Evaluation of Associative Effects of In Vitro Gas Production and Fermentation Profile Caused by Variation in Ruminant Diet Constituents
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design and Substrates
2.2. In Vitro Fermentation Procedure and Measurement of Gas Production (Assay 1)
2.3. Fermentation Parameters (Assay 2)
2.4. Chemical Composition
2.5. Models and Calculations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Agriculture, forestry and other land use: Emissions from livestock and manure management. IPCC Guidel. Natl. Greenh. Gas Invent. 2006, 4, 10.1–10.87. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). The physical science basis. In Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauls, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminantes: Its contribuiton to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Na, Y.; Li, D.H.; Choi, Y.; Kim, K.H.; Lee, S.R. Effects of feeding level on nutrient digestibility and enteric methane production in growing goats (Capra hircus hircus) and Sika deer (Cervus nippon hortulorum). Asian-Australas. J. Anim. Sci. 2018, 31, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed. Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Jiao, H.P.; Dale, A.J.; Carson, A.F.; Murray, S.; Gordon, A.W.; Ferris, C.P. Effect of concentrate feed level on methane emissions from grazing dairy cows. J. Dairy Sci. 2014, 97, 7043–7053. [Google Scholar] [CrossRef]
- Schulman, M.D.; Valentino, D. Factors Influencing Rumen Fermentation: Effect of Hydrogen on Formation of Propionate. J. Dairy Sci. 1975, 59, 1444–1451. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994; pp. 334–336. [Google Scholar] [CrossRef]
- Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Patra, A.K.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhury, P.K.; Carro, M.D.; Griffith, G.W.; Dagar, S.S.; Puniya, M.; Calabro, S.; Ravella, S.R.; Dhewa, T.; Upadhyay, R.C.; et al. New aspects and strategies for methane mitigation from ruminants. Appl. Microbiol. Biotechnol. 2014, 98, 31–44. [Google Scholar] [CrossRef]
- DeVries, T.J.; Schwaiger, T.; Beauchemin, K.A.; Penner, G.B. Impact of severity of ruminal acidosis on feed-sorting behaviour of beef cattle. Anim. Prod. Sci. 2014, 54, 1238–1242. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Beauchemin, K. Physically Effective Fiber: Method of Determination and Effects on Chewing, Ruminal Acidosis, and Digestion by Dairy Cows. J. Dairy Sci. 2006, 89, 2618–2633. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Sniffen, C.; Van Soest, P. Effect of Carbohydrate Limitation on Degradation and Utilization of Casein by Mixed Rumen Bacteria. J. Dairy Sci. 1983, 66, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.M.; Stockdale, C.R. Associative effects between forages and grains: Consequences for feed utilisation. Aust. J. Agric. Res. 1999, 50, 757–774. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.; DePeters, E.; Taylor, S. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed. Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Robinson, P.; Getachew, G.; Cone, J. Evaluation of the extent of associative effects of two groups of four feeds using an in vitro gas production procedure. Anim. Feed. Sci. Technol. 2009, 150, 9–17. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Wang, Y.; Li, S.; Cao, Z.; Ji, S.; He, Y.; Zhang, H. Effect of Dietary Forage to Concentrate Ratios on Dynamic Profile Changes and Interactions of Ruminal Microbiota and Metabolites in Holstein Heifers. Front. Microbiol. 2017, 8, 2206. [Google Scholar] [CrossRef]
- Sun, L.; Lee, M.; Jeon, S.; Seo, S. Evaluation of the Associative Effects of Rice Straw with Timothy Hay and Corn Grain Using an In Vitro Ruminal Gas Production Technique. Animals 2020, 10, 325. [Google Scholar] [CrossRef]
- Upadhyay, A.; Lama, J.P.; Tawata, S. Utilization of Pineapple Waste: A Review. J. Food Sci. Technol. Nepal 2010, 6, 10–18. [Google Scholar] [CrossRef]
- Ososanya, T.O.; Adewumi, M.K.; Jinadu, K.B. Impact of pineapple waste silage on intake, digestibility and fermentation patterns of West African Dwarf sheep. Afr. J. Biotechnol. 2014, 13, 2575–2581. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitsch 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ruiz, D.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.; O’kiely, P.; Reynolds, C.; Schwarm, A.; Shingfield, K.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed. Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- McDougall, E.I. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis (Apparatus Reagents, Procedures and Some Applications). In Agricultural Handbook No. 379; United States Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Abreu, M.L.C.; Vieira, R.A.M.; Rocha, N.S.; Araujo, R.P.; Glória, L.S.; Fernandes, A.M.; de Lacerda, P.D.; Júnior, A.G. Clitoria ternatea L. as a Potential High Quality Forage Legume. Asian-Australas. J. Anim. Sci. 2014, 27, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Fenner, H. Method for Determining Total Volatile Bases in Rumen Fluid by Steam Distillation. J. Dairy Sci. 1965, 48, 249–251. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemistry: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Thiex, N.J.; Anderson, S.; Gildemeister, B.; Adcock, W.; Boedigheimer, J.; Bogren, E.; Coffin, R.; Conway, K.; DeBaker, A.; Frankenius, E.; et al. Crude Fat, Hexanes Extraction, in Feed, Cereal Grain, and Forage (Randall/Soxtec/Submersion Method): Collaborative Study. J. AOAC Int. 2003, 86, 899–908. [Google Scholar] [CrossRef]
- Thiex, N.J.; Manson, H.; Anderson, S.; Persson, J.A. Determinantion of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distrillation into boric acid: Collaborative study. J. AOAC Int. 2002, 85, 309–317. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; Editora UFV: Viçosa, Brazil, 2006. [Google Scholar]
- Brody, S. Bioenergetics and growth. In With Special Reference to the Efficiency Complex in Domestic Animals; Reinhold Publishing Co.: New York, NY, USA, 1945. [Google Scholar]
- López, S.; France, J.; Dhanoa, M.S.; Mould, F.; Dijkstra, J. Comparison of mathematical models to describe disappearance curves obtained using the polyester bag technique for incubating feeds in the rumen. J. Anim. Sci. 1999, 77, 1875–1888. [Google Scholar] [CrossRef][Green Version]
- López, S.; France, J.; Gerrits, W.J.J.; Dhanoa, M.S.; Humphries, D.J.; Dijkstra, J. A generalized Michaelis-Menten equation for the analysis of growth. J. Anim. Sci. 2000, 78, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed. Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer-Verlag Inc.: New York, NY, USA, 2000. [Google Scholar]
- Sugiura, N. Further Analysis of the Data by Akaike’s Information Criterion and the Finite Corrections. Commun. Stat. Theory Method 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Gemeda, B.S.; Hassen, A. Methane production of two roughage and total mixed ration as influenced by cellulase and xylanase enzyme addition. Sci. Agricola 2015, 72, 11–19. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. Rumen Microbes, Enzymes and Feed Digestion—A Review. Asian-Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Lanzas, C.; Sniffen, C.; Seo, S.; Tedeschi, L.; Fox, D. A revised CNCPS feed carbohydrate fractionation scheme for formulating rations for ruminants. Anim. Feed. Sci. Technol. 2007, 136, 167–190. [Google Scholar] [CrossRef]
- Hartley, R.D.; Akin, D.E. Effect of forage cell wall phenolic acids and derivatives on rumen microflora. J. Sci. Food Agric. 1989, 49, 405–411. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; De La Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.S.; Lana, R.P.; Pereira, J.C.; Guimarães, G.; Faleiro Neto, J.A. Growth rate of mixed ruminal bacteria as a function of energetic substrate concentration in bath culture. Pesq. Agropec. Trop. 2011, 41, 39–43. [Google Scholar] [CrossRef][Green Version]
- Ungerfeld, E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: A meta-analysis. Front. Microbiol. 2015, 6, 37. [Google Scholar] [CrossRef]
- Mertens, D.; Loften, J. The Effect of Starch on Forage Fiber Digestion Kinetics In Vitro. J. Dairy Sci. 1980, 63, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, S.; Tan, Z. Modeling in vitro gas production kinetics: Derivation of Logistic–Exponential (LE) equations and comparison of models. Anim. Feed. Sci. Technol. 2011, 165, 137–150. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Maccarana, L.; Cattani, M.; Tagliapietra, F.; Bailoni, L.; Schiavon, S. Influence of main dietary chemical constituents on the in vitro gas and methane production in diets for dairy cows. J. Anim. Sci. Biotechnol. 2016, 7, 54. [Google Scholar] [CrossRef]
- McAllister, T.A.; Bae, H.D.; Jones, G.A.; Cheng, K.-J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [CrossRef]
- Leng, R.A. Unravelling methanogenesis in ruminants, horses and kangaroos: The links between gut anatomy, microbial biofilms and host immunity. Anim. Prod. Sci. 2018, 58, 1175. [Google Scholar] [CrossRef]
- Alstrup, L.; Søegaard, K.; Weisbjerg, M. Effects of maturity and harvest season of grass-clover silage and of forage-to-concentrate ratio on milk production of dairy cows. J. Dairy Sci. 2016, 99, 328–340. [Google Scholar] [CrossRef]
- Bharanidharan, R.; Arokiyaraj, S.; Kim, E.B.; Lee, C.H.; Woo, Y.W.; Na, Y.; Kim, D.; Kim, K.H. Ruminal methane emissions, metabolic, and microbial profile of Holstein steers fed forage and concentrate, separately or as a total mixed ration. PLoS ONE 2018, 13, e0202446. [Google Scholar] [CrossRef] [PubMed]
- Penner, G.; Taniguchi, M.; Guan, L.; Beauchemin, K.; Oba, M. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J. Dairy Sci. 2009, 92, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Kohn, R.A.; Dunlap, T.F. Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. J. Anim. Sci. 1998, 76, 1702–1709. [Google Scholar] [CrossRef]
- Heldt, J.S.; Cochran, R.C.; Stokka, G.L.; Farmer, C.G.; Mathis, C.P.; Titgemeyer, E.C.; Nagaraja, T.G. Effects of different supplemental sugars and starch fed in combination with degradable intake protein on low-quality forage use by beef steers. J. Anim. Sci. 1999, 77, 2793–2802. [Google Scholar] [CrossRef]
- Heldt, J.S.; Cochran, R.C.; Mathis, C.P.; Woods, B.C.; Olson, K.C.; Titgemeyer, E.C.; Nagaraja, T.G.; Vanzant, E.S.; Johnson, D.E. Effects of level and source of carbohydrate and level of degradable intake protein on intake and digestion of low-quality tallgrass-prairie hay by beef steers. J. Anim. Sci. 1999, 77, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001.
- Sandoval-Castro, C.; Capetillo-Leal, C.; Cetina-Góngora, R.; Ramirez-Avilés, L. A mixture simplex design to study associative effects with an in vitro gas production technique. Anim. Feed. Sci. Technol. 2002, 101, 191–200. [Google Scholar] [CrossRef]
- Firkins, J. Invited Review: Advances in rumen efficiency. Appl. Anim. Sci. 2021, 37, 388–403. [Google Scholar] [CrossRef]
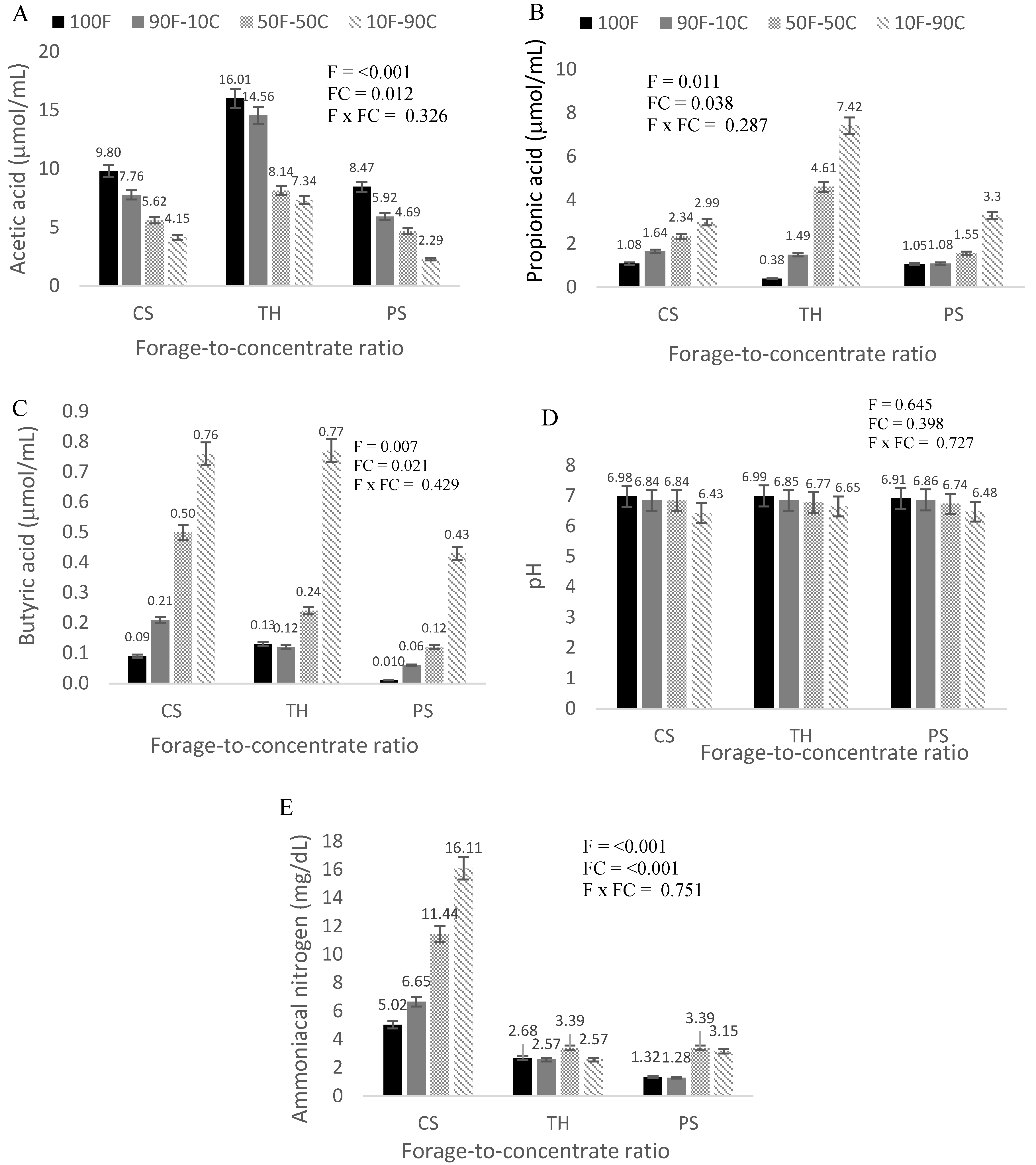
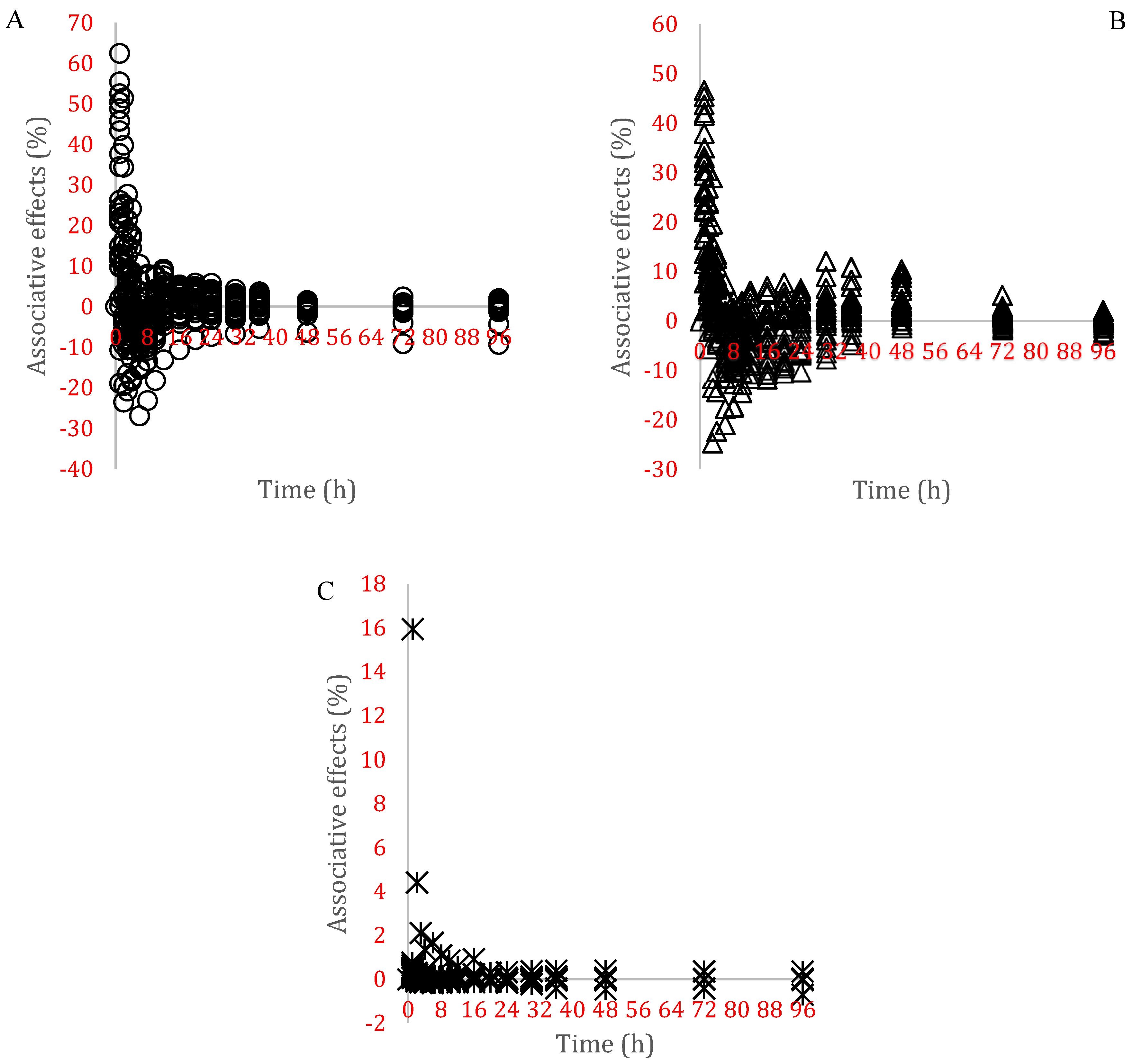
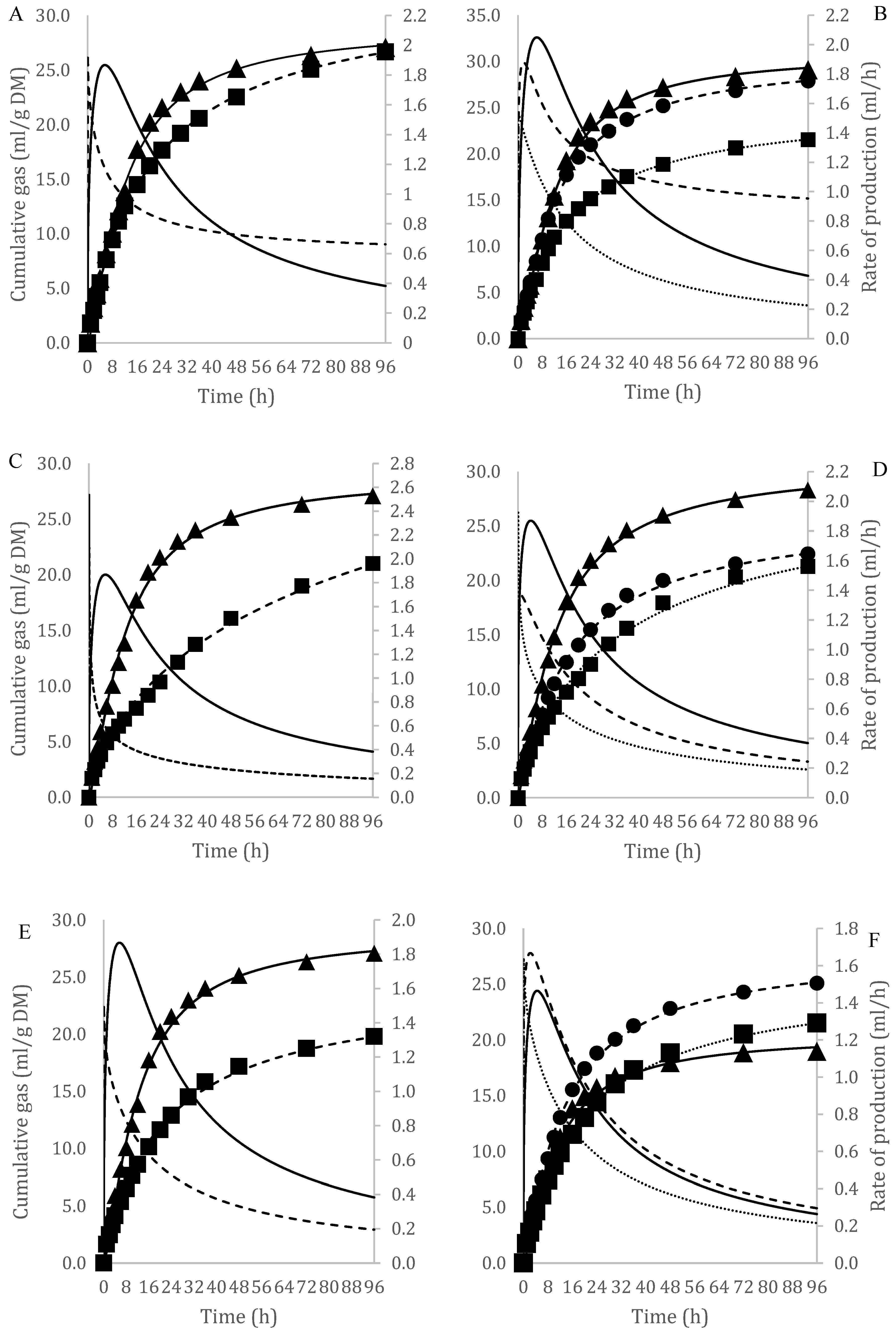
| Substrate | DM | Ash | CP | CF | NDF | ADF | LIG | NFC | LIG/NDF |
|---|---|---|---|---|---|---|---|---|---|
| Corn silage (CS) | |||||||||
| 100-CS | 301.7 | 44.81 | 79.18 | 14.76 | 411.59 | 250.21 | 28.93 | 449.66 | 7.029 |
| 90CS-10C | 365.39 | 44.77 | 95.22 | 33.41 | 378.64 | 230.09 | 26.46 | 447.96 | 6.987 |
| 80CS-20C | 429.08 | 42.33 | 107.15 | 21.72 | 360.57 | 223.33 | 25.35 | 468.24 | 7.03 |
| 70CS-30C | 492.77 | 35.45 | 123.04 | 39.33 | 317.69 | 196.97 | 22.91 | 484.48 | 7.213 |
| 60CS-40C | 556.45 | 39.22 | 146.95 | 28.83 | 279.84 | 175.76 | 21.86 | 505.15 | 7.813 |
| 50CS-50C | 620.14 | 35.22 | 160.36 | 23.41 | 255.36 | 156.8 | 18.82 | 525.65 | 7.37 |
| 40CS-60C | 683.83 | 35.86 | 191.61 | 32.09 | 210.23 | 138.49 | 15.41 | 530.21 | 7.328 |
| 30CS-70C | 747.51 | 37.19 | 189.18 | 24.17 | 187.96 | 120.17 | 17.56 | 561.49 | 9.341 |
| 20CS-80C | 811.2 | 32.42 | 226.08 | 31.76 | 154.77 | 96.74 | 16.61 | 554.97 | 10.729 |
| 10CS-90C | 874.89 | 33.99 | 222.68 | 28.32 | 106.86 | 66.77 | 6.44 | 608.14 | 6.027 |
| SEM | 57.847 | 1.338 | 15.767 | 2.096 | 30.446 | 18.2 | 1.964 | 15.728 | 0.407 |
| Tifton hay (TH) | |||||||||
| 100-TH | 867.4 | 66.59 | 48.01 | 15.09 | 777.85 | 417.55 | 52.05 | 92.45 | 6.691 |
| 90TH-10C | 874.52 | 62.66 | 55.44 | 13.41 | 730.69 | 379.6 | 50.72 | 137.79 | 6.941 |
| 80TH-20C | 881.63 | 57.68 | 85.63 | 15.43 | 634.96 | 334.15 | 41.38 | 206.3 | 6.517 |
| 70TH-30C | 888.75 | 57.48 | 102.77 | 23.94 | 555.13 | 300.24 | 37.58 | 260.68 | 6.77 |
| 60TH- 40C | 895.87 | 55.67 | 137.64 | 22.38 | 504.33 | 248.96 | 30.74 | 279.98 | 6.096 |
| 50TH-50C | 902.99 | 50.42 | 166.13 | 15.79 | 431.9 | 235.7 | 29.1 | 335.76 | 6.737 |
| 40TH-60C | 910.11 | 40.41 | 177.5 | 14.13 | 354.68 | 202.93 | 24 | 413.27 | 6.767 |
| 30TH-70C | 917.22 | 40.78 | 195.59 | 24.45 | 266.55 | 171.49 | 20.42 | 472.62 | 7.659 |
| 20TH-80C | 924.34 | 37.16 | 202.15 | 28.88 | 231.16 | 130.54 | 14.33 | 500.65 | 6.199 |
| 10TH-90C | 931.46 | 36.59 | 224.27 | 25.59 | 155.92 | 86.16 | 8.35 | 557.63 | 5.354 |
| SEM | 6.465 | 3.319 | 19 | 1.708 | 64.022 | 32.275 | 4.403 | 47.333 | 0.181 |
| Pineapple silage (PS) 1 | |||||||||
| 100-PS | 191.45 | 62.63 | 89.01 | 45.65 | 595.13 | 381.01 | 56.56 | 207.57 | 9.505 |
| 90PS-10C | 266.17 | 56.07 | 105.2 | 33.05 | 502.25 | 334.11 | 50.02 | 303.43 | 9.959 |
| 80PS-20C | 340.88 | 50.42 | 112 | 47 | 471.17 | 310.64 | 43.68 | 319.4 | 9.27 |
| 70PS-30C | 415.59 | 47.19 | 123.77 | 49.13 | 411.53 | 290.04 | 41.72 | 368.38 | 10.138 |
| 60PS-40C | 490.3 | 45.7 | 161.22 | 43.71 | 345.93 | 248.57 | 38.57 | 403.44 | 11.149 |
| 50PS-50C | 565.01 | 43.11 | 163.56 | 32.29 | 355.23 | 213.06 | 26.72 | 405.81 | 7.522 |
| 40PS-60C | 639.73 | 43.42 | 177.18 | 35.06 | 283.7 | 180.61 | 21.63 | 460.64 | 7.624 |
| 30PS-70C | 714.44 | 40.79 | 200.2 | 34.62 | 229.59 | 138.99 | 21.18 | 494.79 | 9.225 |
| 20PS-80C | 789.15 | 38.42 | 214.33 | 33.4 | 190.34 | 109.79 | 14.2 | 523.51 | 7.461 |
| 10PS-90C | 863.86 | 37.75 | 227.35 | 29 | 143.64 | 81.92 | 9.37 | 562.27 | 6.524 |
| SEM | 67.861 | 2.374 | 14.492 | 2.178 | 43.436 | 30.244 | 4.777 | 32.848 | 0.442 |
| 100-Concentrate (C) | 938.58 | 31.71 | 242.34 | 30.82 | 95.01 | 50.37 | 5.3 | 600.11 | 5.67 |
| Soybean meal | 869.04 | 66.38 | 485.64 | 18.86 | 122.51 | 47.42 | 5.86 | 306.62 | 4.78 |
| Ground corn | 856.3 | 11.01 | 84.93 | 31.54 | 77.3 | 24.12 | 8.16 | 795.23 | 10.562 |
| SEM | 20.877 | 13.188 | 95.167 | 3.356 | 10.739 | 6.777 | 0.716 | 115.941 | 1.468 |
| Model | AICc | Δr | Wr | ERr |
|---|---|---|---|---|
| Brody, Hom. var. | 403.43 | 191.61 | 2.4 × 10−42 | 4.1 × 1041 |
| Brody, corCar1 | 218.77 | 6.96 | 0.03 | 32.4 |
| Gompertz, Hom. var. | 478.36 | 266.55 | 1.3 × 10−58 | 7.6 × 1057 |
| Gompertz, corCar1 | 268.93 | 57.12 | 3.8 × 10−13 | 2.5 × 1012 |
| Logistic, Hom. var. | 300.13 | 88.32 | 6.4 × 10−20 | 1.5 × 1019 |
| Logistic, corCar1 | 223.35 | 11.53 | 3.0 × 10−03 | 318.9 |
| Michaelis-Menten, Hom. var. | 372.13 | 160.32 | 1.5 × 10−35 | 6.5 × 1034 |
| Michaelis-Menten, corCar1 | 211.82 | 0.0 | 0.97 | 1.0 |
| Substrate | A | Lower | Upper | c | Lower | Upper | K | Lower | Upper |
|---|---|---|---|---|---|---|---|---|---|
| Corn silage (CS) | |||||||||
| 100-CS | 32.81 | 29.01 | 36.61 | 0.95 j | 0.87 | 1.03 | 20.67 a | 17.22 | 24.12 |
| 90CS-10C | 25.17 | 21.59 | 28.75 | 1.00 i | 0.91 | 1.10 | 16.15 b | 13.43 | 18.87 |
| 80CS-20C | 31.09 | 27.57 | 34.62 | 1.07 h | 0.99 | 1.15 | 15.18 c | 13.36 | 17.00 |
| 70CS-30C | 28.49 | 25.01 | 31.97 | 1.13 f | 1.04 | 1.22 | 14.75 d | 13.02 | 16.49 |
| 60CS-40C | 30.85 | 27.37 | 34.33 | 1.10 g | 1.02 | 1.18 | 13.67 e | 12.15 | 15.19 |
| 50CS-50C | 30.54 | 27.10 | 33.97 | 1.17 e | 1.09 | 1.25 | 12.72 f | 11.46 | 13.97 |
| 40CS-60C | 30.17 | 26.74 | 33.59 | 1.18 d | 1.09 | 1.26 | 12.29 g | 11.09 | 13.50 |
| 30CS-70C | 30.80 | 27.40 | 34.20 | 1.24 c | 1.16 | 1.33 | 12.00 gh | 10.95 | 13.05 |
| 20CS-80C | 31.57 | 28.17 | 34.96 | 1.28 b | 1.19 | 1.37 | 12.09 g | 11.11 | 13.08 |
| 10CS-90C | 30.86 | 27.49 | 34.23 | 1.40 a | 1.30 | 1.49 | 11.74 h | 10.89 | 12.59 |
| p-value | 0.059 | <0.001 | <0.001 | ||||||
| Tifton hay (TH) | |||||||||
| 100-TH | 45.10 a | 31.12 | 59.07 | 0.71 j | 0.61 | 0.81 | 117.54 a | 22.59 | 212.49 |
| 90TH-10C | 30.24 bc | 25.46 | 35.02 | 0.85 i | 0.75 | 0.95 | 34.73 b | 23.58 | 45.87 |
| 80TH-20C | 25.23 d | 21.54 | 28.93 | 1.01 g | 0.90 | 1.12 | 19.91 d | 16.18 | 23.65 |
| 70TH-30C | 27.36 bcd | 23.64 | 31.09 | 1.00 h | 0.90 | 1.10 | 20.35 c | 16.71 | 24.00 |
| 60TH-40C | 25.98 cd | 22.44 | 29.52 | 1.05 d | 0.96 | 1.15 | 15.49 f | 13.19 | 17.79 |
| 50TH-50C | 26.17 bcd | 22.58 | 29.76 | 1.04 f | 0.94 | 1.14 | 16.97 e | 14.29 | 19.65 |
| 40TH-60C | 28.82 bcd | 25.31 | 32.34 | 1.05 e | 0.97 | 1.14 | 14.22 g | 12.36 | 16.07 |
| 30TH-70C | 30.09 bc | 26.63 | 33.55 | 1.13 c | 1.05 | 1.22 | 13.40 h | 11.96 | 14.84 |
| 20TH-80C | 30.44 bc | 26.99 | 33.89 | 1.14 b | 1.06 | 1.23 | 13.31 i | 11.92 | 14.69 |
| 10TH-90C | 30.69 b | 27.28 | 34.10 | 1.24 a | 1.16 | 1.33 | 12.61 j | 11.50 | 13.72 |
| p-value | <0.001 | <0.001 | <0.001 | ||||||
| Pineapple silage (PS) 1 | |||||||||
| 100-PS | 24.59 | 20.75 | 28.43 | 0.94 j | 0.84 | 1.05 | 21.52 a | 16.54 | 26.49 |
| 90PS-10C | 26.14 | 22.40 | 29.88 | 0.96 i | 0.86 | 1.06 | 19.38 b | 15.53 | 23.23 |
| 80PS-20C | 27.12 | 23.48 | 30.75 | 1.00 h | 0.91 | 1.10 | 17.68 c | 14.72 | 20.64 |
| 70PS-30C | 27.26 | 23.71 | 30.80 | 1.05 g | 0.96 | 1.15 | 15.77 d | 13.51 | 18.04 |
| 60PS-40C | 24.75 | 21.26 | 28.23 | 1.06 f | 0.96 | 1.15 | 13.19 ef | 11.27 | 15.12 |
| 50PS-50C | 27.92 | 24.46 | 31.38 | 1.12 d | 1.03 | 1.21 | 13.42 e | 11.84 | 14.99 |
| 40PS-60C | 29.09 | 25.63 | 32.54 | 1.12 e | 1.03 | 1.20 | 12.99 f | 11.53 | 14.46 |
| 30PS-70C | 28.59 | 25.19 | 31.99 | 1.22 c | 1.13 | 1.31 | 11.61 g | 10.49 | 12.73 |
| 20PS-80C | 29.51 | 26.12 | 32.91 | 1.24 b | 1.15 | 1.33 | 11.62 g | 10.56 | 12.67 |
| 10PS-90C | 20.45 | 17.10 | 23.80 | 1.31 a | 1.20 | 1.42 | 10.71 h | 9.62 | 11.79 |
| p-value | 0.464 | <0.001 | <0.001 | ||||||
| 100-Concentrate (C) | 28.92 | 25.55 | 32.30 | 1.35 a | 1.25 | 1.44 | 11.94 b | 10.97 | 12.92 |
| Soybean meal | 24.97 | 21.55 | 28.39 | 1.09 c | 1.00 | 1.18 | 10.17 c | 8.86 | 11.49 |
| Ground corn | 31.04 | 27.64 | 34.43 | 1.41 b | 1.30 | 1.51 | 15.34 a | 14.20 | 16.48 |
| p-value | 0.089 | <0.001 | <0.001 |
| Substrate | V24 | V48 | µ0.5 (/h) | AF (%) |
|---|---|---|---|---|
| Corn silage (CS) | ||||
| 100-CS | 17.57 ± 0.83 bc | 9.83 ± 2.47 bc | 9.83 ± 2.47 a | −0.7 ± 0.37 b |
| 90CS-10C | 15.05 ± 1.35 c | 8.09 ± 2.13 c | 8.09 ± 2.13 c | −2.17 ± 1.42 b |
| 80CS-20C | 19.29 ± 1.52 abc | 8.13 ± 1.60 abc | 8.13 ± 1.60 bc | −0.25 ± 0.45 b |
| 70CS-30C | 18.06 ± 1.63 abc | 8.32 ± 1.66 abc | 8.32 ± 1.66 b | −1.76 ± 0.22 b |
| 60CS-40C | 20.04 ± 1.72 abc | 7.51 ± 1.39 abc | 7.51 ± 1.39 e | 0.23 ± 0.55 b |
| 50CS-50C | 20.69 ± 1.91 ab | 7.44 ± 1.27 ab | 7.44 ± 1.27 e | 1.58 ± 1.03 b |
| 40CS-60C | 20.73 ± 1.97 ab | 7.23 ± 1.23 ab | 7.23 ± 1.23 f | 1.84 ± 1.18 ab |
| 30CS-70C | 21.65 ± 2.07 ab | 7.46 ± 1.17 ab | 7.46 ± 1.17 e | 3.29 ± 3.15 ab |
| 20CS-80C | 22.29 ± 2.1 a | 7.74 ± 1.15 a | 7.74 ± 1.15 d | 5.11 ± 8.33 ab |
| 10CS-90C | 22.56 ± 2.25 a | 8.21 ± 1.15 a | 8.21 ± 1.15 bc | 9.89 ± 1.35 a |
| p-value | <0.001 | <0.001 | <0.001 | 0.003 |
| Tifton hay (TH) | ||||
| 100-TH | 10.99 ± 3.63 f | 15.59 ± 2.75 d | 41.88 ± 39.67 a | 0.17 ± 3.75 |
| 90TH-10C | 12.75 ± 0.27 ef | 17.20 ± 0.91 cd | 14.83 ± 6.48 b | 1.24 ± 0.89 |
| 80TH-20C | 13.8 ± 0.96 def | 17.89 ± 2.12 cd | 10.07 ± 2.96 c | 2.79 ± 2.14 |
| 70TH-30C | 14.81 ± 0.91 de | 19.21 ± 2.07 bc | 10.18 ± 2.83 c | 1.99 ± 0.76 |
| 60TH-40C | 15.94 ± 1.46 cd | 19.93 ± 2.49 bc | 8.16 ± 1.97 e | 2.17 ± 1.22 |
| 50TH-50C | 15.42 ± 1.29 cd | 19.54 ± 2.37 bc | 8.81 ± 2.23 d | 2.31 ± 2.13 |
| 40TH-60C | 18.28 ± 1.60 bc | 22.55 ± 2.58 ab | 7.47 ± 1.58 g | 0.67 ± 0.31 |
| 30TH-70C | 19.83 ± 1.79 ab | 24.35 ± 2.73 a | 7.59 ± 1.38 g | 2.23 ± 1.73 |
| 20TH-80C | 20.17 ± 1.81 ab | 24.74 ± 2.75 a | 7.61 ± 1.38 g | 2.15 ± 1.02 |
| 10TH-90C | 21.18 ± 2.0 a | 25.8 ± 2.89 a | 7.84 ± 1.25 f | 4.84 ± 2.15 |
| p-value | <0.001 | <0.001 | <0.001 | 0.659 |
| Pineapple silage (PS) 1 | ||||
| 100-PS | 12.93 ± 0.75 | 16.74 ± 1.90 | 10.16 ± 3.50 a | 0.44 ± 0.91 b |
| 90PS-10C | 14.41 ± 0.96 | 18.42 ± 2.08 | 9.30 ± 2.80 b | 0.31 ± 20.55 b |
| 80PS-20C | 15.62 ± 1.17 | 19.83 ± 2.26 | 8.86 ± 2.32 c | 0.77 ± 1.19 b |
| 70PS-30C | 17.8 ± 1.71 | 21.71 ± 2.69 | 8.30 ± 1.92 d | 9.54 ± 4.81 b |
| 60PS-40C | 16.16 ± 1.73 | 19.72 ± 2.65 | 6.97 ± 1.65 h | 1.47 ± 0.56 b |
| 50PS-50C | 18.37 ± 1.78 | 22.54 ± 2.72 | 7.54 ± 1.49 e | 1.88 ± 1.03 b |
| 40PS-60C | 19.35 ± 1.82 | 23.61 ± 2.74 | 7.27 ± 1.38 f | 2.08 ± 1.17 b |
| 30PS-70C | 20.25 ± 2.10 | 24.19 ± 2.93 | 7.08 ± 1.21 gh | 3.54 ± 2.28 b |
| 20PS-80C | 20.99 ± 2.12 | 25.19 ± 2.95 | 7.22 ± 1.17 fg | 3.96 ± 2.33 b |
| 10PS-90C | 15.17 ± 2.31 | 17.93 ± 3.01 | 7.00 ± 1.30 h | 57.29 ± 20.97 a |
| p-value | 0.133 | 0.279 | <0.001 | 0.003 |
| 100-concentrate (C) | 20.8 ± 2.18 | 25.07 ± 3.01 | 8.04 ± 1.24 b | |
| Soybean meal | 17.93 ± 2.13 | 21.08 ± 2.88 | 5.54 ± 1.17 c | |
| Ground corn | 20.24 ± 1.80 | 25.84 ± 2.88 | 10.78 ± 1.57 a | |
| p-value | 0.075 | 0.073 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baffa, D.F.; Oliveira, T.S.; Fernandes, A.M.; Camilo, M.G.; Silva, I.N.; Meirelles Júnior, J.R.; Aniceto, E.S. Evaluation of Associative Effects of In Vitro Gas Production and Fermentation Profile Caused by Variation in Ruminant Diet Constituents. Methane 2023, 2, 344-360. https://doi.org/10.3390/methane2030023
Baffa DF, Oliveira TS, Fernandes AM, Camilo MG, Silva IN, Meirelles Júnior JR, Aniceto ES. Evaluation of Associative Effects of In Vitro Gas Production and Fermentation Profile Caused by Variation in Ruminant Diet Constituents. Methane. 2023; 2(3):344-360. https://doi.org/10.3390/methane2030023
Chicago/Turabian StyleBaffa, Danielle F., Tadeu S. Oliveira, Alberto M. Fernandes, Michelle G. Camilo, Ismael N. Silva, José R. Meirelles Júnior, and Elon S. Aniceto. 2023. "Evaluation of Associative Effects of In Vitro Gas Production and Fermentation Profile Caused by Variation in Ruminant Diet Constituents" Methane 2, no. 3: 344-360. https://doi.org/10.3390/methane2030023
APA StyleBaffa, D. F., Oliveira, T. S., Fernandes, A. M., Camilo, M. G., Silva, I. N., Meirelles Júnior, J. R., & Aniceto, E. S. (2023). Evaluation of Associative Effects of In Vitro Gas Production and Fermentation Profile Caused by Variation in Ruminant Diet Constituents. Methane, 2(3), 344-360. https://doi.org/10.3390/methane2030023






