Abstract
Measuring antimicrobial use (AMU) in animal production can provide useful data for monitoring AMU over time, which will promote antimicrobial resistance (AMR) reduction. This study involved the daily collation and validation of active primary drug sales and prescription data from veterinary outlets and clinics of the Kaduna metropolis. In total, 83.7% of the identified antimicrobials were in the form of oral medication, and most were registered antibiotics (52.8%). Parenteral and topical forms were also identified, with 94% also being antibiotics. The estimated AMU was 282 mg/kg population correction unit (PCU). Poultry represented the most significant population, constituting 99% (31,502,004) of the study population. The class-specific AMU was antibiotics, with 274 mg/kg PCU. The antiprotozoal AMU was 418 mg/kg PCU. The anthelminthic AMU was the highest at 576 mg/kg PCU. This study has provided useful and practical information on the trends in antimicrobial use in animals, with poultry being the most important animal population involved in AMU and oxytetracycline being the most abused antibiotic in animal production. Antimicrobial stewardship (AMS) should be targeted at poultry populations, with an emphasis on reducing antibiotic use/consumption.
1. Introduction
The use of antimicrobials in animals is a global practice against infections and the enhancement of productivity. It has been established that a linear relationship exists between antimicrobial use/consumption (AMU/AMC) and antimicrobial resistance (AMR) [1]. Much of the effort in monitoring AMU/AMR in animal production has been driven by the consensus that AMU/AMR in animal production contributes to the overall burden of AMR in humans [2]; as the global data indicate, consumption of antimicrobials in food animals far exceeds consumption in human medicine because of larger biomass and non-therapeutic AMU in food animals. This is in addition to the fact that AMU in animals has become a worldwide concern because in most countries, more than 50% of the important antimicrobials used in humans are being used in livestock [3]. These have led to the establishment of several initiatives globally, regionally, and nationally to promote responsible use of antimicrobials, reduce excessive AMU in animal production and support surveillance systems for monitoring AMU/AMR [4]. Incorrect use and abuse of antimicrobials such as frequent and prolonged use are key drivers for the spread and emergence of antimicrobial resistance. High levels of such antimicrobial resistance have a negative impact on livestock production either by reducing productivity, or high costs of treatment on farmers/owners [5]. This increased AMR in animals conversely affects patients (human) health outcomes as it results in concomitant emergence or increase in antimicrobial resistance in humans, rendering human infectious diseases harder or more difficult to treat, in addition to the increase costs of human healthcare treatments [6]. Measuring AMU in animal production may address several challenges including providing data useful for monitoring AMU overtime that will assist in setting benchmarks to promote AMU reduction. Also, measuring AMU is fundamental to investigating the qualitative and quantitative associations between AMU and AMR [7].
This study aimed to measure antimicrobial usage (consumption) in animal production using active primary drug sales and prescription data within Kaduna Metropolises. The objectives were to compile qualitative prevalence of usage of specific antimicrobials and their classes in Kaduna metropolis; to measure quantitative amounts of antimicrobial active principles.
2. Materials and Methods
This study was carried out in the four (4) local government areas (LGAs) that make up the Kaduna metropolis including Igabi, Kaduna North, Kaduna South and Chikun LGAs. The study was conducted for twelve (12) weeks from April 2022 to July 2022. The study was a cross-sectional surveillance to generate qualitative and quantitative data on antimicrobials used or consumed in animals (prevention or treatment of disease and growth promotion) through structured interview questionnaire administration at veterinary clinics and veterinary pharmaceuticals outlets (retail and wholesale) daily to buyers or end users of these antimicrobials. Forty-one (41) pre-identified points of which two (2) trademark points (wholesale), twenty-two (22) retail veterinary drug stores, and eight (8) prescription points (clinics) participated in the study (78% of the survey population). The survey questionnaire was designed in English containing two (2) sections: (i) demographic information of the respondent and (ii) veterinary care information with the antimicrobial and animal production information. To include AMU questions in the questionnaire, AMR expert documents of FAO were consulted, and eleven (11) questions suggested to gather information on AMU in animal production were included. These questions included purpose of using the antibiotics, frequency of usage, who advises on use of antibiotics, number of animals, the species treated and source of drugs. The questionnaire was administered to each respondent as a one-on-one interview at the point of drug purchase, administration or prescription. Data obtained were summarized for clearing, processing, and analysis. All data regarding demographic features and AMU (sale, prescription) were analyzed through descriptive statistics. Quantitative data were analyzed by calculating the population correction unit (PCU, with some modification) as follows: the standardized average weight in kilograms (kg) of all animals at time of treatment multiplied by the number of animals based on national statistics (live and/or slaughter) (here we used actual head count of animals in the survey) to estimate the relative amount of antimicrobial active ingredient used for every kilogram body weight at times of treatment over the course of the study period covered (12 weeks) (Table 1). Class-specific PCU and AMU were thus estimated for antibiotics, antiprotozoals, and anthelminthics in addition to the overall AMU.

Table 1.
Animals’ population distribution and the relative prevalence of antimicrobials used per group.
3. Results
The total livestock, poultry, and fish PCU was 112.8 mt for all antimicrobials and total AMU was 282 mg/PCU (Table 2). The PCU of cattle, which measures the biomass of animals that may receive antibiotics, was 8383.03 tonnes, while for anthelminthics, it was 2399.46 tonnes. However, the AMU coefficient, which measures the amount of antibiotics used per PCU, was low at 3.6 mg/PCU, while it was 3 times higher for anthelminthics at 11.6 mg/PCU. As a result, the total amount of antibiotics used in the 28,907 cattle of the study area was estimated at 30,387.6 tonnes in the 12-week study period. Most of this increase was driven by the higher AMU coefficient rather than the higher PCU. Despite increasing production from mechanization and intensification, the poultry industry remains the biggest threat in the increasing AMU in animals bred for food. According to the data, the total PCU of poultry was the highest (79.2%) at 46,796.95 tonnes. Accordingly, the antimicrobial AMU in this group was 31.6 mt during the study period, meaning that the average antibiotic AMU in the poultry of the study area was 653.3 mg/PCU, anthelminthics AMU was 2227 mg/PCU, and antiprotozoal AMU was estimated at 1509.6 mg/PCU. This high AMU shows the absence in the poultry industry of a more responsible and prudent use of antimicrobials. According to the data, the PCU of pigs was low at 34.44 tonnes. The average AMU per pig also fluctuated, increasing from 13.9 mg/PCU for antibiotics to 46.9 mg/PCU for anthelminthics, and then levelled to 0 for the recorded use of antiprotozoals in pigs of the study area (Table 3). The changes in AMU reflect the combined effects of changes in PCU and mg/PCU.

Table 2.
Class-specific and total antimicrobial use (AMU) in animals of the study for the study period (12 weeks).

Table 3.
Animal-specific AMU and total AMU in animal groups of the study for the study period (12 weeks).
We adjusted the PCU calculation by considering the weight variation within each animal category. Table 2 illustrates that the selected animals consumed 31,765.2 tonnes of antimicrobials within the 12-week study period, with antibiotics accounting for the largest share estimated at 30,387.6 tonnes corresponding to 112,786.4 tonnes and 110,899.5 tonnes of PCU, respectively.
Among the eight types of livestock, poultry accounted for the largest share of AMU, with 31,638 tonnes, representing 99.6% of the total AMU and 46,796.9 tonnes of PCU. Fish followed with 63.5 tonnes of AMU (0.2%). This was followed by the ruminant livestock (cattle and sheep/goats) with 31.8 tonnes each (0.1% each) AMU. Rabbits and horses followed with 0.004% and 0.002%, respectively, at 1.27 tonnes and 635.3 kg of AMU, respectively, with 2.5 tonnes and 129.8 tonnes of PCU, respectively. Pigs had the smallest AMU of 317.7 kg, with only 0.001% of the total, and 34.4 tonnes of PCU (Table 3).
4. Discussion
This study has provided useful information on trends of antimicrobial usage in food animals. In comparing this survey with other surveillance systems undertaken by other countries, certain observations have been noted. The antimicrobial groups showing the largest sales, in terms of weight, were the antibiotics compared to anthelminthics and antiprotozoals. The terramycines constituted 24.1% (14.3% of the total volume of all antimicrobials), with reference specifically to oxytetracycline and doxycycline. These antibiotics are used for the treatment and prevention of diseases such as mycoplasmosis in poultry and as growth promoters in food animals. The extensive usage of all these drugs, especially florfenicol, tylosin, and ciprofloxacin, is a cause for great concern because their main route of administration is through the feed and water at sub-therapeutic levels for prophylaxis (Figure 1). This form of administration promotes the potential for resistance to related antimicrobials administered in human medicine. These antimicrobials are very important in human health and have the potential to add to the development of cross-resistance to antimicrobials administered in human health. Fluoroquinolone usage in food animals is a cause for great concern with the emergence of quinolone-resistant strains in zoonotic Salmonella, and Campylobacter being reported in western Europe and the United States. Resistance to this group leaves few treatment options available, as multiple cross-resistance is now commonplace [14].
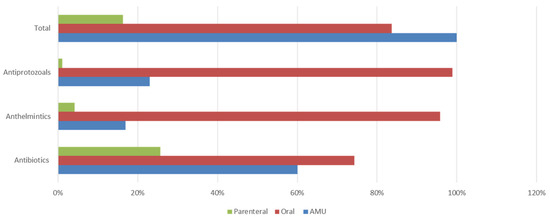
Figure 1.
Proportion of class-specific antimicrobial usage and the two modes of administration (oral vs. parenteral).
Anthelminthic antimicrobials were also commonplace for albendazole, piperazine, and levamisole. Albendazole is the most used treatment as it is a broad-spectrum anthelminthic, cheap, and the most readily available of the anthelminthics with a wide safety margin (Figure 2). The antimicrobials with the largest sales/usage were triazenetriones; diclazuril and toltrazuril (17.1% of the total volume). This is unsurprising as coccidiosis is a major disease of concern in poultry and even small ruminants with most of the treatment almost always administered as prophylaxis. In addition, it also reflects the poor control measures established for such a common and important disease (Figure 3).
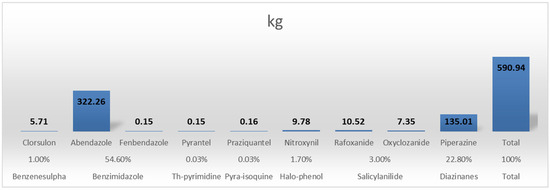
Figure 2.
Quantities and class proportions of anthelminthic agents consumed/used in animals within the study period (12 weeks).
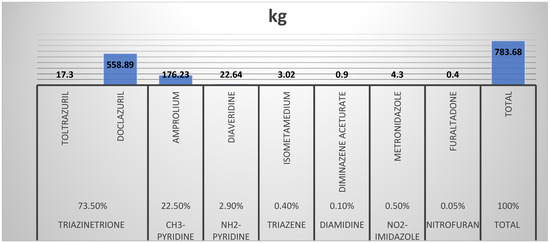
Figure 3.
Quantities and class proportions of antiprotozal agents consumed/used in animals within the study period (12 weeks).
The observation that oral dosing constituted 83.7% of the total of antimicrobial usage (either infeed or water) is significant (Figure 1). In humans, treatment is directed at the individual patient, but for animals, entire groups may be treated with the administration of medicated feed or water. The dosages included for prophylaxis are usually at low concentrations for extended time periods. Both practices, in combination, have the potential to accelerate the emergence of resistant bacteria in the animals concerned, which can then infect humans, through contact or via the food chain.
5. Conclusions
Due to the concerns expressed above, antimicrobial usage should be reviewed by the regulatory authorities. These should be reviewed in order of priority, relating to the documented evidence for their ability to increase antimicrobial resistance in other groups of antimicrobials, or because of their structural relatedness to antimicrobials in human medicine. Concern is not as great for anthelminthic antimicrobials administered mostly orally, which are not registered as antimicrobial growth promoters (AGPs) like antibiotics. This is because the concentrations administered are broad-spectrum and the longer acting salts facilitate convenient and one-off administration. Longitudinal study designs such as this allow insights into repeated behaviour of consumption over time (especially when consecutive cycles are investigated). Such studies may also provide insights into treatment practices for different diseases or types of animals.
This study provided useful and practical information on the trends in antimicrobial usage in animals, with poultry being the most important animal population involved in the AMU and oxytetracycline being the most abused antibiotic in animal production (Figure 4). Antimicrobial stewardship (AMS) should be targeted toward poultry populations, with an emphasis on reducing antibiotic usage.
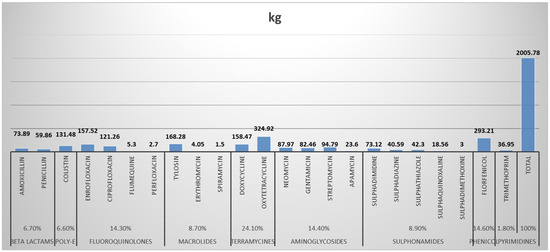
Figure 4.
Quantities and class proportions of antibiotics consumed/used in animals within the study period (12 weeks).
Author Contributions
Conceptualization, A.A.; methodology, A.A.; validation, A.A., M.O.A. and H.A.K.; formal analysis, A.A.; investigation, M.O.A. and H.A.K.; resources, A.A., M.O.A. and H.A.K.; data curation, A.A.; writing—original draft preparation, A.A.; writing—review and editing, A.A.; supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable as the study did not involve humans or animals.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMU | Antimicrobial Use |
| PCU | Population Correction Unit |
| SMW | Standardised Mean Weight |
| mg | Milligram |
| kg | Kilogram |
| AMS | Antimicrobial Stewardship |
References
- Sadiq, M.B.; Syed-Hussain, S.S.; Ramanoon, S.Z.; Ahmad, N.I.; Zin, N.M.; Khalid, S.; Naseeha, D.; Syahirah, A.; Mansor, R. Knowledge, attitude and perception regarding antimicrobial resistance and usage among ruminant farmers in Selangor, Malaysia. Prev. Vet. Med. 2018, 15, 76–83. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance; HM Government and Wellcome Trust: London, UK, 2016. [Google Scholar]
- Umair, M.; Abdullah, R.M.; Aslam, B.; Nawaz, M.H.; Ali, Q.; Fatima, F.; Ali, J.; Zahoor, M.A.; Mohsin, M. First Case Report on Quantification of Antimicrobial Use in Corporate Dairy Farms in Pakistan. Front. Vet. Sci. 2020, 7, 575848. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Battaglia, D.; García, A.D.; Tempelman, K.; Bullon, C.; Motriuc, N.; Caudell, M.; Cahill, S.; Song, J.; LeJeune, J. Achieving antimicrobial stewardship on the global scale: Challenges and Opportunities. Microorganisms 2022, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Olsen, J.E. Association between antimicrobial usage and resistance in Salmonella from poultry farms in Nigeria. BMC Vet. Res. 2021, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Choisy, M.; Van Cuong, N.; Bao, T.D.; Kiet, B.T.; Hien, B.V.; Thu, H.V.; Chansiripornchai, N.; Setyawan, E.; Thwaites, G.; Rushton, J.; et al. Assessing antimicrobial misuse in small-scale chicken farms in Vietnam from an observational study. BMC Vet. Res. 2019, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Babayemi, O.J.; Ajayi, M.O.; Olona, J.F.; Anurudu, N.F.; Ajayi, F.T. Livestock value chain: Prediction of live weight and out yield of three indigenous breeds of cattle in Nigeria. Niger. J. Anim. Prod. 2018, 45, 265–272. [Google Scholar] [CrossRef]
- Lawal-Adebowale, O.A. Dynamics of Ruminant Livestock Management in the Context of the Nigerian Agricultural System; Javed, K., Ed.; Livestock Production; Intech Open: London, UK, 2012; p. 61. [Google Scholar]
- Mahmud, M.A.; Shaba, P.; Onu, J.E.; Sani, S.A.; Danmaigoro, A. Gross morphological and morphomeric studies of the oviduct in three genotypes of Nigerian indigenous laying chickens. J. Dairy Vet. Anim. Res. 2017, 5, 138–142. [Google Scholar]
- Saidu, B.; Ishaq, A.; Ibrahim, H.; Dahiru, A.; Abdullahi, A.; Onwuchekwa, C.; Abduazeez, N.; Pilau, N.; Abdulrasheed, A.; Bamaiyi, A. Comparison of electrocardiographic parameters of racing and non-racing horses in Sokoto Nigeria. Sokoto J. Vet. Sci. 2020, 18, 33–38. [Google Scholar] [CrossRef]
- Weka, R.; Bwala, D.; Adedeji, Y.; Ifende, I.; Davou, A.; Ogo, N.; Luka, P. Tracing the Domestic Pigs in Africa; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Agaviezor, B.O.; Ologbose, F.I. Breed and doe’s body weight effect on litter weight and number of rabbits raised in South-South Nigeria. Anim. Res. Int. 2020, 17, 3572–3577. [Google Scholar]
- White, T.C.; Holleman, S.; Dy, F.; Mirels, L.F.; Stevens, D.A. Resistance Mechanisms in Clinical Isolates of Candida albicans. Antimicrob. Agents Chemother. 2002, 46, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).