Enterococcus faecalis Biofilm: A Clinical and Environmental Hazard †
Abstract
1. Introduction
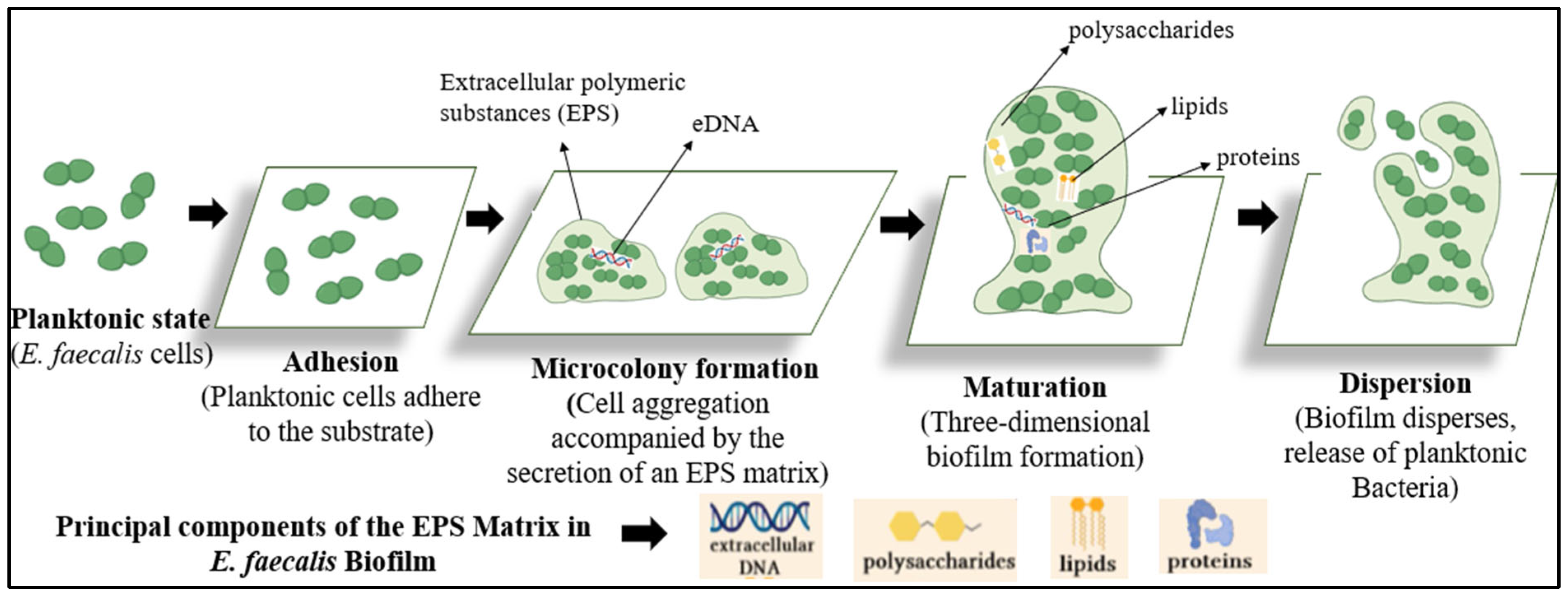
2. Stages of Biofilm Formation in Enterococcus faecalis
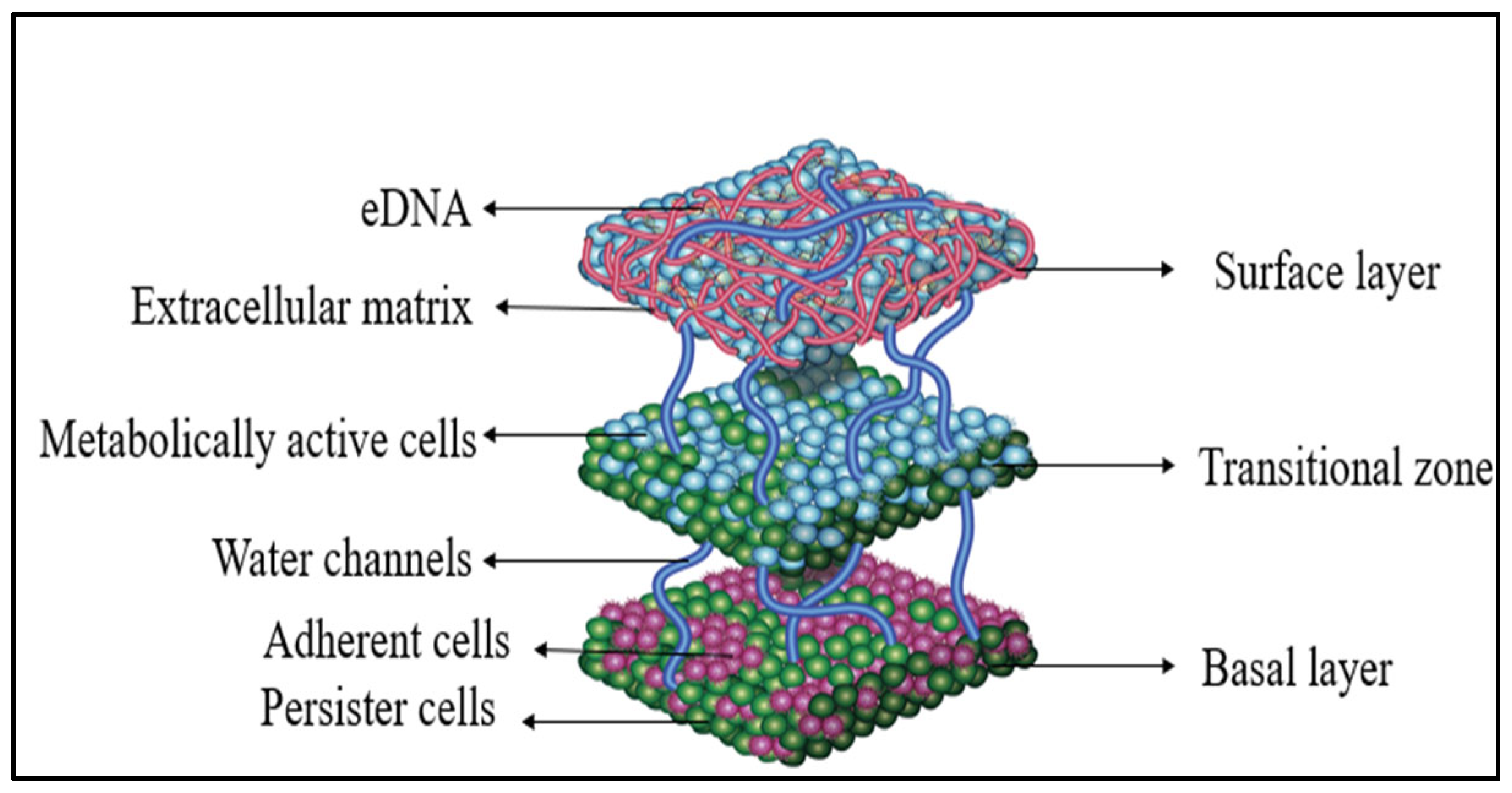
3. Architecture of Enterococcus faecalis Biofilm
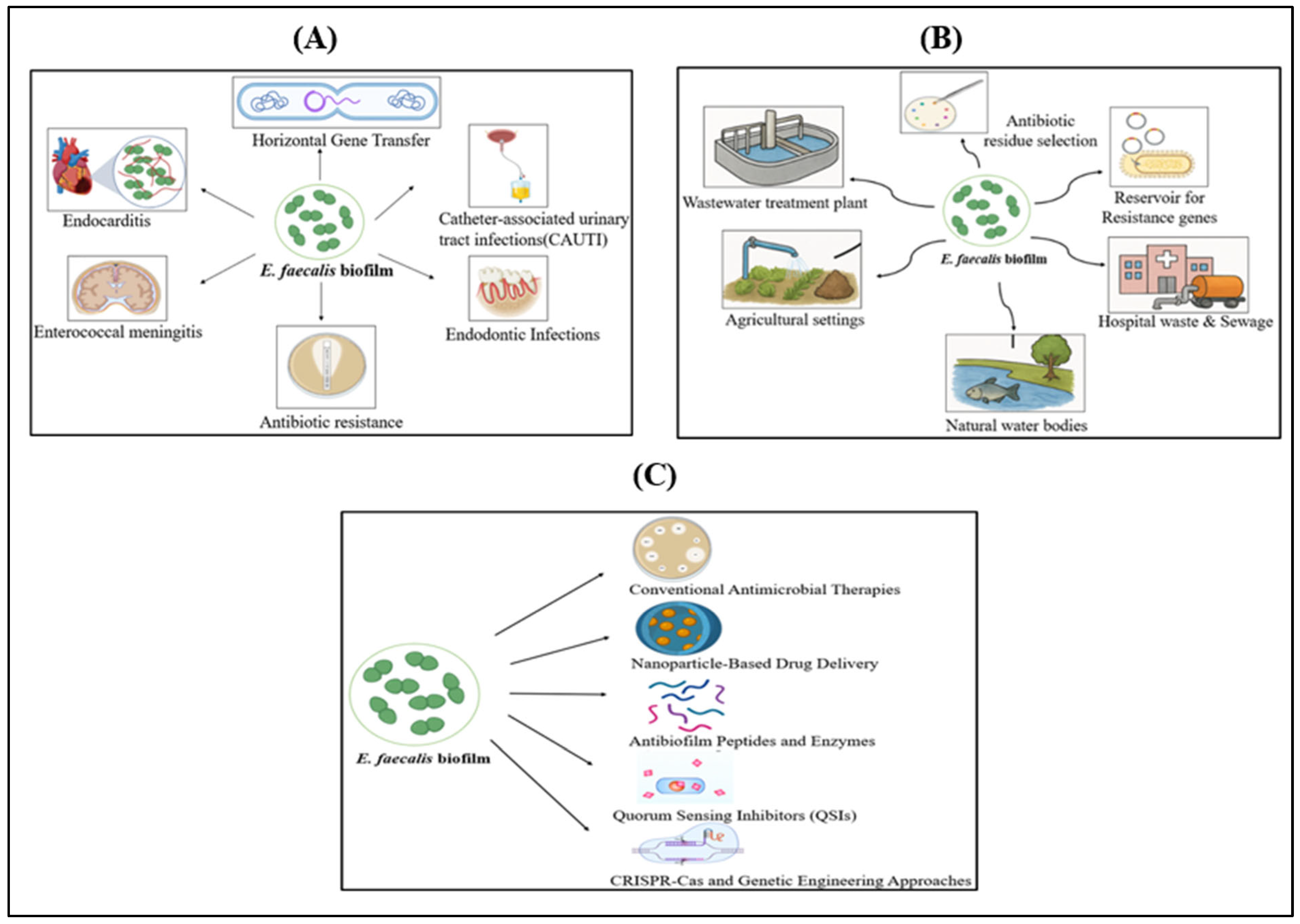
4. Clinical Hazards Caused by E. faecalis Biofilm
5. Environmental Hazards Caused by E. faecalis Biofilm
6. Emerging Strategies for Prevention and Treatment of E. faecalis Biofilm
6.1. Conventional Antimicrobial Therapies
6.2. Nanoparticle-Based Antimicrobial Delivery
6.3. Plant-Based Alternative Therapy
6.4. Antibiofilm Peptides
6.5. Enzyme-Based Biofilm Disruption
6.6. Quorum Sensing Inhibitors (QSIs)
6.7. Anti-Biofilm Surface Coatings
6.8. CRISPR-Cas and Genetic Engineering Approaches
7. Advanced Strategies for Disrupting E. faecalis Biofilm
7.1. Bacteriophage Therapy
7.2. Energy-Based Disruption Methods
- Bioelectric and Acoustic Methods
- Shock Wave Therapy
- Non-Thermal Plasma (NTP)
- Photodynamic therapy (PDT)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| Esp | Enterococcal surface protein |
| QSIs | Quorum-sensing inhibitors |
| Agg | Aggregation substance |
| Ebp | Endocarditis and biofilm-associated pili |
| fsr | Faecalis system regulator |
| AtlA | Autolysins |
| GelE | Gelatinase |
| SprE | Serine protease |
| eDNA | Extracellular DNA |
| EPS | Extracellular polymeric substance |
| vanA | Vancomycin |
| ermB | Erythromycin ribosomal methylase |
| NTP | Non-Thermal Plasma |
| PDT | Photodynamic therapy |
References
- Archambaud, C.; Nunez, N.; da Silva, R.A.; Kline, K.A.; Serror, P. Enterococcus faecalis: An overlooked cell invader. Microbiol. Mol. Biol. Rev. 2024, 88, e00069-24. [Google Scholar]
- Sadanandan, B.; Vaniyamparambath, V.; Lokesh, K.N.; Shetty, K.; Joglekar, A.P.; Ashrit, P.; Hemanth, B. Candida albicans biofilm formation and growth optimization for functional studies using response surface methodology. J. Appl. Microbiol. 2022, 132, 3277–3292. [Google Scholar] [CrossRef] [PubMed]
- Ashrit, P.; Sadanandan, B.; Kyathsandra Natraj, L.; Shetty, K.; Vaniyamparambath, V.; Raghu, A.V. A microplate-based Response Surface Methodology model for growth optimization and biofilm formation on polystyrene polymeric material in a Candida albicans and Escherichia coli co-culture. Polym. Adv. Technol. 2022, 33, 2872–2875. [Google Scholar] [CrossRef]
- Sadanandan, B.; Ashrit, P.; Nataraj, L.K.; Shetty, K.; Jogalekar, A.P.; Vaniyamparambath, V.; Hemanth, B. High-throughput comparative assessment of biofilm formation of Candida glabrata on polystyrene material. Korean J. Chem. Eng. 2022, 39, 1277–1286. [Google Scholar] [CrossRef]
- Willett, J.L.E.; Dunny, G.M. Insights into Ecology, Pathogenesis, and Biofilm Formation of Enterococcus faecalis from Functional Genomics. Microbiol. Mol. Biol. Rev. 2025, 89, e00081-23. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Beig, M.; Wang, L.; Navidifar, T.; Moradi, S.; Tabaei, F.M.; Teymouri, Z.; Moghadam, M.A.; Sedighi, M. Global Status of Antimicrobial Resistance in Clinical Enterococcus faecalis Isolates: Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 80. [Google Scholar] [CrossRef]
- Ghazvinian, M.; Asgharzadeh Marghmalek, S.; Gholami, M.; Amir Gholami, S.; Amiri, E.; Goli, H.R. Antimicrobial resistance patterns, virulence genes, and biofilm formation in enterococci strains collected from different sources. BMC Infect. Dis. 2024, 24, 274. [Google Scholar] [CrossRef]
- Hashem, Y.A.; Abdelrahman, K.A.; Aziz, R.K. Phenotype–Genotype Correlations and Distribution of Key Virulence Factors in Enterococcus faecalis Isolated from Patients with Urinary Tract Infections. Infect. Drug Resist. 2021, 14, 1713–1723. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Revisiting the Virulence Hallmarks of Pseudomonas aeruginosa: A Chronicle through the Perspective of Quorum Sensing. Environ. Microbiol. 2022, 24, 2630–2656. [Google Scholar] [CrossRef]
- Hakim, T.A.; Zaki, B.M.; Mohamed, D.A.; El-Shatoury, E.H.; Farrag, H.A. Novel Strategies for Vancomycin-Resistant Enterococcus faecalis Biofilm Control: Bacteriophage (vB_EfaS_ZC1), Propolis, and Their Combined Effects in an Ex Vivo Endodontic Model. Ann. Clin. Microbiol. Antimicrob. 2025, 24, 24. [Google Scholar] [CrossRef]
- Erol, H.B.; Kaskatepe, B.; Gocmen, D.; Ziraman, F.G. The Treatment of Enterococcus faecalis-Related Root Canal Biofilms with Phage Therapy. Microb. Pathog. 2024, 197, 107081. [Google Scholar] [CrossRef]
- Azzam, A.; Elkafas, H.; Khaled, H.; Ashraf, A.; Yousef, M.; Elkashef, A.A. Prevalence of Vancomycin-Resistant Enterococci (VRE) in Egypt (2010–2022): A Systematic Review and Meta-Analysis. J. Egypt. Public Health Assoc. 2023, 98, 8. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Freitas, C.; Ribeiro, J.; Igrejas, G.; Poeta, P. Comparative Analysis of Antibiotic Resistance and Biofilm Formation in Enterococcus spp. across One Health Domains. FEMS Microbes. 2025, 6, xtaf005. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Boeder, A.M.; Spiller, F.; Carlstrom, M.; Izídio, G.S. Enterococcus faecalis: Implications for Host Health. World J. Microbiol. Biotechnol. 2024, 40, 190. [Google Scholar] [CrossRef]
- Mubarak, A.G.; El-Zamkan, M.A.; Younis, W.; Saleh, S.O.; Abd-Elhafeez, H.H.; Yoseef, A.G. Phenotypic and genotypic characterization of Enterococcus faecalis and Enterococcus faecium isolated from fish, vegetables, and humans. Sci. Rep. 2024, 14, 21741. [Google Scholar] [CrossRef]
- Namaki Kheljan, M.; Teymorpour, R.; Peeri Doghaheh, H.; Arzanlou, M. Antimicrobial biocides susceptibility and tolerance-associated genes in Enterococcus faecalis and Enterococcus faecium isolates collected from human and environmental sources. Curr. Microbiol. 2022, 79, 170. [Google Scholar] [CrossRef]
- Șchiopu, P.; Toc, D.A.; Colosi, I.A.; Costache, C.; Ruospo, G.; Berar, G.; Todea, D.A. An overview of the factors involved in biofilm production by the Enterococcus genus. Int. J. Mol. Sci. 2023, 24, 11577. [Google Scholar] [CrossRef]
- Gaeta, C.; Marruganti, C.; Ali, I.A.; Fabbro, A.; Pinzauti, D.; Santoro, F.; Grandini, S. The presence of Enterococcus faecalis in saliva as a risk factor for endodontic infection. Front. Cell Infect Microbiol. 2023, 13, 1061645. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Anthony, D.C. Vancomycin Resistant E. faecium: Addressing Global and Clinical Challenges. Antibiotics 2025, 14, 522. [Google Scholar]
- Samani, R.J.; Tajbakhsh, E.; Momtaz, H.; Samani, M.K. Prevalence of virulence genes and antibiotic resistance pattern in Enterococcus faecalis isolated from urinary tract infection in Shahrekord, Iran. Rep. Biochem. Mol. Biol. 2021, 10, 50. [Google Scholar] [CrossRef]
- Nkemngong, C.; Teska, P. Biofilms, mobile genetic elements and the persistence of pathogens on environmental surfaces in healthcare and food processing environments. Front. Microbiol. 2024, 15, 1405428. [Google Scholar] [CrossRef] [PubMed]
- Oli, A.K.; Javaregowda, P.K.; Jain, A.; Kelmani, C.R. Mechanism involved in biofilm formation of Enterococcus faecalis. In Focus on Bacterial Biofilms; Das, T., Ed.; IntechOpen: London, UK, 2022; Chapter 5; pp. 179–200. ISBN 978-1-80355-797-4. [Google Scholar]
- Woitschach, F.; Kloss, M.; Schlodder, K.; Borck, A.; Grabow, N.; Reisinger, E.C.; Sombetzki, M. Bacterial adhesion and biofilm formation of Enterococcus faecalis on zwitterionic methylmethacrylate and polysulfones. Front. Cell Infect. Microbiol. 2022, 12, 868338. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V. The multifaceted lifestyle of enterococci: Genetic diversity, ecology and risks for public health. Curr. Opin. Microbiol. 2022, 65, 73–80. [Google Scholar] [CrossRef]
- Khan, N.; Ishfaq, M.; Mufti, I.U.; Ishfaq, S. A Short Review on Enterococcus faecalis. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2024, 67, 298–310. [Google Scholar]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance in Enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Mohamed, J.A.; Huang, D.B.; Murray, B.E. High-Level Aminoglycoside Resistance in Enterococci. Antimicrob. Agents Chemother. 2023, 67, e00733-23. [Google Scholar]
- Thomas, V.C.; Hiromasa, Y.; Harms, N.; Thurlow, L.R.; Tomich, J.; Hancock, L.E. A Signaling Peptide-Dependent Quorum Sensing System Regulates Enterococcus faecalis Biofilm Formation and Virulence. J. Bacteriol. 2023, 205, e00362-22. [Google Scholar]
- García-Aljaro, C.; Ballesté, E.; Muniesa, M.; Jofre, J. Determination of crAssphage in water samples and applicability for tracking human faecal pollution. Microb. Biotechnol. 2017, 10, 1775–1780. [Google Scholar] [CrossRef]
- Zdragas, A.; Partheniou, P.; Kotzamanidis, C.; Psoni, L.; Koutita, O.; Moraitou, E.; Tzanetakis, N.; Yiangou, M. Molecular characterization of low-level vancomycin-resistant enterococci found in coastal water of Thermaikos Gulf, Northern Greece. Water Res. 2008, 42, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. The Rise of the Enterococcus: Beyond Vancomycin Resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Tang, Y.; Zhou, Q.; Hu, B. Lysozyme Reduces Biofilm Viability of Vancomycin-Resistant Enterococcus faecalis via Non-Enzymatic Antimicrobial Activity. Front. Microbiol. 2022, 13, 963431. [Google Scholar]
- He, Z.; Zhang, Y.; Sun, Y.; Li, X. Lysozyme-Induced Reduction of Enterococcus faecalis Biofilms and Enhancement of Antibiotic Susceptibility. J. Dent. Res. Rev. 2023, 14, 34–42. [Google Scholar]
- Hemmati, F.; Rezaee, M.A.; Ebrahimzadeh, S.; Yousefi, L.; Nouri, R.; Kafil, H.S.; Gholizadeh, P. Novel strategies to combat bacterial biofilms. Mol. Biotechnol. 2021, 63, 569–586. [Google Scholar] [CrossRef]
- Yang, S.; Meng, X.; Zhen, Y.; Baima, Q.; Wang, Y.; Jiang, X.; Xu, Z. Strategies and mechanisms targeting Enterococcus faecalis biofilms associated with endodontic infections: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1433313. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, Y.; Liu, J.; Zhang, T. Antibiofilm Strategies Combining Enzymatic Degradation and Antimicrobial Agents against Multidrug-Resistant Pathogens. J. Biomed. Sci. 2023, 30, 12. [Google Scholar]
- Wang, Y.; Fang, L.; Wang, P.; Qin, L.; Jia, Y.; Cai, Y.; Liu, F.; Zhou, H.; Wang, S. Antibacterial Effects of Silica Nanoparticles Loading Nano-Silver and Chlorhexidine in Root Canals Infected by Enterococcus faecalis. J. Endod. 2025, 51, 54–63. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, Z.; Hong, X.; Xu, Z.; Xu, Y.; He, Y.; Chen, S. Novel Approaches on Root Canal Disinfection Methods Against E. faecalis. J. Oral Microbiol. 2025, 17, 2475947. [Google Scholar] [CrossRef]
- Sadanandan, B.; Vijayalakshmi, V.; Ashrit, P.; Babu, U.V.; Sharath Kumar, L.M.; Sampath, V.; Shetty, K.; Joglekar, A.P.; Awaknavar, R. Aqueous Spice Extracts as Alternative Antimycotics to Control Highly Drug Resistant Extensive Biofilm Forming Clinical Isolates of Candida albicans. PLoS ONE 2023, 18, e0281035. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.A.; da Silva, B.R.; Andrade, A.L.; de Souza, J.V.; Cavalcante, R.M.S.; de Almeida, J.A.C.; Oliveira, M.P. Antimicrobial and Antibiofilm Activity of Synthetic Peptide [W7]KR12-KAEK Against Enterococcus faecalis Strains. Curr. Microbiol. 2023, 80, 325. [Google Scholar] [CrossRef] [PubMed]
- Güneş, B.; Akçelik, N. The Role of eDNA in Biofilm Structure of Enterococcus faecalis and Investigation of the Efficiency of Enzyme and Antibiotic Application in Biofilm Eradication. Microbiol. Bull. 2022, 56, 606–619. [Google Scholar]
- Shao, C.; Bai, J.; Gao, C.; Wang, Y.; Zhang, Y. Quorum Sensing and Biofilm Formation in Enterococcus faecalis: Potential Targets for Infection Control. Front. Cell. Infect. Microbiol. 2022, 12, 857611. [Google Scholar]
- Sadanandan, B.; Vijayalakshmi, V.; Shetty, K.; Rathish, A.; Shivkumar, H.; Gundreddy, M.; Narendra, N.K.; Devaiah, N.M. In Situ Aqueous Spice Extract-Based Antifungal Lock Strategy for Salvage of Foley’s Catheter Biofouled with Candida albicans Biofilm Gel. Gels 2025, 11, 23. [Google Scholar] [CrossRef]
- Sewid, A.H.; Sharaf, M.; El-Demerdash, A.S.; Ragab, S.M.; Al-Otibi, F.O.; Yassin, M.T.; Liu, C.G. Hexagonal Zinc Oxide Nanoparticles: A Novel Approach to Combat Multidrug-Resistant Enterococcus faecalis Biofilms in Feline Urinary Tract Infections. Front. Cell. Infect. Microbiol. 2025, 14, 1505469. [Google Scholar] [CrossRef]
- Hullahalli, K.; Rodrigues, M.; Palmer, K.L. CRISPR-Cas-Mediated Genome Editing and Antimicrobial Strategies in Enterococcus faecalis: Progress and Perspectives. Front. Microbiol. 2023, 14, 1139215. [Google Scholar]
- Rodriguez, A.M.; Wang, B.; Liu, Y.; Lu, T.K. Phage-Delivered CRISPR-Cas Systems for Precision Antimicrobials against Drug-Resistant Enterococcus faecalis. Nat. Commun. 2023, 14, 3264. [Google Scholar]
- Khalifa, L.; Gelman, D.; Shlezinger, M.; Dessal, A.L.; Coppenhagen-Glazer, S.; Beyth, S.; Hazan, R.; Yerushalmy, O.; Que, Y.A.; Ben-Porat, S.; et al. Defeating Antibiotic- and Phage-Resistant Enterococcus faecalis Using a Phage Cocktail In Vitro and in a Clot Model. Front. Microbiol. 2018, 9, 326. [Google Scholar] [CrossRef]
- Tesema, M.Y. Outlooks of Endolysins with Innolysins Therapeutic Potentials against Antimicrobial Resistance. Discov. Med. 2025, 2, 188. [Google Scholar] [CrossRef]
- Zhu, M.; Dang, J.; Dong, F.; Zhang, Y.; Liu, Y.; Huang, C. Antimicrobial and Cleaning Effects of Ultrasonic-Mediated Plasma-Loaded Microbubbles on Enterococcus faecalis Biofilm: An In Vitro Study. BMC Oral Health 2023, 23, 133. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, T. Strategies for Detecting Bacterial Biofilms: Unveiling the Hidden World of Microbial Aggregates. Egypt. J. Soil Sci. 2024, 64, 1069–1096. [Google Scholar] [CrossRef]
- Assadian, H.; Fathollahi, S.; Pourhajibagher, M.; Solimei, L.; Benedicenti, S.; Chiniforush, N. Effectiveness of Activated Sodium Hypochlorite Irrigation by Shock Wave-Enhanced Emission Photoacoustic Streaming, Sonic and Ultrasonic Devices in Removing Enterococcus faecalis Biofilm from Root Canal System. J. Clin. Med. 2024, 13, 6278. [Google Scholar] [CrossRef]
- Scholtz, V.; Vaňková, E.; Kašparová, P.; Premanath, R.; Karunasagar, I.; Julák, J. Non-Thermal Plasma Treatment of ESKAPE Pathogens: A Review. Front. Microbiol. 2021, 12, 737635. [Google Scholar] [CrossRef]
- Gupta, T.T.; Ayan, H. Application of Non-Thermal Plasma on Biofilm: A Review. Appl. Sci. 2019, 9, 3548. [Google Scholar] [CrossRef]
- López-Jiménez, L.; Fusté, E.; Martínez-Garriga, B.; Arnabat-Domínguez, J.; Vinuesa, T.; Viñas, M. Effects of Photodynamic Therapy on Enterococcus faecalis Biofilms. Lasers Med. Sci. 2015, 30, 1519–1526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadanandan, B.; Yogendraiah, K.M. Enterococcus faecalis Biofilm: A Clinical and Environmental Hazard. Med. Sci. Forum 2025, 35, 5. https://doi.org/10.3390/msf2025035005
Sadanandan B, Yogendraiah KM. Enterococcus faecalis Biofilm: A Clinical and Environmental Hazard. Medical Sciences Forum. 2025; 35(1):5. https://doi.org/10.3390/msf2025035005
Chicago/Turabian StyleSadanandan, Bindu, and Kavyasree Marabanahalli Yogendraiah. 2025. "Enterococcus faecalis Biofilm: A Clinical and Environmental Hazard" Medical Sciences Forum 35, no. 1: 5. https://doi.org/10.3390/msf2025035005
APA StyleSadanandan, B., & Yogendraiah, K. M. (2025). Enterococcus faecalis Biofilm: A Clinical and Environmental Hazard. Medical Sciences Forum, 35(1), 5. https://doi.org/10.3390/msf2025035005





