The Utilization of Seed Priming as a Tool to Overcome Salt and Drought Stresses: Is Still a Long Way to Go?
Abstract
1. Introduction
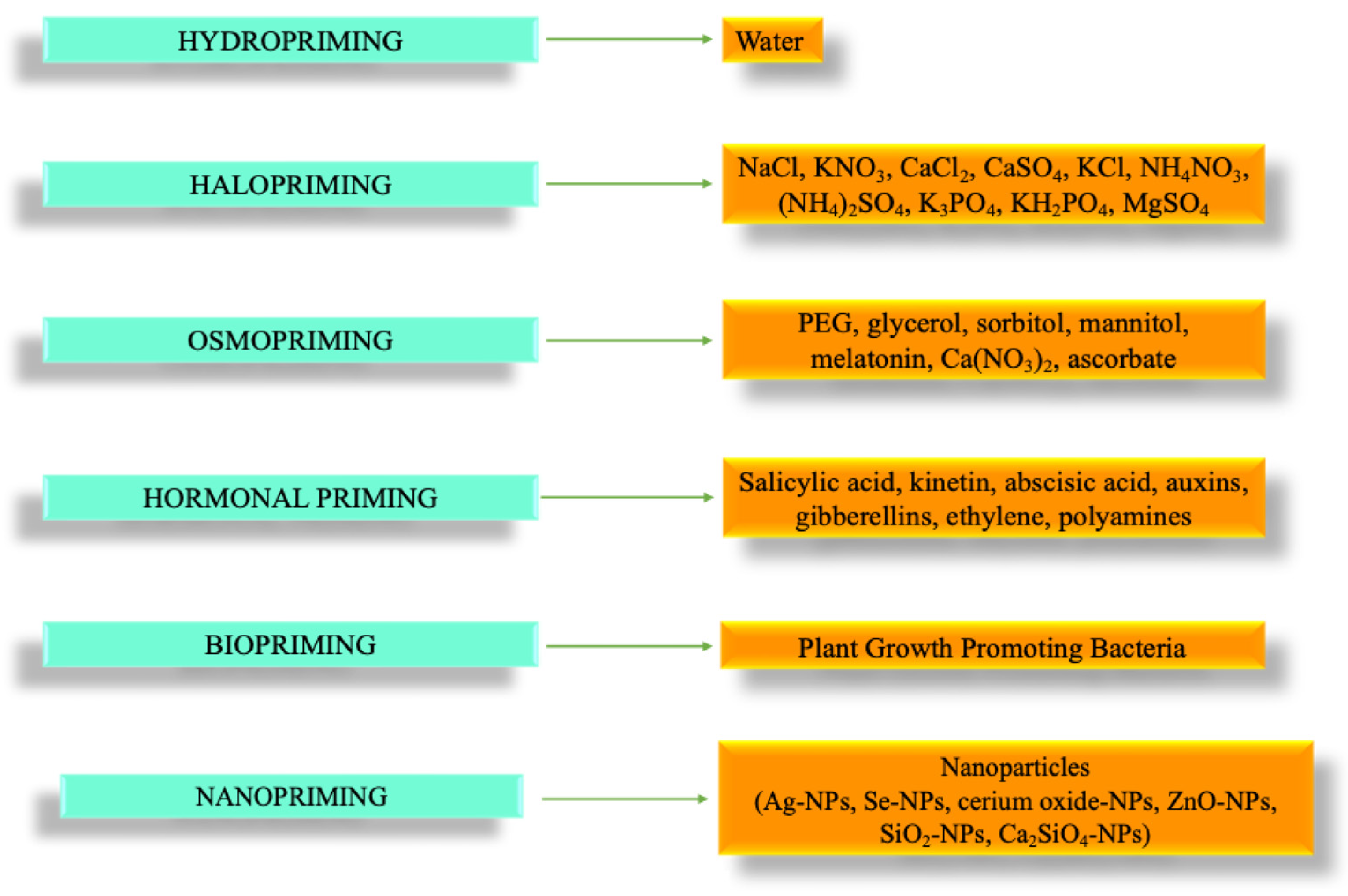
2. Overview and Conventional Priming Methods
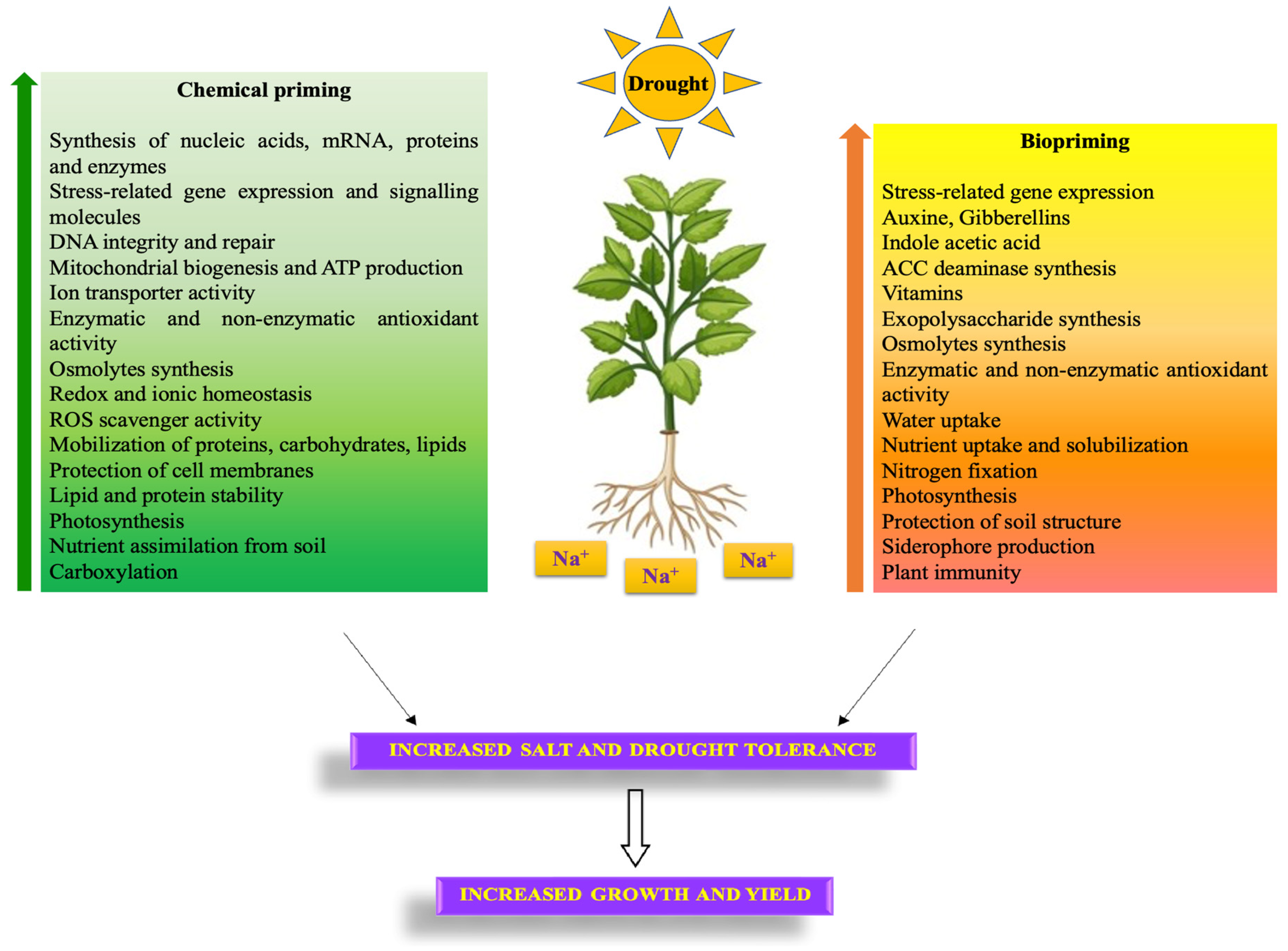
3. Advanced Methods: Biopriming and Nanopriming
4. Salt and Drought Stress Memory
5. Application of Seed Priming Protocols to Crop Species and the Application in Agriculture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javaid, M.H.; Khan, A.R.; Salam, A.; Neelam, A.; Azhar, W.; Ulhassan, Z.; Gan, Y. Exploring the Adaptive Responses of Plants to Abiotic Stresses Using Transcriptome Data. Agriculture 2022, 12, 211. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Zeeshan Fakhar, A.; Sharif, R.; Chen, H.; Zhang, C.; Ju, L.; Fotopoulos, V.; Siddique, K.H.M.; Singh, R.K.; et al. Smart reprograming of plants against salinity stress using modern biotechnological tools. Crit. Rev. Biotech. 2022, 15, 1035–1062. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Mason, C.J.; Jones, A.G.; Felton, G.W. Co-option of microbial associates by insects and their impact on plant-folivore interactions. Plant. Cell Environ. 2019, 42, 1078–1086. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant. Sci. 2005, 24, 23–28. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Gianella, M.; Pagano, A.; Forti, C.; Guzzon, F.; Mondoni, A.; de Sousa Araújo, S.; Macovei, A.; Balestrazzi, A. Chapter 6—Molecular aspects of seed priming as a means of progress in crop improvement. In Advances in Crop Improvement Techniques; Woodhead Publishing: Sawston, UK, 2020; pp. 89–100. [Google Scholar]
- Pradhan, N.; Prakash, P.; Tiwari, S.K.; Manimurugan, C.; Sharma, R.P.; Singh, P.M. Osmopriming of tomato genotypes with polyethylene glycol 6000 induces tolerance to salinity stress. Trends Biosci. 2014, 7, 4412–4417. [Google Scholar]
- Borromeo, I.; Domenici, F.; Del Gallo, M.; Forni, C. Role of Polyamines in the Response to Salt Stress of Tomato. Plants 2023, 12, 1855. [Google Scholar] [CrossRef]
- Khan, H.A.; Ayub, C.M.; Pervez, M.A.; Bilal, R.M.; Shahid, M.A.; Ziaf, K. Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009, 28, 81–87. [Google Scholar]
- Khan, H.A.; Pervez, M.A.; Ayub, C.M.; Ziaf, K.; Balal, R.M.; Shahid, M.A.; Akhtar, N. Hormonal priming alleviates salt stress in hot pepper (Capsicum annuum L.). Soil Environ. 2009, 28, 130–135. [Google Scholar]
- Nasri, N.; Kaddour, R.; Mahmoudi, H.; Baatour, O.; Bouraoui, N.; Lachaâl, M. The effect of osmopriming on germination, seedling growth and phosphatase activities of lettuce under saline condition. Afr. J. Biotechnol. 2011, 10, 14366–14372. [Google Scholar] [CrossRef]
- Abraha, B.; Yohannes, G. The role of seed priming in improving seedling growth of maize (Zea mays L.) under salt stress at field conditions. Agric. Sci. 2013, 4, 666–672. [Google Scholar] [CrossRef]
- Tabatabaei, S. The effect of priming on germination indexes and seed reserve utilization of maize seeds under salinity stress. Seed Res. (J. Seed Sci. Technol.) 2014, 3, 44. [Google Scholar]
- Dkhil, B.B.; Issa, A.; Denden, M. Germination and seedling emergence of primed okra (Abelmoschus esculentus L.) seeds under salt stress and low temperature. Am. J. Plant Physiol. 2014, 9, 38–45. [Google Scholar] [CrossRef]
- Naz, F.; Gul, H.; Hamayun, M.; Sayyed, A.; Khan, H.; Sherwani, S. Effect of NaCl stress on Pisum sativum germination and seedling growth with the influence of seed priming with potassium (KCl and KOH). Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 1304–1311. [Google Scholar] [CrossRef]
- Aloui, H.; Souguir, M.; Hannachi, C. Determination of an optimal priming duration and concentration protocol for pepper seeds (Capsicum annuum L.). Acta Agric. Slov. 2015, 103, 213–221. [Google Scholar] [CrossRef]
- Zavariyan, A.M.; Rad, M.Y.; Asghari, M. Effect of seed priming by potassium nitrate on germination and biochemical indices in Silybum marianum L. under salinity stress. Int. J. Life Sci. 2015, 9, 23–29. [Google Scholar] [CrossRef]
- Miladinov, Z.J.; Balešević-Tubić, S.N.; Đorđević, V.B.; Đukić, V.H.; Ilić, A.D.; Čobanović, L.M. Optimal time of soybean seed priming and primer effect under salt stress conditions. J. Agric. Sci. 2015, 60, 109–117. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Enhancement of Brassica napus Tolerance to High Saline Conditions by Seed Priming. Plants 2021, 10, 403. [Google Scholar] [CrossRef]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Paolo, B.; Lukasz, W.S.K.S.; Robert, P. Seed priming: New comprehensive approaches for an old empirical technique. In New Challenges in Seed Biology-Basic and Translational Research Driving Seed Technology; Intech Open: Rijeka, Croatia, 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Abd-ur-Rahman, H.; Asif, M.; Hussain, M.; Bilal, H.M.; Adnan, M.; Rehman, F.; Ahmad, S.; Khalid, M. Seed Priming; An Effective Way to Improve Plant Growth. EC Agric. 2020, 6, 01–05. [Google Scholar]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- Ansari, O.; Azadi, M.S.; Sharif-Zadeh, F.; Younesi, E. Effect of hormone priming on germination characteristics and enzyme activity of mountain rye (Secale montanum) seeds under drought stress conditions. J. Stress Physiol. Biochem. 2013, 9, 61–71. [Google Scholar]
- Chunthaburee, S.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 405–413. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Phys. 2015, 183, 1–12. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biol. Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Mouradi, M.; Bouizgaren, A.; Farissi, M.; Latrach, L.; Qaddoury, A.; Ghoulam, C. Seed osmopriming improves plant growth, nodulation, chlorophyll fluorescence and nutrient uptake in alfalfa (Medicago sativa L.)–rhizobia symbiosis under drought stress. Sci. Hortic. 2016, 213, 232–242. [Google Scholar] [CrossRef]
- Hasan, M.; Salam, M.; Chowdhury, M.; Sultana, M.; Islam, N. Effect of osmopriming on germination of rice seed. Bang. J. Agril. Res. 2016, 41, 451–460. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Li, X.; Zhu, X.; Liu, S.; Song, F.; Liu, F.; Wang, Y.; Qi, X.; Wang, F.; Zuo, Z.; et al. Salt acclimation induced salt tolerance is enhanced by abscisic acid priming in wheat. Plant Soil. Environ. 2017, 63, 307–314. [Google Scholar] [CrossRef]
- Ulfat, A.; Majid, S.A.; Hameed, A. Hormonal seed priming improves wheat (Triticum aestivum L.) field performance under drought and non-stress conditions. Pak. J. Bot. 2017, 49, 1239–1253. [Google Scholar]
- Abid, M.; Hakeem, A.; Shao, Y.; Liu, Y.; Zahoor, R.; Fan, Y.; Suyu, J.; Tahir Ata-Ul-Karim, S.; Tian, Z.; Jiang, D.; et al. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2018, 145, 12–20. [Google Scholar] [CrossRef]
- Gharbi, E.; Lutts, S.; Dailly, H.; Quinet, M. Comparison between the impacts of two different modes of salicylic acid application on tomato (Solanum lycopersicum) responses to salinity. Plant Signal. Behav. 2018, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Akter, L.; Fakir, O.A.; Alam, M.K.; Islam, M.U.; Chakraborti, P.; Alam, M.J.; Rashid, M.; Begum, M.; Kader, M. Amelioration of salinity stress in maize seed germination and seedling growth attributes through seed priming. Open J. Soil. Sci. 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Robledo, D.A.R. Effects of halopriming on seed germination and seedling emergence of Capsicum frutescens. J. Bot. Res. 2020, 3, 114–118. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; Qabil, N.; Awad, M.F.; Mansour, E. Seed Halo-Priming Improves Seedling Vigor, Grain Yield, and Water Use Efficiency of Maize under Varying Irrigation Regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Hidayah, A.; Nisak, R.R.; Susanto, F.A.; Nuringtyas, T.R.; Yamaguchi, N.; Purwestri, Y.A. Seed Halopriming Improves Salinity Tolerance of Some Rice Cultivars During Seedling Stage. Bot. Stud. 2022, 63, 24. [Google Scholar] [CrossRef]
- Taghvaei, M.; Nasrolahizadehi, A.; Mastinu, A. Effect of Light, Temperature, Salinity, and Halopriming on Seed Germination and Seedling Growth of Hibiscus sabdariffa under Salinity Stress. Agronomy 2022, 12, 2491. [Google Scholar] [CrossRef]
- Vidak, M.; Lazarević, B.; Nekić, M.; Šatović, Z.; Carović-Stanko, K. Effect of hormonal priming and osmopriming on germination of winter savory (Satureja montana L.) natural population under drought stress. Agronomy 2022, 12, 1288. [Google Scholar] [CrossRef]
- Bisht, S.; Sharma, V.; Kumari, N. Biosynthesized magnetite nanoparticles from Polyalthia longifolia leaves improve photosynthetic performance and yield of Trigonella foenum-graecum under drought stress. Plant Stress 2022, 5, 100090. [Google Scholar] [CrossRef]
- Cifuentes, L.; Gonzàlez, M.; Pinto-Irish, K.; Alvarez, R.; Coba de la Peña, T.; Ostria-Gallardo, E.; Franck, N.; Fischer, S.; Barros, G.; Castro, C.; et al. Metabolic imprint induced by seed halo-priming promotes a differential physiological performance in two contrasting quinoa ecotypes. Front. Plant Sci. 2023, 13, 1034788. [Google Scholar] [CrossRef]
- Corbineau, F.; Picard, M.A.; Fougereux, J.A.; Ladonne, F.; Côme, D. Effects of dehydration conditions on desiccation tolerance of developing pea seeds as related to oligosaccharide content and cell membrane properties. Seed Sci. Res. 2000, 10, 329–339. [Google Scholar] [CrossRef]
- Benamar, A.; Tallon, C.; Macherel, D. Membrane Integrity and Oxidative Properties of Mitochondria Isolated from Imbibing Pea Seeds after Priming or Accelerated Ageing. Seed Sci. Res. 2003, 13, 35–45. [Google Scholar] [CrossRef]
- Pandita, V.K.; Anand, A.; Nagarajan, S. Enhancement of seed germination in hot pepper following pre-sowing treatments. Seed Sci. Technol. 2007, 35, 282–290. [Google Scholar] [CrossRef]
- Sung, Y.; Cantliffe, D.J.; Nagata, R.T.; Nascimento, W.M. Structural Changes in Lettuce Seed During Germination at High Temperature Altered by Genotype, Seed Maturation Temperature, and Seed Priming. J. Am. Soc. Hortic. Sci. 2008, 133, 300–311. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci. 2011, 180, 212–220. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Fundamental Processes Involved in Seed Priming. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant. Biosyst. 2022, 156, 370–383. [Google Scholar] [CrossRef]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2013, 2, 6. [Google Scholar] [CrossRef]
- Brígido, C.; Nascimento, F.X.; Duan, J.; Glick, B.R.; Oliveira, S. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Mesorhizobium spp. reduces the negative effects of salt stress in chickpea. FEMS Microbiol. Lett. 2013, 349, 46–53. [Google Scholar] [CrossRef]
- Tittabutr, P.; Piromyou, P.; Longtonglang, A.; Noisa-Ngiam, R.; Boonkerd, N.; Teaumroong, N. Alleviation of the effect of environmental stresses using co-inoculation of mung bean by Bradyrhizobium and rhizobacteria containing stress-induced ACC deaminase enzyme. Soil. Sci. Plant. Nutr. 2013, 59, 559–571. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Nazli, F.; Akram, F.; Arshad, M.; Khalid, M. Effectiveness of halo-tolerant, auxin producing Pseudomonas and Rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.). Braz. J. Microbiol. 2013, 44, 1341–1348. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Yao, L.; Yue, H.; Li, H.; Li, C. Effect of IAA produced by Klebsiella oxytoca Rs-5 on cotton growth under salt stress. J. Gen. Appl. Microbiol. 2013, 59, 59–65. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus licheniformis K11 in Pepper. Plant. Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Bangash, N.; Khalid, A.; Mahmood, T.; Siddique, M.T. Screening rhizobacteria containing ACC-deaminase for growth promotion of wheat under water stress. Pak. J. Bot. 2013, 45, 91–96. [Google Scholar]
- Aamir, M.; Aslam, A.; Khan, M.Y.; Usman, M. Co-inoculation with Rhizobium and plant growth promoting rhizobacteria (PGPR) for inducing salinity tolerance in mung bean under field condition of semi-arid climate. Asian J. Agric. Biol. 2013, 1, 7. [Google Scholar]
- Jha, Y.; Subramanian, R.B. Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline condition. Chil. J. Agric. Res. 2013, 73, 213–219. [Google Scholar] [CrossRef]
- Saghafi, K.; Ahmadi, J.; Asghar-zadeh, A.; Esmailizad, A. An Evaluation of the Influence of PGPR on Wheat Growth Indices under Saline Stress. J. Sol. Biol. 2013, 1, 47–59. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant. Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Panitlurtumpai, N.; Sangdee, A.; Sakulpone, N.; Sirisom, P.; Pimthong, A. Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: Identification and effect on rice under saline conditions. J. Plant Interact. 2014, 9, 379–387. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr. Microbiol. 2014, 69, 484–494. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Nasir, A.; Aon, M.; Hussain, S.; Ahmad, M.; Naz, I. Seed inoculation with Pseudomonas fluorescens and Pseudomonas syringae enhanced maize growth in a compacted saline-sodic soil. Phyton 2018, 87, 25–31. [Google Scholar]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, J.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef]
- Yan, J.M.; Smith, M.D.; Glick, B.R.; Liang, Y. Effects of ACC deaminase containing rhizobacteria on plant growth and expression of Toc GTPases in tomato (Solanum lycopersicum) under salt stress. Botany 2014, 92, 775–781. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. Sci. 2015, 22, 773–779. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Kim, Y.J.; Choi, E.S.; Koh, S.C.; Lee, S.W.; Kim, Y.J.; Yang, D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015, 172, 7–15. [Google Scholar] [CrossRef]
- Bhatt, S.; Pandhi, N.; Raghav, R. Improved salt tolerance and growth parameters of groundnut (Arachis hypogaea L.) employing Halotolerant Bacillus cereus SVSCD1 isolated from Saurashtra Region, Gujarat. Gujarat. Ecol. Environ. Conserv. 2020, 26, S199–S212. [Google Scholar]
- Ni, H.; Wu, Y.; Zong, R.; Ren, S.; Pan, D.; Yu, L.; Li, J.; Qu, Z.; Wang, Q.; Zhao, G.; et al. Combination of Aspergillus niger MJ1 with Pseudomonas stutzeri DSM4166 or mutant Pseudomonas fluorescens CHA0-nif improved crop quality, soil properties, and microbial communities in barrier soil. Front. Microbiol. 2023, 14, 1064358. [Google Scholar] [CrossRef]
- Han, Y.; Wang, R.; Yang, Z.; Zhan, Y.; Ma, Y.; Ping, S.; Zhang, L.; Lin, M.; Yan, Y. 1-Aminocyclopropane-1-Carboxylate Deaminase from Pseudomonas stutzeri A1501 Facilitates the Growth of Rice in the Presence of Salt or Heavy Metals. J. Microbiol. Biotechnol. 2015, 25, 1119–1128. [Google Scholar] [CrossRef]
- Saleem, A.R.; Bangash, N.; Mahmood, T.; Khalid, A.; Centritto, M.; Siddique, M.T. Rhizobacteria capable of producing ACC deaminase promote growth of velvet bean (Mucuna pruriens) under water stress condition. Int. J. Agric. Biol. 2015, 17, 663–667. [Google Scholar] [CrossRef]
- Kiani, M.Z.; Ali, A.; Sultan, T.; Ahmad, R.; Hydar, S.I. Plant growth promoting rhizobacteria having 1-aminocyclopropane-1-carboxylic acid deaminase to induce salt tolerance in sunflower (Helianthus annus L.). Nat. Res. 2015, 6, 391–397. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Sundaram, S.; Chandrasekaran, M.; Kim, K.; Selvakumar, G.; Sa, T. Halotolerant bacteria with ACC deaminase activity alleviate salt stress effect in canola seed germination. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 237–241. [Google Scholar] [CrossRef]
- Lee, G.W.; Lee, K.J.; Chae, J.C. Herbaspirillum sp. strain GW103 alleviates salt stress in Brassica rapa L. ssp. pekinensis. Protoplasma 2016, 253, 655–661. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef]
- Vimal, S.R.; Patel, V.K.; Singh, J.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Kim, J.E.; Woo, O.G.; Bae, Y.; Keum, H.L.; Chung, S.; Sul, W.J.; Lee, J.H. Enhanced drought and salt stress tolerance in Arabidopsis by Flavobacterium crocinum HYN0056T. Plant Biol. 2020, 63, 63–71. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, J.M.; Choi, C.G.; Lee, H.; Moon, J.C.; Chung, N. Effect of plant growth-promoting rhizobacterial treatment on growth and physiological characteristics of Triticum aestivum L. under salt stress. Appl. Biol. Chem. 2021, 64, 89. [Google Scholar] [CrossRef]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Imran, M.; Kang, S.-M.; Pande, A.; Adhikari, A.; Kwon, E.-H.; Lee, I.-J. Biopriming of Maize Seeds with a Novel Bacterial Strain SH-6 to Enhance Drought Tolerance in South Korea. Plants 2022, 11, 1674. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Oyedoh, O.P.; Yang, W.; Dhanasekaran, D.; Santoyo, G.; Glick, B.R.; Babalola, O.O. Sustainable Agriculture: Rare-Actinomycetes to the Rescue. Agronomy 2023, 13, 666. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Pagnani, G.; Rossi, M.; D’Egidio, S.; Del Gallo, M.; Forni, C. Daucus carota L. seed inoculation with a consortium of bacteria improves plant growth and soil fertility status and microbial community. Appl. Sci. 2021, 11, 3274. [Google Scholar] [CrossRef]
- Djebaili, R.; Pellegrini, M.; Rossi, M.; Forni, C.; Smati, M.; Del Gallo, M.; Kitouni, M. Characterization of Plant Growth-Promoting traits and inoculation effects on Triticum durum of Actinomycetes isolates under salt stress conditions. Soil. Syst. 2021, 5, 26. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Salehi, H.; Abbas, S.; Saeed, F.; Poinern, G.E.J.; Siddique, K.H.M.; Varshney, R.K. Nano-enabled stress-smartagriculture: Can nanotechnology deliver drought and salinity-smart crops? J. Sustain. Agric. Environ. 2023, 2, 189–214. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Green-Synthesized Nanoparticles Enhanced Seedling Growth, Yield, and Quality of Onion (Allium cepa L.). ACS Sustain. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as potential hallmarks of drought stress tolerance in plants. Physiol. Plant 2022, 174, e13665. [Google Scholar] [CrossRef]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015, 83, 149–159. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Prasad, S.S.; Mitra, J.; Siddiqui, N.; Sahoo, L.; Kobayashi, Y.; Koyama, H. How do plants remember drought? Planta 2022, 256, 7. [Google Scholar] [CrossRef]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, N.; Virlouvet, L.; Riethoven, J.J.; Fromm, M.; Avramova, Z. Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Virlouvet, L.; Fromm, M. Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol. 2015, 205, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Van Hulten, M.; Pelser, M.; Van Loon, L.C.; Pieterse, C.M.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [PubMed]
- Galviz, Y.C.F.; Ribeiro, R.V.; Souza, G.M. Yes, plants do have memory. Theor. Exp. Plant Physiol. 2020, 32, 195–202. [Google Scholar] [CrossRef]
- Soja, G.; Eid, M.; Gangl, H.; Redl, H. Ozone sensitivity of grapevine (Vitis vinifera L.): Evidence for a memory effect in a perennial crop plant? Phyton. Ann. Rev. Bot. 1997, 37, 265–270. [Google Scholar]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual Exposure to Salinity Improves Tolerance to Salt Stress in Rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, M.; Renton, M.; Depczynski, M.; Mancuso, S. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 2014, 175, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Burritt, D.J.; Fujita, M. Cross-stress tolerance in plants: Molecular mechanisms and possible involvement of reactive oxygen species and methylglyoxal detoxification systems. In Abiotic Stress Response in Plants; Tuteja, N., Gill, S.S., Eds.; Wiley: New York, NY, USA, 2016; pp. 323–375. [Google Scholar] [CrossRef]
- Galis, I.; Gaquerel, E.; Pandey, S.P.; Baldwin, I.T. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ. 2009, 32, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Gong, M. Mechanical stimulation-induced cross-adaptation in plants: An overview. J. Plant Biol. 2011, 54, 358–364. [Google Scholar] [CrossRef]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Physiological, proteomic and transcriptional responses of wheat to combination of drought or waterlogging with late spring low temperature. Funct. Plant Biol. 2014, 41, 690–703. [Google Scholar] [CrossRef]
- Faralli, M.; Lektemur, C.; Rosellini, D.; Gürel, F. Effects of heat shock and salinity on barley growth and stress-related gene transcription. Biol. Plant 2015, 59, 537–546. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhong, J.; Zhou, Q.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Drought priming induces thermo-tolerance to post-anthesis high-temperature in offspring of winter wheat. Environ. Exp. Bot. 2016, 127, 26–36. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef]
- Gerke, J. Phytate (Inositol hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A Review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Nafees, M.; Chen, J.; Darras, A.; Ferrante, A.; Hancock, J.T.; Ashraf, M.; Zaid, A.; Latif, N.; Corpas, F.J.; et al. Chemical priming enhances plant tolerance to salt stress. Front. Plant Sci. 2022, 13, 946922. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef] [PubMed]
| Plant | Stress | Priming | Priming Agent | Growth Conditions | Limits | Reference |
|---|---|---|---|---|---|---|
| Wheat | Drought | Osmopriming | Ascorbic acid | Greenhouse | Lack of enzymatic analysis. No information about fruit and yield. | [27] |
| Wheat | Salt | Hormopriming | Gibberellin acid | Botanical Garden | Lack of enzymatic analysis. | [28] |
| Mountain Rye | Drought | Hormopriming | Gibberellic acid Salicylic acid | Laboratory | No information about fruit and yield. No metabolic analysis. | [29] |
| Rice | Salt | Hormopriming | Polyamines Gibberellin acid | Greenhouse | No enzymatic analysis. No information about fruit and yield. | [30] |
| Rapeseed | Salt | Osmopriming | PEG | Laboratory | No information about fruit and yield. No metabolic analysis. | [31] |
| Faba bean | Salt | Osmopriming | Melatonin | Laboratory | No information on yield and fruits. | [32] |
| Alfalfa | Drought | Osmopriming | PEG | Greenhouse | No biochemical analysis. No information about fruit and yield. | [33] |
| Tomato | Salt | Osmopriming | PEG | Laboratory | Lack of enzymatic analysis. No information about fruit and yield. | [34] |
| Wheat | Salt | Hormopriming | Abscisic acid | Greenhouse | Lack of metabolic analysis. No information about fruit and yield. | [35] |
| Wheat | Drought | Hormopriming | Gibberellic acid Salicylic acid | Field | No enzymatic analysis. | [36] |
| Wheat | Drought | Osmopriming | PEG | Experimental station | No metabolic analysis. | [37] |
| Tomato | Salt | Hormopriming | Salicylic acid | Growth chamber | No enzymatic analysis. No information about fruit and yield. | [38] |
| Maize | Salt | Halopriming Osmopriming | NaCl Sugar | Net house | Lack of metabolic and enzymatic analyses. No information about fruit and yield. | [39] |
| Chili pepper | Salt | Halopriming | NaCl KNO3 | Laboratory | Growth limited only to 2 weeks. | [40] |
| Rapeseed | Salt | Hormopriming | Polyamines | Growth chamber | No information about fruit and yield. | [21] |
| Soybean | Salt | Hormopriming | Jasmonic acid | Greenhouse | No information about fruit and yield. | [41] |
| Maize | Drought | Halopriming | NaCl | Growth chamber | No biochemical analysis. | [42] |
| Rice | Salt | Halopriming | NaCl KNO3 CaCl2 KCl | Greenhouse | No information about fruit and yield. Lack of enzymatic analysis. | [43] |
| Hibiscus tea | Light Temperature Salinity | Halopriming | NaCl | Laboratory | Tests performed exclusively on seeds. | [44] |
| Winter savory | Drought | Osmopriming | PEG | Laboratory | Tests performed exclusively on seeds. | [45] |
| Trigonella foenum-graecum | Drought | Nanopriming | Magnetite nanoparticles from leaves of Pulmonaria longifolia | Growth chamber | Lack of metabolic and enzymatic analyses. No information about fruit and yield. | [46] |
| Quinoa | Salt | Halopriming | NaCl | Greenhouse | No information about fruit and yield. No enzymatic analysis. | [47] |
| Tomato | Salt | Hormopriming | Polyamines | Greenhouse | No information about fruit and yield. | [10] |
| Plant | Bacteria | Stress | Conditions | Limits | Reference |
|---|---|---|---|---|---|
| Wheat | Hallobacillus sp., Bacillus halodenitrificans | Salt | Laboratory | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [60] |
| Chickpea | Mesorhizobium ciceri | Salt | Growth chamber | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [61] |
| Mung bean Bean Peanut | Bradyrhizobium sp., Enterobacter sp., Chryseobacterium sp. | Salt Drought | Growth chamber | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [62] |
| Mung bean | Pseudomonas, Rhizobium | Salt | Growth chamber | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [63] |
| Cotton | Klebsiella oxytoca | Salt | Greenhouse | Lack of enzymatic analysis. No information about fruit and yield. | [64] |
| Pepper | Bacillus licheniformis | Drought | Growth chamber | Lack of enzymatic analysis. No information about fruit and yield. | [65] |
| Wheat | Serratia spp., Aerococcus spp. | Drought | Jars with soil | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [66] |
| Mung bean | Rhizobium sp., PGPR | Salt | Field | Lack of enzymatic analysis. | [67] |
| Rice | Bacillus pumilus, Pseudomonas pseudoalcaligenes | Salt | Greenhouse | No information about fruit and yield. | [68] |
| Wheat | Azosprillium lipoferum, Pseudomonas fluorescens | Salt | Greenhouse | Lack of enzymatic analysis. No information about fruit and yield. | [69] |
| Rice | Bacillus amyloliquefaciens | Salt | Greenhouse | Lack of enzymatic analysis. No information about fruit and yield. | [70] |
| Rice | Serratia sp. | Salt | Greenhouse | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [71] |
| Sunflower | Pseudomonasaeruginosa | Salt | Growth chamber | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [72] |
| Wheat | Bacillus thuringiensis | Drought | Growth chamber | No information about fruit and yield. | [73] |
| Maize | Pseudomonas syringae, Pseudomonas fluorescens | Drought, salt | Field | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [74] |
| Limonium sinense (Girard) Kuntze | Bacillus Arthrobacter Streptomyces Isoptericola | Salt | Greenhouse | Lack of enzymatic analysis. No information about fruit and yield. | [75] |
| Tomato Arabidopsis | Enterobacter sp. EJ01 | Salt | Growth chamber | Lack of metabolic analysis. No information about fruit and yield. | [76] |
| Tomato | Pseudomonasputida | Salt | Greenhouse | Lack of enzymatic analysis. No information about fruit and yield. | [77] |
| Tomato | Pseudomonas fluorescens Pseudomonas migulae | Salt | Greenhouse | Lack of enzymatic analysis. | [56] |
| Cotton | Pseudomonas putida Pseudomonas chlororaphis | Salt | Growth chamber | Lack of enzymatic and metabolic analysis. No information about fruit and yield. | [78] |
| Arabidopsis thaliana | Micrococcus yunnanensis, Paenibacillus barengoltzii | Salt Drought | Growth chamber | Lack of enzymatic and metabolic analysis. | [79] |
| Groundnut | Bacillus cereus SVSCD1 | Salt | Controlled conditions | Lack of enzymatic analysis. No information about fruit and yield. | [80] |
| Cucumber Lattuce | Aspergillus niger MJ1 Pseudomonas stutzeri DSM4166 Pseudomonas fluorescens CHA0-nif | Salt | Field | Lack of metabolic and enzymatic analysis. | [81] |
| Rice | Pseudomonasstutzeri | Salt | Growth chamber | Lack enzymatic analysis. No information about fruit and yield. | [82] |
| Velvet bean | Rhizobacteria | Drought | Growth chamber | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [83] |
| Sunflower | PGPB | Salt | Greenhouse | Lack of metabolic and enzymatic analysis. | [84] |
| Canola | Brevibacterium epidermidis Bacillus aryabhattai | Salt | Laboratory | Tests performed on seeds. | [85] |
| Chinese cabbage | Herbaspirillum sp. | Salt | Growth chamber | Lack of enzymatic analysis. No information about fruit and yield. | [86] |
| Chickpea | Bacillus amyloliquefaciens Pseudomonas putida | Drought | Greenhouse | Lack of metabolic analysis. No information about fruit and yield. | [87] |
| Rice | Bacillus amyloliquefaciens | Salt | Growth chamber | Lack of enzymatic analysis. No information about fruit and yield. | [88] |
| Paddy plants | Curtobacterium albidum | Salt | Greenhouse | No information about fruit and yield. | [89] |
| Arabidopsis | Flavobacterium crocinum HYN0056T | Salt Drought | Controlled-environment chamber | Lack of metabolic and enzymatic analysis. | [90] |
| Triticum aestivum | Paenibacillus pabuli Pseudomonas nitroreducens Bacillus megaterium | Salt | Growth chamber | Lack of metabolic and enzymatic analysis. No information about fruit and yield. | [91] |
| Brassica napus | Azospirillum brasilense Arthrobacter globiformis Burkholderia ambifaria Herbaspirillum seropedicae Pseudomonas sp. | Salt | Growth chamber | No information about fruit and yield. | [58] |
| Brassica napus | Arthrobacter globiformis | Salt | Growth chamber | No information about fruit and yield. | [59] |
| Maize | Novel Bacterial Strain SH-6 | Drought | Laboratory | Lack of metabolic analysis and enzymatic analysis. No information about fruit and yield. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forni, C.; Borromeo, I. The Utilization of Seed Priming as a Tool to Overcome Salt and Drought Stresses: Is Still a Long Way to Go? Seeds 2023, 2, 406-420. https://doi.org/10.3390/seeds2040031
Forni C, Borromeo I. The Utilization of Seed Priming as a Tool to Overcome Salt and Drought Stresses: Is Still a Long Way to Go? Seeds. 2023; 2(4):406-420. https://doi.org/10.3390/seeds2040031
Chicago/Turabian StyleForni, Cinzia, and Ilaria Borromeo. 2023. "The Utilization of Seed Priming as a Tool to Overcome Salt and Drought Stresses: Is Still a Long Way to Go?" Seeds 2, no. 4: 406-420. https://doi.org/10.3390/seeds2040031
APA StyleForni, C., & Borromeo, I. (2023). The Utilization of Seed Priming as a Tool to Overcome Salt and Drought Stresses: Is Still a Long Way to Go? Seeds, 2(4), 406-420. https://doi.org/10.3390/seeds2040031







