-Ocampo, J.Á.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. Cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef] [PubMed]
- Divya, P.; Durga, K.K.; Sunil, N.; Rajasri, M.; Keshavulu, K.; Udayababu, P. Modified Atmosphere Storage Technique for the Management of Pulse Beetle, Callosobruchus Chinensis in Horse Gram. Legume Res.-Int. J. 2016, 39, 474–478. [Google Scholar] [CrossRef]
- Hashem, M.Y.; Risha, E.-S.M. Post-Harvest Losses Caused by Southern Cowpea Beetle Callosobruchus maculatus (F.) in Faba Bean Vicia faba, and Its Control Using Modified Atmospheres/Lagerverluste Durch Den Vierfleckigen Bohnenkäfer (Callosbruchus maculatus (F.)) Bei Ackerbohnen (Vicia faba) Und Die Bekämfung Durch Modifizierte Atmosphären. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2000, 107, 205–211. [Google Scholar]
- Ingabire, J.P.; Hategekimana, A.; Bhuvaneswari, K.; Mohan, S.; Ganapathy, S. Management of Pulse Beetle, Callosobruchus maculatus (F) Population by Nitrogen Based Modified Atmosphere. J. Entomol. Zool. Stud. 2013, 1, 48–52. [Google Scholar]
- Ingabire, J.P.; Hategekimana, A.; Bhuvaneswari, K.; Erler, F. Effectiveness of Various Combinations of Three Main Gases (Oxygen, Carbon Dioxide and Nitrogen) through Modified Atmospheres on Pulse Beetle, Callosobruchus maculatus (F) Population in Stored Green Grams. Int. J. Trop. Insect Sci. 2021, 41, 3233–3240. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J.; Riudavets, J.; Del Toro-Sánchez, C.L.; Rueda-Puente, E.O.; Martínez-Cruz, O.; Wong-Corral, F.J. Effect of Controlled Atmospheres on the Insect Callosobruchus maculatus Fab. in Stored Chickpea. J. Stored Prod. Res. 2016, 69, 78–85. [Google Scholar] [CrossRef]
- Kutbay, F.; Varol, İ.; Bayram, M.; Ozdemir, A. The Effect of Carbon Dioxide at High Pressure under Different Developmental Stages of Callosobruchus maculatus (F.) Hosting on Chickpeas. Afr. J. Biotechnol. 2011, 10, 2053–2057. [Google Scholar]
- Pascua, G.F.S.; Bayogan, E.R.V.; Salaipeth, L.; Photchanachai, S. Pretreating Callosobruchus maculatus (F.) Eggs in Mung Bean with Modified Atmosphere Conditions Influence Its Adult Emergence and Survival. J. Stored Prod. Res. 2021, 91, 101771. [Google Scholar] [CrossRef]
- Wong-Corral, F.J.; Castañé, C.; Riudavets, J. Lethal Effects of CO2-Modified Atmospheres for the Control of Three Bruchidae Species. J. Stored Prod. Res. 2013, 55, 62–67. [Google Scholar] [CrossRef]
- Moncini, L.; Simone, G.; Romi, M.; Cai, G.; Guerriero, G.; Whittaker, A.; Benedettelli, S.; Berni, R. Controlled Nitrogen Atmosphere for the Preservation of Functional Molecules during Silos Storage: A Case Study Using Old Italian Wheat Cultivars. J. Stored Prod. Res. 2020, 88, 101638. [Google Scholar] [CrossRef]
- Pisuttu, C.; Risoli, S.; Moncini, L.; Nali, C.; Pellegrini, E.; Sarrocco, S. Sustainable Strategies to Counteract Mycotoxins Contamination and Cowpea Weevil in Chickpea Seeds during Post-Harvest. Toxins 2023, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Moisture Measurement—Unground Grain and Seeds. Available online: https://dergipark.org.tr/tr/download/issue-full-file/41582 (accessed on 30 November 2023). [CrossRef]
- International Seed Testing Association. International Rules for Seed Testing; International Seed Testing Association: Wallisellen, Switzerland, 2024; Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing.html (accessed on 8 December 2023).
- ISO 4833-1:2013; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 30 November 2023).
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38276.html (accessed on 30 November 2023).
- Al-mentafji, H.N. Official Methods of Analysis of Aoac International; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.R.; Raju, S.V.S.; Dhanapal, R.; Sharma, K.R. Storage of Chickpea Grains (Cicer arietinum L.) in Triple Layer Bags Prevent Losses Caused by Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) under Laboratory Conditions. J. Stored Prod. Res. 2020, 88, 101685. [Google Scholar] [CrossRef]
- Bhattarai, B.; Walker, C.K.; Wallace, A.J.; Nuttall, J.G.; Hepworth, G.; Panozzo, J.F.; Partington, D.L.; Fitzgerald, G.J. Modified Storage Atmosphere Prevents the Degradation of Key Grain Quality Traits in Lentil. Agronomy 2023, 13, 2160. [Google Scholar] [CrossRef]
- Al-Haidari, R.A.; Al-Oqail, M.M. New Benzoic Acid Derivatives from Cassia Italica Growing in Saudi Arabia and Their Antioxidant Activity. Saudi Pharm. J. 2020, 28, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, Y.; Yang, X.; Liao, A.; Wu, J. The Role of Indole Derivative in the Growth of Plants: A Review. Front. Plant Sci. 2023, 13, 1120613. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, J.; Zheng, C.; Yang, Y.; Wang, L.; Zhang, R.; Ren, X.; Wei, S.; Aziz, U.; Du, J.; et al. Abscisic Acid Inhibits Primary Root Growth by Impairing ABI4-Mediated Cell Cycle and Auxin Biosynthesis. Plant Physiol 2023, 191, 265–279. [Google Scholar] [CrossRef]
- Senaratna, T.; Merritt, D.; Dixon, K.; Bunn, E.; Touchell, D.; Sivasithamparam, K. Benzoic Acid May Act as the Functional Group in Salicylic Acid and Derivatives in the Induction of Multiple Stress Tolerance in Plants. Plant Growth Regul. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Mirali, M.; Purves, R.W.; Vandenberg, A. Phenolic Profiling of Green Lentil (Lens culinaris Medic.) Seeds Subjected to Long-Term Storage. Eur. Food Res. Technol. 2016, 242, 2161–2170. [Google Scholar] [CrossRef]
- Lindemann, I.d.S.; Lang, G.H.; Ferreira, C.D.; Colussi, R.; Elias, M.C.; Vanier, N.L. Cowpea Storage under Nitrogen-Modified Atmosphere at Different Temperatures: Impact on Grain Structure, Cooking Quality, in Vitro Starch Digestibility, and Phenolic Extractability. J. Food Process. Preserv. 2020, 44, e14368. [Google Scholar] [CrossRef]
- Vanier, N.L.; Rupollo, G.; Paraginski, R.T.; de Oliveira, M.; Elias, M.C. Effects of Nitrogen-Modified Atmosphere Storage on Physical, Chemical and Technological Properties of Carioca Bean. Curr. Agric. Sci. Technol. 2014, 20, 10–20. [Google Scholar]
- Nasar-Abbas, S.M.; Plummer, J.A.; Siddique, K.H.M.; White, P.F.; Harris, D.; Dods, K. Nitrogen Retards and Oxygen Accelerates Colour Darkening in Faba Bean (Vicia faba L.) during Storage. Postharvest Biol. Technol. 2008, 47, 113–118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).