Pre-Sowing Static Magnetic Field Treatment of Vegetable Seeds and Its Effect on Germination and Young Seedlings Development
Abstract
:1. Introduction
2. Materials and Methods
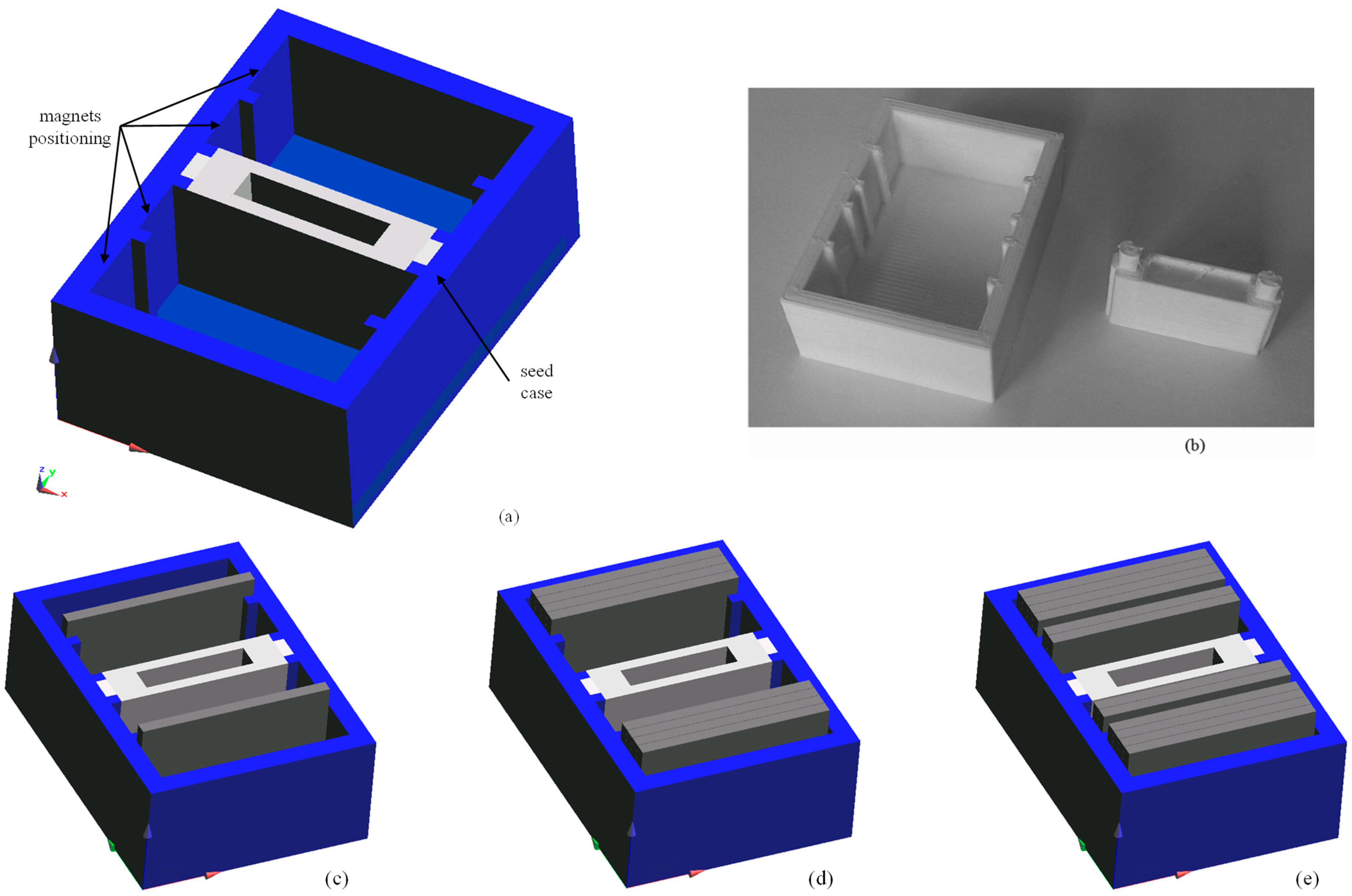
2.1. Exposure Set-Up
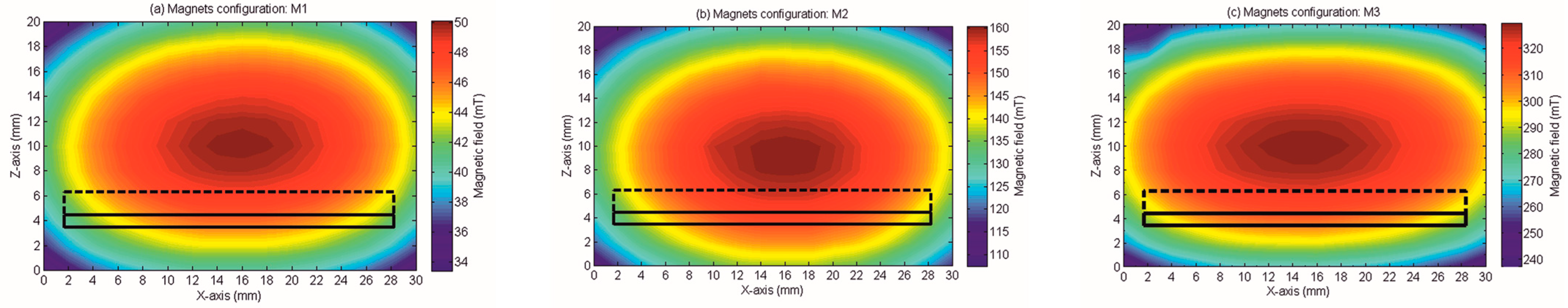
2.2. Magnetic Field Characterization
2.3. Experimental Design
2.4. Germination Parameters
2.5. Growth Parameters
2.6. Statistical Analysis
3. Results
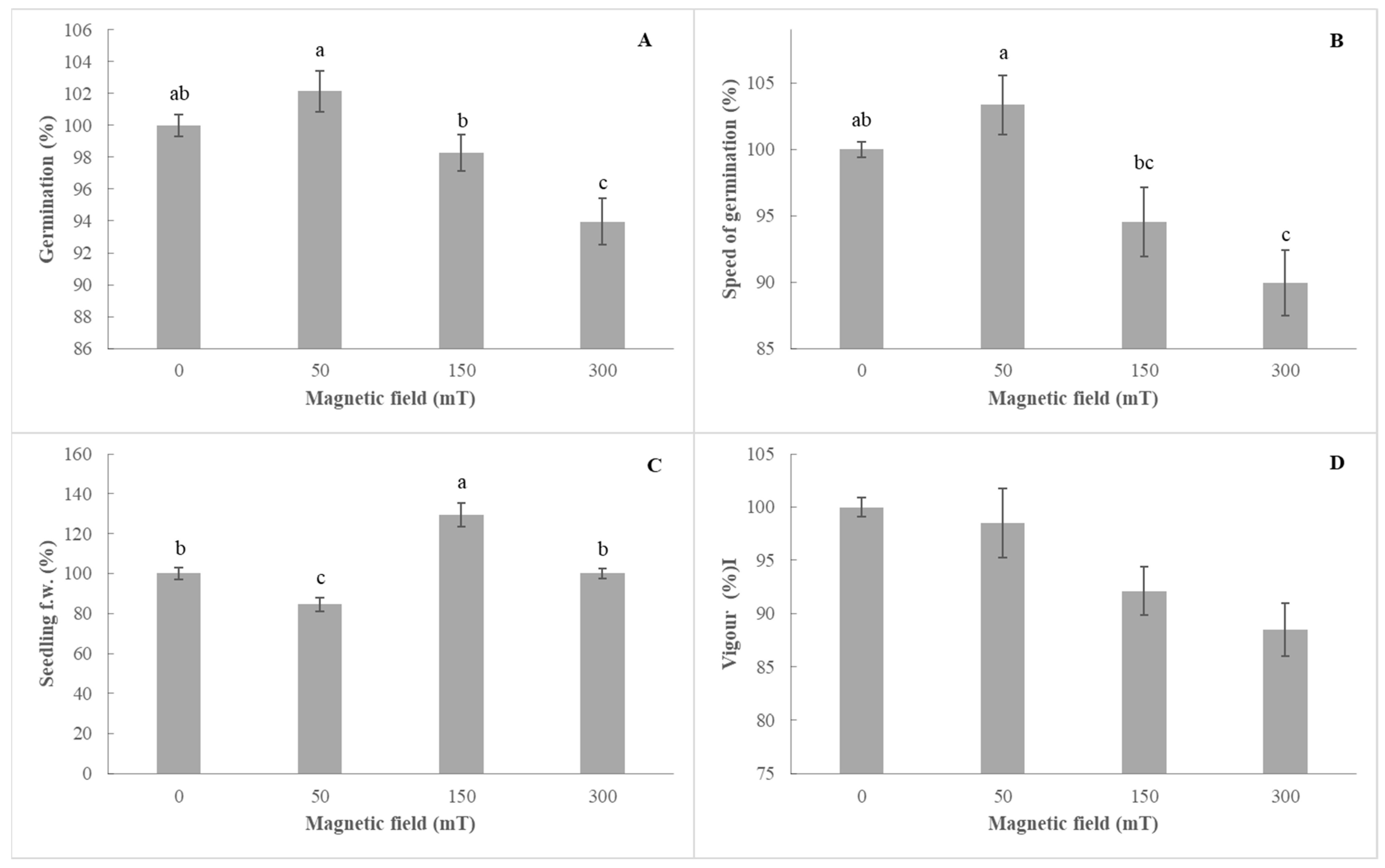
3.1. Effect of Pre-Sowing Treatment on Tomato (Solanum lycopersicum L.)
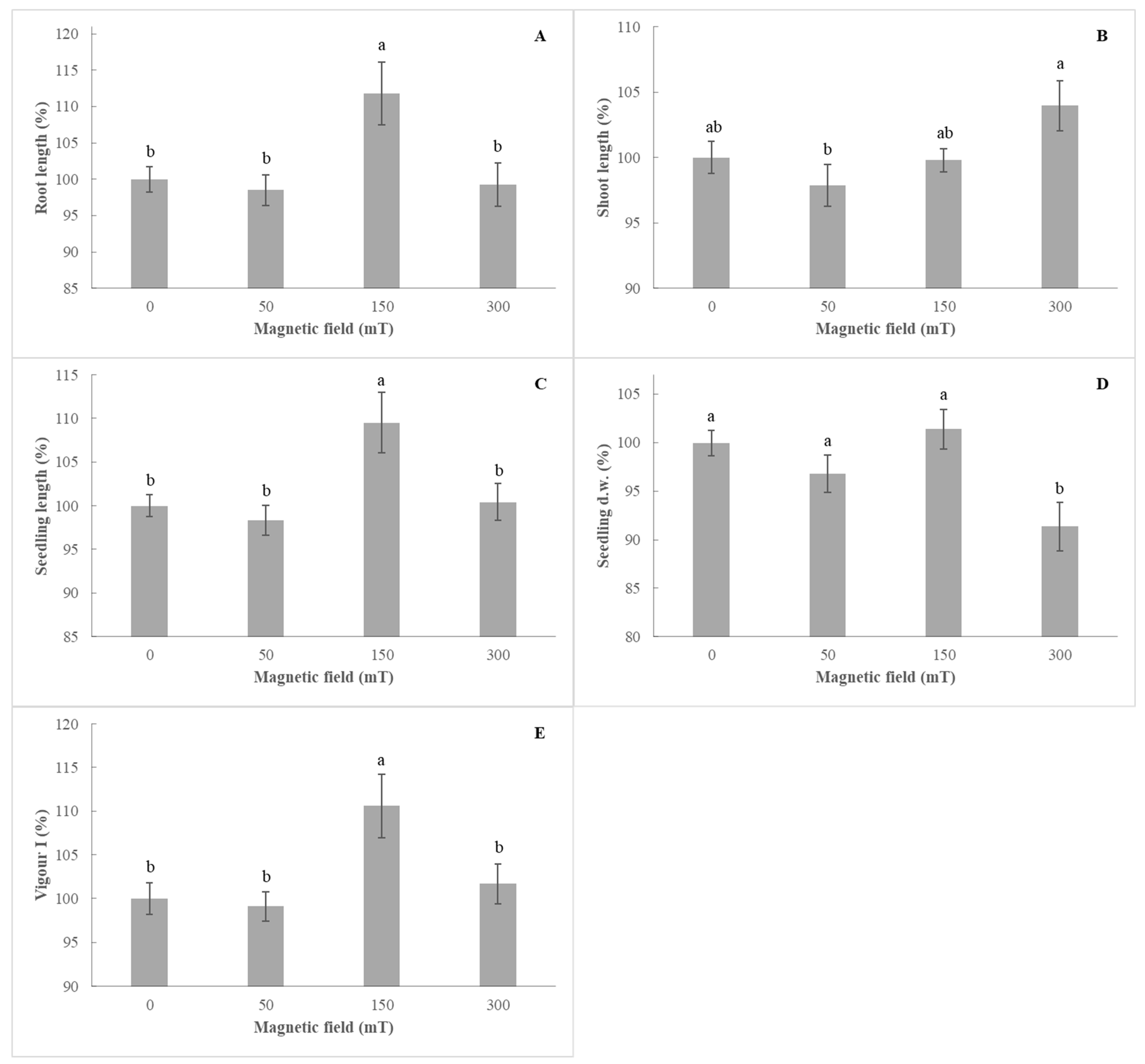
3.2. Effect of Pre-Sowing Treatment on Rocket (Eruca sativa L.)
3.3. Effect of Pre-Sowing Treatment on Lettuce (Lactuca sativa L.)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cliffe, D.O. Production and scheduling of lettuce transplants for commercial crop production. Acta Hortic. 1988, 247, 49–51. [Google Scholar] [CrossRef]
- Kozai, T. Introduction, 1–5. In Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Springer: Dordrecht, The Netherlands, 2005; p. 315. [Google Scholar]
- Thomas, B.M. Overview of the seedling, incorporated, transplant industry operation. HortTechnology 1993, 3, 406–408. [Google Scholar] [CrossRef]
- Guzman, C.; Sanchez, A.; Nagata, R.T. A comparison of transplanted and direct-seeded lettuce at various levels of soil fertility. Soil Crop Sci. Soc. Fla. Proc. 1989, 48, 26–28. [Google Scholar]
- Klassen, P. Economics dictate using transplants. Am. Veg. Grower 1986, 34, 9–14. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Martinez, E. Magnetic field as a method of improving the quality of sowing material: A review. Int. Agrophysics 2015, 29, 377–389. [Google Scholar] [CrossRef]
- Araújo, S.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.; Parola-Contreras, I.; Montoya-Gómez, L.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Vashisth, A.; Meena, N.; Krishnan, P. Magnetic field affects growth and yield of sunflower under different moisture stress conditions. Bioelectromagnetics 2021, 42, 473–483. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. Physical factors for plant growth stimulation improve food quality. In Food Production—Approaches, Challenges and Tasks; Aladjadjiyan, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 145–168. [Google Scholar]
- Koukounaras, A.; Tsouvaltzis, P.; Siomos, A.S. Effect of root and foliar application of amino acids on the growth and yield of greenhouse tomato in different fertilization levels. J. Food Agric. Environm. 2013, 11, 644–648. [Google Scholar]
- Siomos, A.S.; Koukounaras, A. Quality and postharvest physiology of rocket leaves. Fresh Produce 2007, 1, 59–65. [Google Scholar]
- Barillari, J.; Canistro, D.; Paolini, M.; Ferroni, F.; Pedulli, G.; Iori, R.; Valgimigli, L. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J. Agric. Food Chem. 2005, 53, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Flórez, M.; Carbonell, M.V.; Martínez, E. Exposure of maize seeds to stationary magnetic fields: Effects on germination and early growth. Environ. Exp. Bot. 2007, 59, 68–75. [Google Scholar] [CrossRef]
- Shine, M.; Guruprasad, K.; Anand, A. Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics 2011, 32, 474–484. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Exposure of seeds to static magnetic field enhances germination and early growth characteristics in Chickpea (Cicer arietinum L.). Bioelectromagnetics 2008, 167, 149–156. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Martínez, E.; Carbonell, M.V.; Flórez, M.; Amaya, J.M.; Maqueda, R. Germination of tomato seeds (Lycopersicon esculentum L.) under magnetic field. Int. Agrophysics 2009, 23, 45–49. [Google Scholar]
- Moon, J.D.; Chung, H.S. Acceleration of germination of tomato seed by applying AC electric and magnetic fields. J. Electrost. 2000, 48, 103–114. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Katsenios, N.; Karkanis, A.; Papastylianou, P.; Triantafyllidis, V.; Travlos, I.; Bilalis, D.J. Effects of presowing pulsed electromagnetic treatment of tomato seed on growth, yield, and lycopene content. Sci. World J. 2014, 2014, 369745. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.; Garcí, D.; Sueiro, L.; Gilart, F.; Porras, E.; Licea, L. Pre-sowing magnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics 2006, 27, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, T.; Dumlupinar, R.; Erdal, S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 2010, 31, 120–129. [Google Scholar] [CrossRef] [PubMed]
- NOAA 2015. National Centers for Environmental Information. Magnetic Field Calculators. Available online: http://www.ngdc.noaa.gov/geomag-web (accessed on 1 June 2015).
- Abdul-Baki, A.A.; Anderson, J.D. Vigour determination in soybean by multiple criteria. Crop Sci. 1973, 10, 31–34. [Google Scholar]
- Garcìa Reina, F.; Arza Pascual, L.; Almanza Fundora, I. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: Experimental results. Bioelectromagnetics 2001, 22, 596–602. [Google Scholar] [CrossRef]
- De Souza, A.; Sueiro, L.; González, L.M.; Licea, L.; Porras, E.; Gilart, F. Improvement of the growth and yield of lettuce plants by non-uniform magnetic fields. Electromagn. Biol. Medic. 2008, 27, 173–184. [Google Scholar] [CrossRef]
- Alpsoy, H.C.; Unal, H. Effect of stationary magnetic field on seed germination and crop yield in spinach (Spinacia oleracea L.). Compt. Ren. Acad. Bulgar. Sci. 2019, 72, 687–696. [Google Scholar]
- Michalak, I.; Lewandowska, S.; Niemczyk, K.; Detyna, J.; Bujak, H.; Arik, P.; Bartniczak, A. Germination of soybean seeds exposed to the static/alternating magnetic field and algal extract. Eng. Life Sci. 2019, 19, 986–999. [Google Scholar] [CrossRef]
- Kornarzynski, K.; Sujak, A.; Czernel, G.; Wiacek, D. Effect of Fe3O4 nanoparticles on germination of seeds and concentration of elements in Helianthus annuus L. under constant magnetic field. Sci. Rep. 2020, 10, 8068. [Google Scholar] [CrossRef]
- Hussain, M.S.; Dastgeer, G.; Afzal, A.M.; Hussain, S.; Kanwar, R.R. Eco-friendly magnetic field treatment to enhance wheat yield and seed germination growth. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100299. [Google Scholar] [CrossRef]
- Poinapen, D.; Brown, D.C.W.; Beeharry, G.K. Seed orientation and magnetic field strength have more influence on tomato seed performance than relative humidity and duration of exposure to non-uniform static magnetic fields. J. Plant Physiol. 2013, 170, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lv, Y.; Chen, C.; Zhang, Y.; Wei, S. Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis. Adv. Space Res. 2014, 53, 1118–1124. [Google Scholar] [CrossRef]



| Source of Variance | DF Z | Germination | Speed of Germination | Root Length | Shoot Length | Seedling Length | Seedling f. w. | Seedling d. w. | Vigour I | Vigour II |
|---|---|---|---|---|---|---|---|---|---|---|
| Magnetic field (A) | 3 | *** | *** | NS | NS | NS | *** | NS | *** | NS |
| Exposure time (B) | 4 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| A × B | 12 | NS | NS | * | NS | * | *** | NS | *** | NS |
| Error | 60 |
| Source of Variance | DF Z | Germination | Speed of Germination | Root Length | Shoot Length | Seedling Length | Seedling f. w. | Seedling d. w. | Vigour I | Vigour II |
|---|---|---|---|---|---|---|---|---|---|---|
| Magnetic field (A) | 3 | NS | NS | ** | * | ** | NS | *** | ** | NS |
| Exposure time (B) | 4 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| A × B | 12 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Error | 60 |
| Source of Variance | DF Z | Germination | Speed of Germination | Root Length | Shoot Length | Seedling Length | Seedling f. w. | Seedling d. w. | Vigour I | Vigour II |
|---|---|---|---|---|---|---|---|---|---|---|
| Magnetic field (A) | 3 | *** | * | * | NS | * | NS | NS | NS | NS |
| Exposure time (B) | 4 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| A × B | 12 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Error | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukounaras, A.; Boursianis, A.; Kostas, S.; Theopoulos, A.; Bantis, F.; Samaras, T. Pre-Sowing Static Magnetic Field Treatment of Vegetable Seeds and Its Effect on Germination and Young Seedlings Development. Seeds 2023, 2, 394-405. https://doi.org/10.3390/seeds2040030
Koukounaras A, Boursianis A, Kostas S, Theopoulos A, Bantis F, Samaras T. Pre-Sowing Static Magnetic Field Treatment of Vegetable Seeds and Its Effect on Germination and Young Seedlings Development. Seeds. 2023; 2(4):394-405. https://doi.org/10.3390/seeds2040030
Chicago/Turabian StyleKoukounaras, Athanasios, Achilles Boursianis, Stefanos Kostas, Argyris Theopoulos, Filippos Bantis, and Theodoros Samaras. 2023. "Pre-Sowing Static Magnetic Field Treatment of Vegetable Seeds and Its Effect on Germination and Young Seedlings Development" Seeds 2, no. 4: 394-405. https://doi.org/10.3390/seeds2040030
APA StyleKoukounaras, A., Boursianis, A., Kostas, S., Theopoulos, A., Bantis, F., & Samaras, T. (2023). Pre-Sowing Static Magnetic Field Treatment of Vegetable Seeds and Its Effect on Germination and Young Seedlings Development. Seeds, 2(4), 394-405. https://doi.org/10.3390/seeds2040030










