Maternal Environmental Effects of Temperature and Exogenous Gibberellic Acid on Seed and Seedling Traits of Four Populations of Evening Primrose (Oenothera biennis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Measurement of Morphological Traits of Flowers, Capsules and Seeds
2.3. Measurement of Total Germination and Germination Rate of Seeds
2.4. Measurement of Morphological Traits of Seedlings
2.5. Data Analysis
3. Results
3.1. Morphological Traits of Flowers, Capsules and Seeds
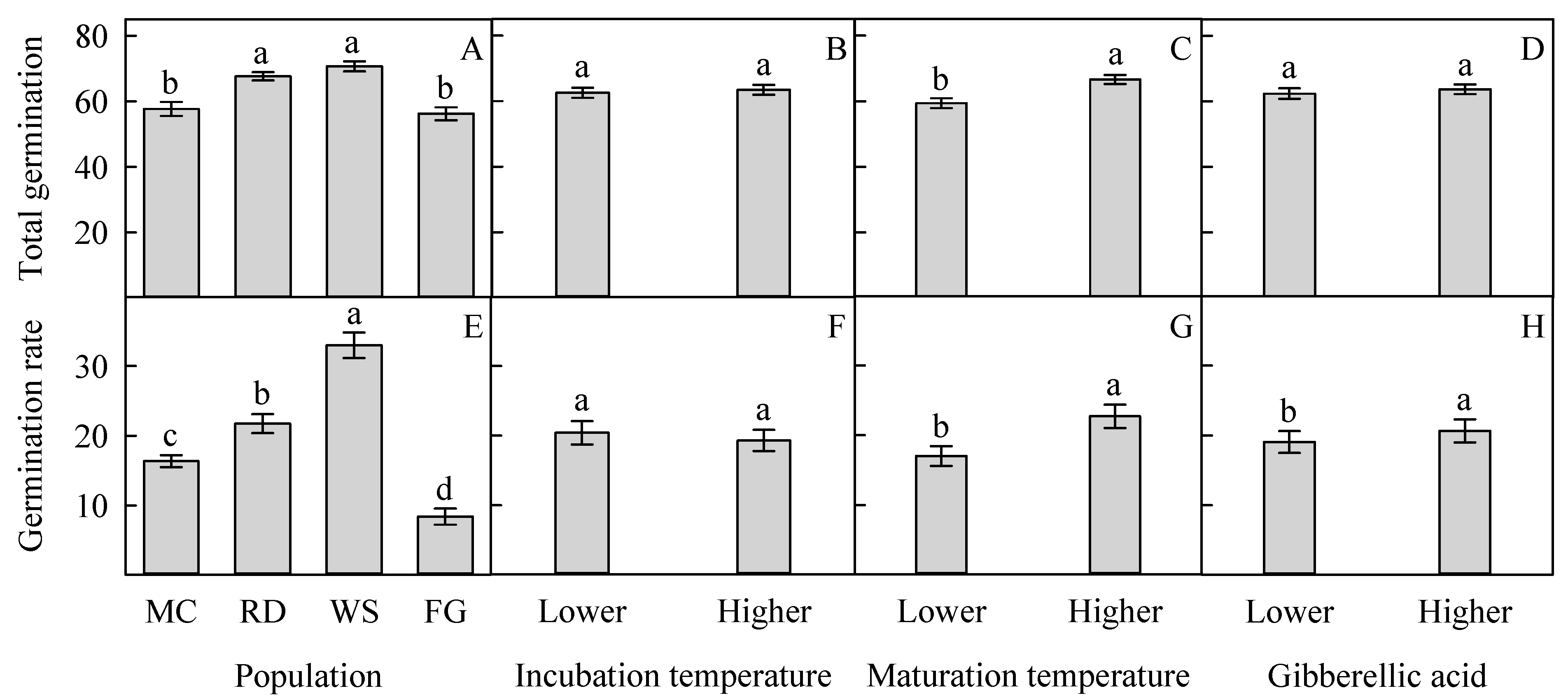
3.2. Total Germination and Germination Rate of Seeds
3.3. Morphological Traits of Seedlings
Relationship between Plant/Seed Traits
4. Discussion
4.1. Interacting Factors Regulate the Morphological Traits of Reproductive Yield
4.2. Maturation Temperature Exerts Major Effects on Subsequent Seed Performance
4.3. Parent Environment Modifies Morphological Traits of Subsequent Seedlings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Alexander, H.M.; Wulff, R.D. Experimental ecological genetics in Plantago: X. The effects of maternal temperature on seed and seedling characters in P. lanceolata. J. Ecol. 1985, 73, 271–282. [Google Scholar] [CrossRef]
- Roach, D.A.; Wulff, R.D. Maternal effects in plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef]
- Singh, J.; Michelangeli, J.A.C.; Gezan, S.A.; Lee, H.; Vallejos, C.E. Maternal effects on seed and seedling phenotypes in reciprocal F1 hybrids of the common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2017, 8, 42. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Chen, J.; Clark, D.; Perez, H.; Huo, H. Effects of maternal environment on seed germination and seedling vigor of Petunia X hybrida under different abiotic stresses. Plants 2021, 10, 581. [Google Scholar] [CrossRef]
- Springthorpe, V.; Penfield, S. Flowering time and seed dormancy control use external coincidence to generate life history strategy. eLife 2015, 4, e05557. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Fitter, A.H.; Hay, R.K.M. Environmental Physiology of Plants, 3rd ed.; Academic Press: London, UK, 2002. [Google Scholar]
- Edwards, B.R.; Burghardt, L.T.; Zapata-Garcia, M.; Donohue, K. Maternal temperature effects on dormancy influence germination responses to water availability in Arabidopsis thaliana. Environ. Exp. Bot. 2016, 126, 55–67. [Google Scholar] [CrossRef]
- Huang, Z.; Footitt, S.; Tang, A.; Finch-Savage, W.E. Predicted global warming scenarios impact on the mother plant to alter seed dormancy and germination behaviour in Arabidopsis. Plant Cell Environ. 2018, 41, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yoong, F.-Y.; O’Neill, C.M.; Penfield, S. Temperature during seed maturation controls seed vigour through ABA breakdown in the endosperm and causes a passive effect on DOG1 mRNA levels during entry into quiescence. New Phytol. 2021, 232, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, W.; Yin, H.; Luo, X.; Chen, W.; Liu, X.; Wang, X.; Meng, Y.; Feng, L.; Qin, Y.; et al. Shading of the mother plant during seed development promotes subsequent seed germination in soybean. J. Exp. Bot. 2020, 71, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, Y. Maternal effects on seeds during development. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 59–84. [Google Scholar]
- Qaderi, M.M.; Cavers, P.B. Variation in germination response within Scotch thistle, Onopordum acanthium L., populations matured under greenhouse and field conditions. Écoscience 2000, 7, 57–65. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B.; Bernards, M.A. Pre- and post-dispersal factors regulate germination patterns and structural characteristics of Scotch thistle (Onopordum acanthium) cypselas. New Phytol. 2003, 159, 263–278. [Google Scholar] [CrossRef]
- Kende, H.; Zeevart, J.A.D. The five “classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef]
- Cleland, R.E. Introduction: Nature, occurrence and functioning of plant hormones. In Biochemistry and Molecular Biology of Plant Hormones; Hooykaas, P.J.J., Hall, M.A., Libbenga, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 33, pp. 3–22. [Google Scholar]
- Piskurewicz, U.; Jikumarua, Y.; Kinoshita, N.; Nambara, E.; Kamiya, Y.; Lopez-Molina, L. The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 2008, 20, 2729–2745. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Cleland, C.F.; Zeevaart, J.A.D. Gibberellins in relation to flowering and stem elongation in the long day plant Silene armeria. Plant Physiol. 1970, 46, 392–400. [Google Scholar] [CrossRef][Green Version]
- Jung, H.; Jo, S.H.; Jung, W.Y.; Park, H.J.; Lee, A.; Moon, J.S.; Seong, S.Y.; Kim, J.-K.; Kim, Y.-S.; Cho, H.-S. Gibberellin promotes bolting and flowering via the floral integrators RsFT and RsSOC1-1 under marginal vernalization in radish. Plants 2020, 9, 594. [Google Scholar] [CrossRef]
- Bose, S.K.; Yadav, R.K.; Mishra, S.; Sangwan, R.S.; Singh, A.K.; Mishra, B.; Srivastava, A.K.; Sangwan, N.S. Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 2013, 66, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.L.; Fkiara, A.; Mackenzie, K.K.; Müller, R.; Lütken, H. Exogenous application of gibberellic acid improves flowering in Kalanchoë. HortScience 2018, 53, 342–346. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhao, D.-D.; Ning, Q.-R.; Wei, J.-P.; Li, Y.; Wang, M.-M.; Liu, X.-L.; Jiang, C.-J.; Liang, Z.-W. A multi-year beneficial effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 2018, 8, 13214. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Cavers, P.B. Interpopulation and interyear variation in germination in Scotch thistle, Onopordum acanthium L., grown in a common garden: Genetics vs environment. Plant Ecol. 2002, 162, 1–8. [Google Scholar] [CrossRef]
- Hall, I.V.; Steiner, E.; Threadgill, P.; Jones, R.W. The biology of Canadian weeds. 84. Oenothera biennis L. Can. J. Plant Sci. 1988, 68, 163–173. [Google Scholar] [CrossRef]
- Christie, W.W. The analysis of evening primrose oil. Ind. Crops Prod. 1999, 10, 73–83. [Google Scholar] [CrossRef]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef]
- Jankowski, W.J.; Stolywho, A. Unusual fatty acid composition of cuticular lipids from leaves of Oenothera. J. Plant Physiol. 1995, 145, 215–220. [Google Scholar] [CrossRef]
- Mendoza de Gyvest, E.; Sparks, C.A.; Sayanova, O.; Lazzeri, P.; Napier, J.A.; Jones, H.D. Genetic manipulation of γ-linolenic acid (GLA) synthesis in a commercial variety of evening primrose (Oenothera sp.). Plant Biotechnol. J. 2004, 2, 351–357. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; López-Martínez, J.C.; Campra-Madrid, P. Gamma-linolenic extraction from seed by SCF and several solvent systems. Int. J. Food Sci. Technol. 2008, 43, 1176–1180. [Google Scholar] [CrossRef]
- Giménez, R.; Sorlino, D.M.; Bertero, H.D.; Ploschuk, E.L. Flowering regulation in the facultative biennial Oenothera biennis L.: Environmental effects and their relation to growth rate. Ind. Crops Prod. 2013, 44, 593–599. [Google Scholar] [CrossRef]
- Greiner, S.; Köhl, K. Growing evening primroses (Oenothera). Front. Plant Sci. 2014, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Godin, V.J.; Reid, D.M. Single and combined effects of temperature and red:far-red light ratio on evening primrose (Oenothera biennis). Botany 2015, 93, 475–483. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbial Properties—Agronomy Monograph No. 9, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Delouche, J.C.; Still, T.W.; Raspet, M.; Lienhard, M. The tetrazolium test for seed viability. Tech. Bull. Miss. Agric. Exp. Stn. 1962, 51, 1–64. [Google Scholar]
- Qaderi, M.M.; Cavers, P.B.; Hamill, A.S.; Bernards, M.A. Effects of collection time and after-ripening on chemical constituents and germinability of Scotch thistle (Onopordum acanthium) cypselas. Botany 2012, 90, 755–762. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide, Version 9.3; SAS Institute: Cary, NC, USA, 2011. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Alm, D.M.; Stoller, E.W.; Wax, L.M. An index model for predicting seed germination and emergence rates. Weed Technol. 1993, 7, 560–569. [Google Scholar] [CrossRef]
- Minitab Inc. Minitab Release 17: Statistical Software for Windows; Minitab Inc.: State College, PA, USA, 2014. [Google Scholar]
- Shin, H.K.; Lieth, J.H.; Kim, S.-H. Effects of temperature on leaf area and flower size in rose. Acta Hortic. 2001, 547, 185–193. [Google Scholar] [CrossRef]
- Pinthus, M.J.; Gale, M.D.; Appleford, N.E.J.; Lenton, J.R. Effect of temperature on gibberellin (GA) responsiveness and on endogenous GA1 content of tall and dwarf wheat genotypes. Plant Physiol. 1989, 90, 854–859. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B.; Hamill, A.S.; Downs, M.P.; Bernards, M.A. Maturation temperature regulates germinability and chemical constituents of Scotch thistle (Onopordum acanthium) cypselas. Can. J. Bot. 2006, 84, 28–38. [Google Scholar] [CrossRef]
- Contreras, S.; Bennett, M.A.; Metzger, J.D.; Tay, D. Maternal light environment during seed development affects lettuce seed weight, germinability, and storability. HortScience 2008, 43, 845–852. [Google Scholar] [CrossRef]
- Figueroa, R.; Herms, D.A.; Cardina, J.; Doohan, D. Maternal environment effects on common groundsel (Senecio vulgaris) seed dormancy. Weed Sci. 2010, 58, 160–166. [Google Scholar] [CrossRef]
- Abo Gamar, M.I.; Qaderi, M.M. Interactive effects of temperature, carbon dioxide and watering regime on seed germinability of two genotypes of Arabidopsis thaliana. Seed Sci. Res. 2019, 29, 12–20. [Google Scholar] [CrossRef]
- Dornbos, D.L., Jr.; McDonald, M.B., Jr. Mass and composition of developing soybean seeds at five reproductive growth stages. Crop Sci. 1986, 26, 624–630. [Google Scholar] [CrossRef]
- Kerdaffrec, E.; Nordborg, M. The maternal environment interacts with genetic variation in regulating seed dormancy in Swedish Arabidopsis thaliana. PLoS ONE 2017, 12, e0190242. [Google Scholar] [CrossRef]
- Hume, L. Maternal environmental effects on plant growth and germination of two strains of Thlaspi arvense L. Int. J. Plant Sci. 1994, 155, 180–186. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Combined effects of temperature and carbon dioxide on plant growth and subsequent seed germinability of Silene noctiflora. Int. J. Plant Sci. 2008, 169, 1200–1209. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Cavers, P.B. Interpopulation variation in germination responses of Scotch thistle, Onopordum acanthium L., to various concentrations of GA3, KNO3, and NaHCO3. Can. J. Bot. 2000, 78, 1156–1163. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Crop responses to elevated carbon dioxide and temperature. In Climate Change and Crops; Singh, S.N., Ed.; Springer: New York, NY, USA, 2009; pp. 1–18. [Google Scholar]

| Population | Latitude and Longitude | Soil pH | Habitat |
|---|---|---|---|
| Mainland Commons Baseball Field (MC) | 44.65993° N 63.66154° W | 7.6 | Open area with no shade, near a gravel path |
| Radcliffe Drive (RD) | 44.66602° N 63.65871° W | 5.9 | On the edge of woods with no shade, near a sidewalk |
| Willet Street (WS) | 44.65791° N 63.65408° W | 6.2 | On the edge of a gravel driveway with no shade |
| Farnham Gate Road (FG) | 44.67212° N 63.67111° W | 6.4 | On the edge of a walking path with no shade |
| Flower | Capsule | Seed (Capsule−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | Area | Diameter | Length | Width | Full Mass | Empty Mass | Number | Mass |
| Population (P) | 99.53 **** | 139.39 **** | 34.31 **** | 6.54 *** | 85.04 **** | 81.12 **** | 19.57 **** | 29.32 **** |
| Maturation temperature (M) | 0.16 | 0.24 | 74.11 **** | 27.75 **** | 9.90 ** | 34.14**** | 0.22 | 0.44 |
| Gibberellic acid (G) | 1.93 | 1.94 | 1.38 | 6.87 ** | 1.03 | 2.90 | 1.25 | 0.00 |
| P × M | 4.53 ** | 12.07 **** | 9.94 **** | 14.18 **** | 10.05 **** | 16.32 **** | 12.67 **** | 4.74 ** |
| P × G | 1.77 | 3.33 * | 1.25 | 5.56 ** | 1.67 | 5.57 ** | 0.89 | 0.77 |
| M × G | 17.62 *** | 13.59 *** | 1.54 | 0.80 | 0.86 | 1.62 | 1.79 | 6.24 * |
| P × M × G | 20.48 **** | 27.78 **** | 1.81 | 3.09 * | 7.04 *** | 9.65 **** | 1.01 | 5.02 ** |
| Source | Seed Total Germination | Seed Germinate Rate |
|---|---|---|
| Population (P) | 31.55 **** | 192.89 **** |
| Incubation temperature (I) | 0.50 | 2.18 |
| Maturation temperature (M) | 31.72 **** | 58.66 **** |
| Gibberellic acid (G) | 1.04 | 4.49 * |
| P × I | 1.74 | 22.94 **** |
| P × M | 1.66 | 4.67 ** |
| P × G | 0.54 | 3.96 * |
| I × M | 5.02 * | 0.00 |
| I × G | 1.72 | 2.06 |
| M × G | 0.01 | 1.24 |
| P × I × M | 5.81 ** | 12.56 **** |
| P × I × G | 0.26 | 3.74 * |
| P × M × G | 3.12 * | 1.94 |
| I × M × G | 25.26 **** | 8.59 ** |
| P × I × M × G | 2.95 * | 3.96 * |
| Population | Seedling Temperature | Maturation Temperature | Gibberellic Acid Level | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | MC | RD | WS | FG | Lower | Higher | Lower | Higher | Lower | Higher |
| Stem height (cm) | 6.35 ± 0.30 a | 5.68 ± 0.31 b | 5.49 ± 0.16 b | 5.67 ± 0.16 b | 5.60 ± 0.18 b | 5.99 ± 0.16 a | 5.46 ± 0.16 b | 6.14 ± 0.18 a | 5.59 ± 0.17 b | 6.00 ± 0.18 a |
| Leaf number (plant−1) | 12.13 ± 0.34 a | 8.79 ± 0.41 b | 8.54 ± 0.26 b | 7.38 ± 0.40 c | 9.79 ± 0.36 a | 8.63 ± 0.34 b | 9.27 ± 0.31 a | 9.15 ± 0.40 a | 8.94 ± 0.34 b | 9.48 ± 0.37 a |
| Leaf area (cm2 plant−1) | 51.15 ± 3.49 b | 48.06 ± 2.18 b | 52.11 ± 3.43 b | 61.07 ± 4.15 a | 55.02 ± 2.78 a | 51.18 ± 2.06 b | 50.88 ± 2.76 b | 55.32 ± 2.10 a | 54.83 ± 2.59 a | 51.37 ± 2.32 b |
| Leaf dry mass (mg plant−1) | 253.55 ± 20.79 ab | 210.92 ± 11.76 bc | 199.47 ± 14.60 c | 257.60 ± 27.93 a | 255.84 ± 16.85 a | 204.93 ± 9.89 b | 209.79 ± 15.58 b | 250.98 ± 12.23 a | 236.87 ± 15.31 a | 223.90 ± 13.20 a |
| Stem dry mass (mg plant−1) | 6.06 ± 0.66 a | 5.58 ± 0.38 a | 5.36 ± 0.44 a | 5.35 ± 0.50 a | 5.59 ± 0.33 a | 5.58 ± 0.38 a | 4.87 ± 0.24 b | 6.31 ± 0.42 a | 5.40 ± 0.36 a | 5.77 ± 0.35 a |
| Root dry mass (mg plant−1) | 213.63 ± 38.06 a | 73.03 ± 10.01 b | 132.94 ± 23.88 b | 72.52 ± 12.66 b | 145.89 ± 22.64 a | 100.17 ± 12.93 b | 109.73 ± 15.73 a | 136.32 ± 21.09 a | 132.27 ± 18.51 a | 113.79 ± 18.80 a |
| Total dry mass (mg plant−1) | 473.23 ± 50.13 a | 289.52 ± 15.58 b | 337.75 ± 29.89 b | 335.43 ± 33.37 b | 407.31 ± 30.89 a | 310.66 ± 17.81 b | 324.37 ± 22.74 b | 393.59 ± 28.26 a | 374.52 ± 25.01 a | 343.45 ± 27.03 a |
| Source | Stem Height | Leaf Number | Leaf Area | Leaf Dry Mass | Stem Dry Mass | Root Dry Mass | Total Dry Mass |
|---|---|---|---|---|---|---|---|
| Population (P) | 13.87 **** | 81.27 **** | 13.20 **** | 6.98 *** | 1.36 | 13.06 **** | 10.37 **** |
| Seedling temperature (S) | 14.22 *** | 26.58 **** | 6.24 * | 20.77 **** | 0.00 | 6.13 * | 15.39 *** |
| Maturation temperature (M) | 44.17 **** | 0.31 | 8.32 ** | 13.59 *** | 25.22 **** | 2.07 | 7.89 ** |
| Gibberellic acid (G) | 16.02 *** | 5.73 * | 5.04 * | 1.35 | 1.68 | 1.00 | 1.59 |
| P × S | 23.51 **** | 6.28 *** | 31.07 **** | 15.39 **** | 28.09 **** | 4.59 ** | 4.93 ** |
| P × M | 16.18 **** | 9.62 **** | 36.86 **** | 9.61 **** | 4.11 ** | 1.59 | 4.90 ** |
| P × G | 34.73 **** | 4.64 ** | 17.21 **** | 10.24 **** | 2.36 | 2.19 | 2.30 |
| S × M | 0.54 | 1.66 | 0.21 | 7.69 ** | 11.85 ** | 3.72 | 0.02 |
| S × G | 0.62 | 0.31 | 1.00 | 0.73 | 3.63 | 0.82 | 0.10 |
| M × G | 4.79 * | 6.64 * | 23.95 **** | 8.74 ** | 4.27 * | 21.32 **** | 23.28 **** |
| P × S × M | 13.76 **** | 2.84 * | 2.18 | 7.03 *** | 9.22 **** | 4.12 ** | 4.08 * |
| P × S × G | 8.22 *** | 11.97 **** | 7.16 *** | 12.99 **** | 3.30 * | 1.78 | 3.81 * |
| S × M × G | 7.21 ** | 3.39 | 3.23 | 0.33 | 6.69 * | 0.78 | 0.18 |
| P × S × M × G | 13.29 **** | 2.21 | 8.41 **** | 0.86 | 4.08 ** | 1.84 | 1.32 |
| Trait | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Flower area | - | |||||||||||||||
| 2 | Flower diameter | 0.993 *** | - | ||||||||||||||
| 3 | Capsule length | 0.184 | 0.196 | - | |||||||||||||
| 4 | Capsule width | 0.300 | 0.279 | 0.506 * | - | ||||||||||||
| 5 | Full capsule mass | 0.150 | 0.127 | 0.427 | 0.044 | - | |||||||||||
| 6 | Empty capsule mass | 0.087 | 0.080 | 0.449 | –0.042 | 0.947 *** | - | ||||||||||
| 7 | Seed number capsule−1 | 0.396 | 0.355 | 0.367 | 0.184 | 0.799 *** | 0.625 * | - | |||||||||
| 8 | Seed mass capsule−1 | 0.215 | 0.173 | 0.299 | 0.169 | 0.866 *** | 0.658 ** | 0.896 *** | - | ||||||||
| 9 | Total germination | 0.230 | 0.271 | –0.328 | –0.156 | –0.619 * | –0.633 ** | –0.505 * | –0.463 | - | |||||||
| 10 | Germination rate | 0.211 | 0.265 | –0.003 | –0.137 | –0.591 * | –0.535 * | –0.573 * | –0.551 * | 0.835 *** | - | ||||||
| 11 | Seedling stem height | 0.094 | 0.088 | –0.089 | 0.036 | 0.214 | 0.135 | 0.205 | 0.290 | 0.055 | –0.038 | - | |||||
| 12 | Leaf number plant1 | –0.065 | –0.054 | 0.412 | 0.031 | 0.551 * | 0.545 * | 0.221 | 0.441 | –0.164 | 0.025 | 0.454 | - | ||||
| 13 | Leaf area plant−1 | –0.543 * | –0.540 * | –0.324 | –0.047 | –0.152 | –0.167 | –0.227 | –0.096 | –0.171 | –0.216 | 0.374 | 0.093 | - | |||
| 14 | Leaf dry mass plant−1 | –0.383 | –0.387 | –0.376 | –0.209 | 0.136 | 0.100 | –0.033 | 0.162 | –0.242 | –0.306 | 0.440 | 0.379 | 0.839 *** | - | ||
| 15 | Stem dry mass plant−1 | 0.114 | 0.100 | –0.399 | –0.126 | –0.004 | –0.061 | –0.050 | 0.085 | 0.229 | 0.119 | 0.621 * | 0.398 | 0.446 | 0.547 * | - | |
| 16 | Root dry mass plant−1 | 0.127 | 0.124 | 0.353 | 0.068 | 0.214 | 0.121 | 0.207 | 0.311 | –0.127 | 0.164 | 0.170 | 0.640 ** | 0.126 | 0.339 | 0.345 | - |
| 17 | Total dry mass plant−1 | –0.098 | –0.103 | 0.060 | –0.056 | 0.219 | 0.135 | 0.130 | 0.302 | –0.207 | –0.033 | 0.345 | 0.647 ** | 0.509 * | 0.742 ** | 0.527 * | 0.882 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LeFait, B.M.; Qaderi, M.M. Maternal Environmental Effects of Temperature and Exogenous Gibberellic Acid on Seed and Seedling Traits of Four Populations of Evening Primrose (Oenothera biennis). Seeds 2022, 1, 110-125. https://doi.org/10.3390/seeds1020010
LeFait BM, Qaderi MM. Maternal Environmental Effects of Temperature and Exogenous Gibberellic Acid on Seed and Seedling Traits of Four Populations of Evening Primrose (Oenothera biennis). Seeds. 2022; 1(2):110-125. https://doi.org/10.3390/seeds1020010
Chicago/Turabian StyleLeFait, Britanie M., and Mirwais M. Qaderi. 2022. "Maternal Environmental Effects of Temperature and Exogenous Gibberellic Acid on Seed and Seedling Traits of Four Populations of Evening Primrose (Oenothera biennis)" Seeds 1, no. 2: 110-125. https://doi.org/10.3390/seeds1020010
APA StyleLeFait, B. M., & Qaderi, M. M. (2022). Maternal Environmental Effects of Temperature and Exogenous Gibberellic Acid on Seed and Seedling Traits of Four Populations of Evening Primrose (Oenothera biennis). Seeds, 1(2), 110-125. https://doi.org/10.3390/seeds1020010







