Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Seaweed Extract Preparation
2.2. Physicochemical Composition of the Seaweed Extracts
2.3. Bioassays: Germination and Growth under Laboratory Conditions
2.4. Statistical Analysis
3. Results
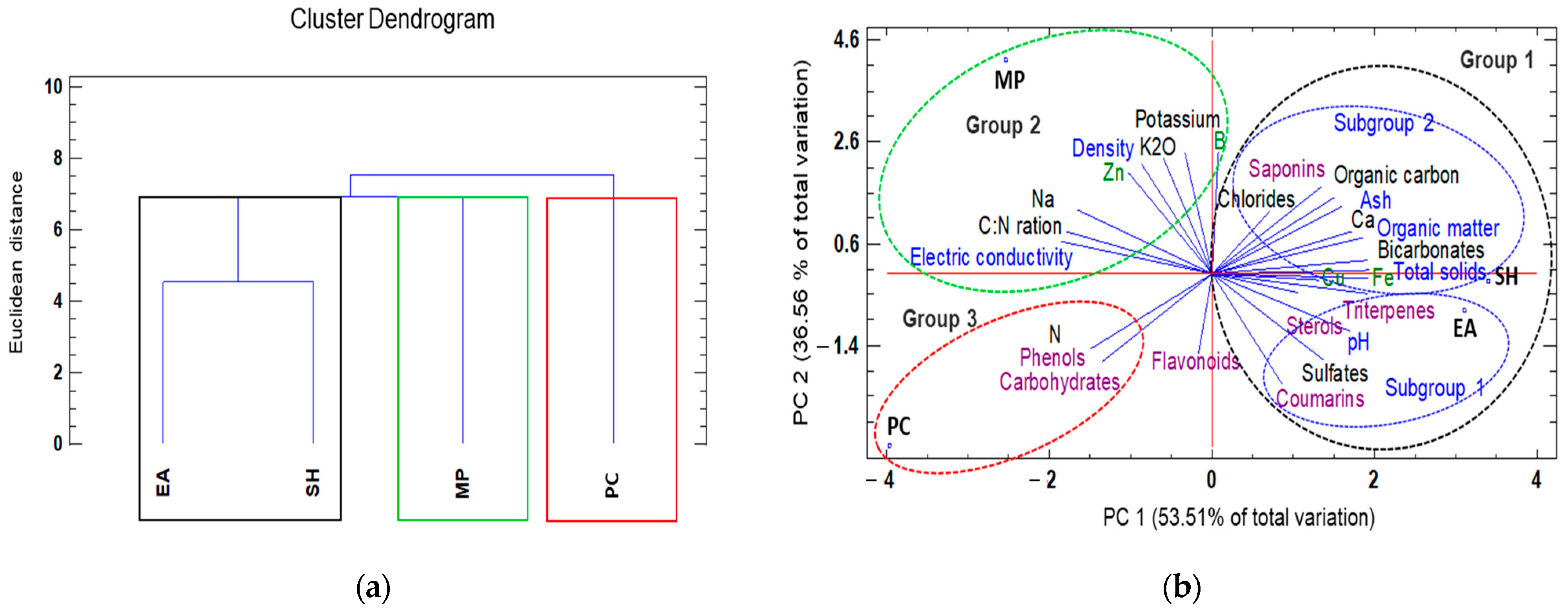
3.1. Chemical Composition of the Seaweed Extracts
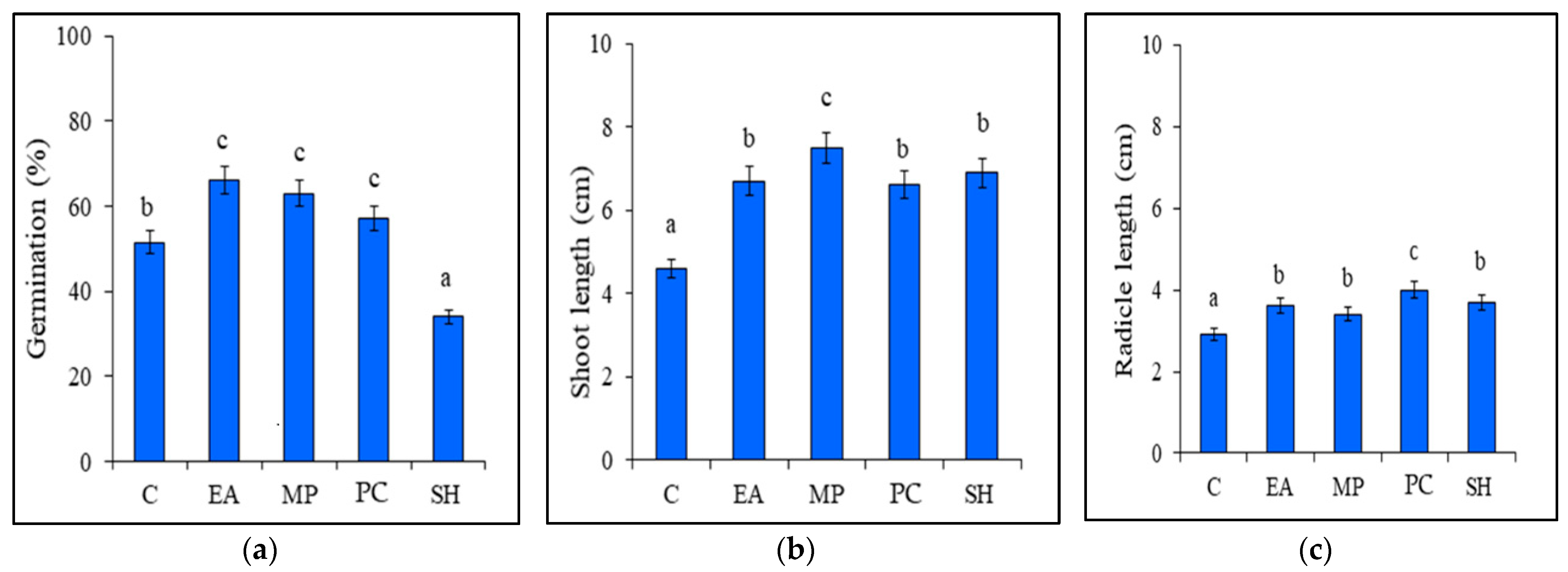
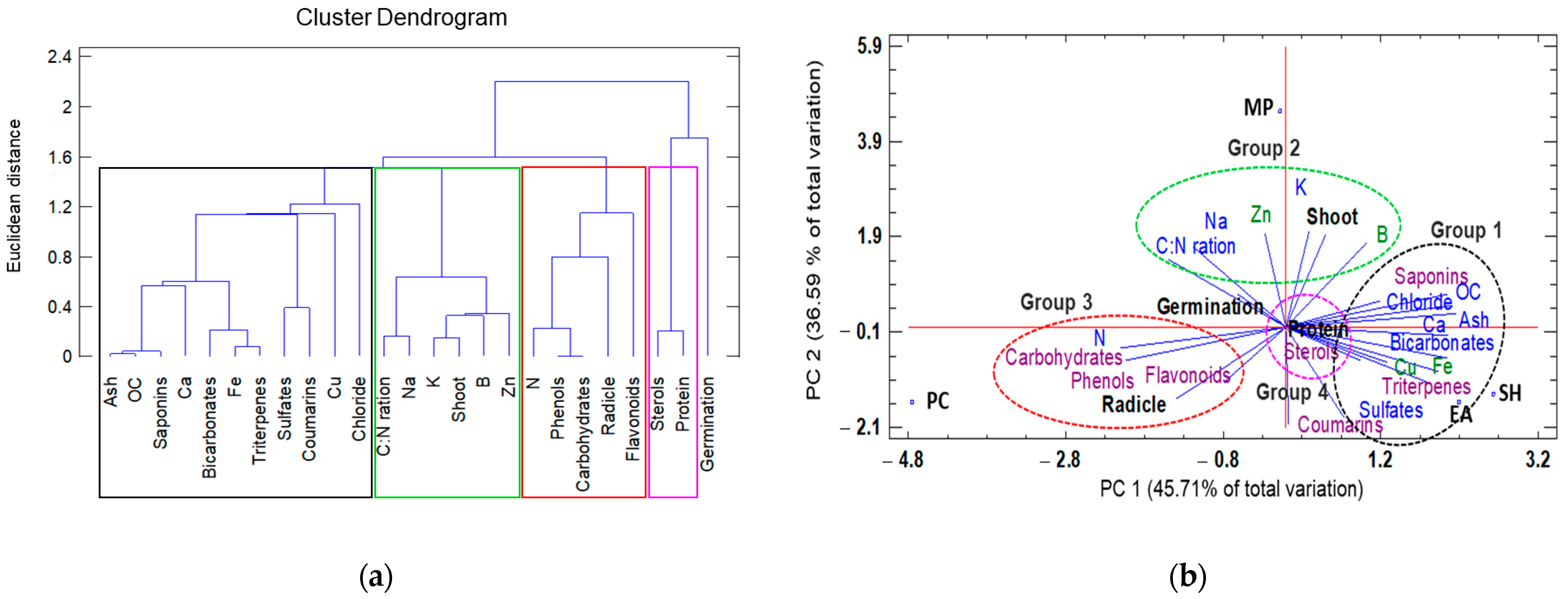
3.2. Germination, Growth, and Protein Content of Tomato Seedlings Treated with Seaweed Extracts
3.3. Understanding Treatment–Variable Interactions through Hierarchical Clustering and PCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- García-Estrada, R.S.; Diaz-Lara, A.; Aguilar-Molina, V.H.; Tovar-Pedraza, J.M. Viruses of Economic Impact on Tomato Crops in Mexico: From Diagnosis to Management—A Review. Viruses 2022, 14, 1251. [Google Scholar] [CrossRef]
- SIAP. Servicio de Información Agroalimentaria y Pesquera. Atlas-Agroalimentario. 2020. Consulted October 2021. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2020/Atlas-Agroalimentario-2020 (accessed on 12 October 2022).
- Branthôme, F.-X. Worldwide (Total Fresh) Tomato Production Exceeds 187 Million Tonnes in 2020. Available online: https://www.tomatonews.com/en/worldwide-total-fresh-tomato-production-exceeds-187-million-tonnes-in-2020_2_1565.html (accessed on 12 October 2022).
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. The Biostimulant Effect of Hydroalcoholic Extracts of Sargassum spp. in Tomato Seedlings under Salt Stress. Plants 2022, 11, 3180. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación (DOF). NORMA Oficial Mexicana NOM-182-SSA1-2010, Etiquetado de Nutrientes Vegetales. Available online: https://www.dof.gob.mx/normasOficiales/4371/salud1a1.htm#:~:text=1.1%20Esta%20norma%20establece%20las,regladores%20de%20crecimiento%20tipo%203 (accessed on 5 March 2023).
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.S.; Netravali, A.N.; Taylor, A.G. A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustainability 2019, 11, 5304. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C.; Novembre, A.D.C.; Chamma, H.M.C.P. Seaweed extract improves the vigor and provides the rapid emergence of dry bean seeds. Am. Eurasian J. Agric. Environ. Sci. 2013, 13, 1104–1107. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruíz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Virgen-Calleros, G.; Ruiz-López, M.A.; Zañudo-Hernández, J.; Délano-Frier, J.P.; Sánchez-Hernández, C. Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus. Alternaria Solani. J. Appl. Phycol. 2014, 26, 1607–1614. [Google Scholar] [CrossRef]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Cragie, S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Osuna-Ruiz, I.; López-Saiz, C.M.; Burgos-Hernández, A.; Velázquez, C.; Nieves-Soto, M.; Hurtado-Oliva, M.A. Antioxidant, antimutagenic and antiproliferative activities in selected seaweed species from Sinaloa, Mexico. Pharm. Biol. 2016, 54, 2196–2210. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Benítez-García, I.; Dueñas-Ledezma, A.K.; Martínez-Montaño, E.; Salazar-Leyva, J.A.; Carrera, E.; Osuna Ruiz, I. Identification and quantification of plant growth regulators and antioxidant compounds in aqueous extracts of Padina durvillaei and Ulva lactuca. Agronomy 2020, 10, 866. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Magallanes, C.; Córdova, C.; Orozco, R. Actividad antibacteriana de extractos etanólicos de macroalgas marinas de la costa central del Perú. Rev. Peru Biol. 2003, 10, 125–132. [Google Scholar] [CrossRef]
- Mascheck, J.A.; Baker, B.J. Macroalgal chemical defenses and their roles in structuring tropical marine communities. In Algal Chemical Ecology; Asmler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Futur. J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Dangariya, M.; Agarwal, P. Seaweed extracts: Potential biodegradable, environmentally friendly resources for regulating plant defence. Algal Res. 2021, 58, 102363. [Google Scholar] [CrossRef]
- Asimakis, E.; Shehata, A.A.; Eisenreich, W.; Acheuk, F.; Lasram, S.; Basiouni, S.; Emekci, M.; Ntougias, S.; Taner, G.; May-Simera, H.; et al. Algae and their metabolites as potential bio-pesticides. Microorganisms 2022, 10, 307. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of plant analysis. In Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1973; pp. 1–32. [Google Scholar]
- AOSA (Association of Official Seed Analysts). Rules for Testing Seed; AOSA: Las Cruces, NM, USA, 2005; pp. 4–113. [Google Scholar]
- ISTA (International Seed Testing Association). International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1996. [Google Scholar]
- Silva, L.D.; Bahcevandziev, K.; Pereira, L. Production of bio-fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Oceanol. Limnol. 2019, 37, 918–927. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts the prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Soares, C.; Švarc-Gajić, J.; Oliva-Teles, M.T.; Pinto, E.; Nastić, N.; Savić, S.; Almeida, A.; Delerue-Matos, C. Mineral composition of subcritical water extracts of Saccorhiza polyschides, a brown seaweed used as fertilizer in the north of Portugal. J. Mar. Sci. Eng. 2020, 8, 244. [Google Scholar] [CrossRef]
- Pohl, A.; Kalisz, A.; Sekara, A. Seaweed extracts’ multifactorial action: Influence on physiological and biochemical status of Solanaceae plants. Acta Agrobot. 2019, 72, 1. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J. Appl. Phycol. 2018, 31, 2025–2037. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Hernández-Herrera, R.M.; Ocampo-Álvarez, H.; Sánchez-Hernández, C.V.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Seaweed liquid extracts induce hormetic growth responses in mung bean plants. J. Appl. Phycol. 2021, 33, 1263–1272. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Fucoidan and alginate on mung bean growth. Hidrobiológica 2022, 32, 353–363. [Google Scholar] [CrossRef]
- Buera, P.; Schebor, C.; Elizalde, B. Effects of carbohydrate crystallization on stability of dehydrated foods and ingredient formulations. J. Food. Eng. 2005, 67, 157–165. [Google Scholar] [CrossRef]
- Baltrusch, K.; Flórez-Fernández, N.; Illera, M.; Torres, M.D.; López-Mosquera, M.E.; Domínguez, H. Potential use of Sargassum muticum as source of plant biostimulants after three different drying methods. J. Appl. Phycol. 2023, 35, 921–933. [Google Scholar] [CrossRef]
- Sohn, S.I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in seaweeds: An overview on biosynthesis to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Ito, M.; Koba, K.; Hikihara, R.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 2018, 255, 147–156. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Durán, A.G.; Calle, J.M.; Butrón, D.; Pérez, A.J.; Macías, F.A.; Simonet, A.M. Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source. Plants 2022, 11, 3378. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Sun, S.; Hu, S.; Zhang, B.; Sun, X.; Xu, N. Allelopathic effects and potential allelochemical of Sargassum fusiforme on red tide microalgae Heterosigma akashiwo. Mar. Pollut. Bull. 2021, 170, 112673. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Ghosh, A. Effect of seaweed saps on growth and yield improvement of green gram. Afr. J. Agric. Res. 2013, 8, 1180–1186. [Google Scholar]
- Howe, P.D. A review of boron effects in the environment. Biol. Trace Elem. Res. 1998, 66, 153–166. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Chapter 7—Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. [Google Scholar]
- Dell, B.; Huang, L.B. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. In Nitrogen Assimilation in Plants; Ohyama, T., Sueyoshi, K., Eds.; Research Singpot: Trivandrum, India, 2010; pp. 1–18. [Google Scholar]
- Drira, M.; Mohamed, J.B.; Hlima, H.B.; Hentati, F.; Michaud, P.; Abdelkafi, S.; Fendri, I. Improvement of Arabidopsis thaliana salt tolerance using a polysaccharidic extract from the brown algae Padina pavonica. Algal Res. 2021, 56, 102324. [Google Scholar] [CrossRef]
- Attia, E.Z.; Youssef, N.H.; Saber, H.; Rushdi, M.I.; Abdel-Rahman, I.A.; Darwish, A.G.; Abdelmohsen, U.R. Halimeda opuntia and Padina pavonica extracts improve growth and metabolic activities in maize under soil-saline conditions. J. Appl. Phycol. 2022, 34, 3189–3203. [Google Scholar] [CrossRef]
- Thriunavukkarasu, R.; Joseph, J.; Aruni, W. Effect of seaweed on seed germination and biochemical constituents of Capsicum annuum. Biocatal. Agric. Biotechnol. 2020, 29, 101761. [Google Scholar]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2016, 68, 2011–2218. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol—A new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. [Google Scholar] [CrossRef]
| Characteristic | EA | MP | PC | SH |
|---|---|---|---|---|
| pH | 7.50 | 5.00 | 5.90 | 7.20 |
| Electric conductivity (dS m−1) | 0.68 | 2.80 | 2.50 | 0.86 |
| Density (g mL−1) | 1.01 | 1.03 | 1.01 | 1.01 |
| Total solids (%) | 2.20 | 0.70 | 0.23 | 2.34 |
| Organic matter (%) | 0.35 | 0.20 | 0.02 | 0.39 |
| Ash (%) | 0.75 | 0.63 | 0.17 | 0.75 |
| C/N ratio (%) | 0.190 | 0.880 | 0.730 | 0.313 |
| Organic carbon (%) | 0.222 | 0.199 | 0.014 | 0.228 |
| Total nitrogen (%) | 0.010 | 0.010 | 0.019 | 0.007 |
| Total phosphorous (%) | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Total potassium (%) | 0.290 | 1.600 | 0.070 | 0.290 |
| Potassium (K2O) (%) | 0.350 | 1.100 | 0.080 | 0.340 |
| Total calcium (%) | 0.051 | 0.040 | 0.011 | 0.074 |
| Total sodium (%) | 0.100 | 0.888 | 0.538 | 0.137 |
| Chloride (%) | 0.040 | 0.060 | 0.039 | 0.075 |
| Sulfates (S-SO4) (%) | 0.047 | 0.010 | 0.033 | 0.042 |
| Bicarbonate (HCO3) (%) | 0.180 | 0.060 | 0.004 | 0.160 |
| Iron (%) | 0.00080 | 0.00030 | 0.00020 | 0.00090 |
| Zinc (%) | 0.00002 | 0.00038 | 0.00010 | 0.00010 |
| Copper (%) | 0.00002 | 0.00001 | 0.00001 | 0.00010 |
| Boron (%) | 0.00040 | 0.00073 | 0.00016 | 0.00040 |
| Metabolite | Assay | EA | MP | PC | SH |
|---|---|---|---|---|---|
| Phenols and tannins | Ferric chloride | (+) | (+) | (++) | (+) |
| Triterpenes and sterols | Salkowski | (+) | (−) | (−) | (+) |
| Flavonoids | Alkaline reagent test | (−) | (−) | (+) | (+) |
| Saponins | Foam | (+) | (+) | (−) | (+) |
| Coumarins | Baljet | (+) | (−) | (+) | (+) |
| Alkaloids | Dragendroffs, Mayers | (−) | (−) | (−) | (−) |
| Proteins | Ninhydrin | (−) | (−) | (−) | (−) |
| Carbohydrates and glycosides | Molisch and Keller Killiani | (+) | (+) | (++) | (+) |
| Control | EA | MP | PC | SH |
|---|---|---|---|---|
| 25.05 ± 0.05 a | 36.46 ± 0.25 a | 29.86 ± 0.55 c | 28.93 ± 0.25 b | 27.34 ± 0.25 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Herrera, R.M.; González-González, M.F.; Velasco-Ramírez, A.P.; Velasco-Ramírez, S.F.; Santacruz-Ruvalcaba, F.; Zamora-Natera, J.F. Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings. Seeds 2023, 2, 436-448. https://doi.org/10.3390/seeds2040033
Hernández-Herrera RM, González-González MF, Velasco-Ramírez AP, Velasco-Ramírez SF, Santacruz-Ruvalcaba F, Zamora-Natera JF. Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings. Seeds. 2023; 2(4):436-448. https://doi.org/10.3390/seeds2040033
Chicago/Turabian StyleHernández-Herrera, Rosalba Mireya, Mario Felipe González-González, Ana Paulina Velasco-Ramírez, Sandra Fabiola Velasco-Ramírez, Fernando Santacruz-Ruvalcaba, and Juan Francisco Zamora-Natera. 2023. "Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings" Seeds 2, no. 4: 436-448. https://doi.org/10.3390/seeds2040033
APA StyleHernández-Herrera, R. M., González-González, M. F., Velasco-Ramírez, A. P., Velasco-Ramírez, S. F., Santacruz-Ruvalcaba, F., & Zamora-Natera, J. F. (2023). Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings. Seeds, 2(4), 436-448. https://doi.org/10.3390/seeds2040033







