Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing †
Abstract
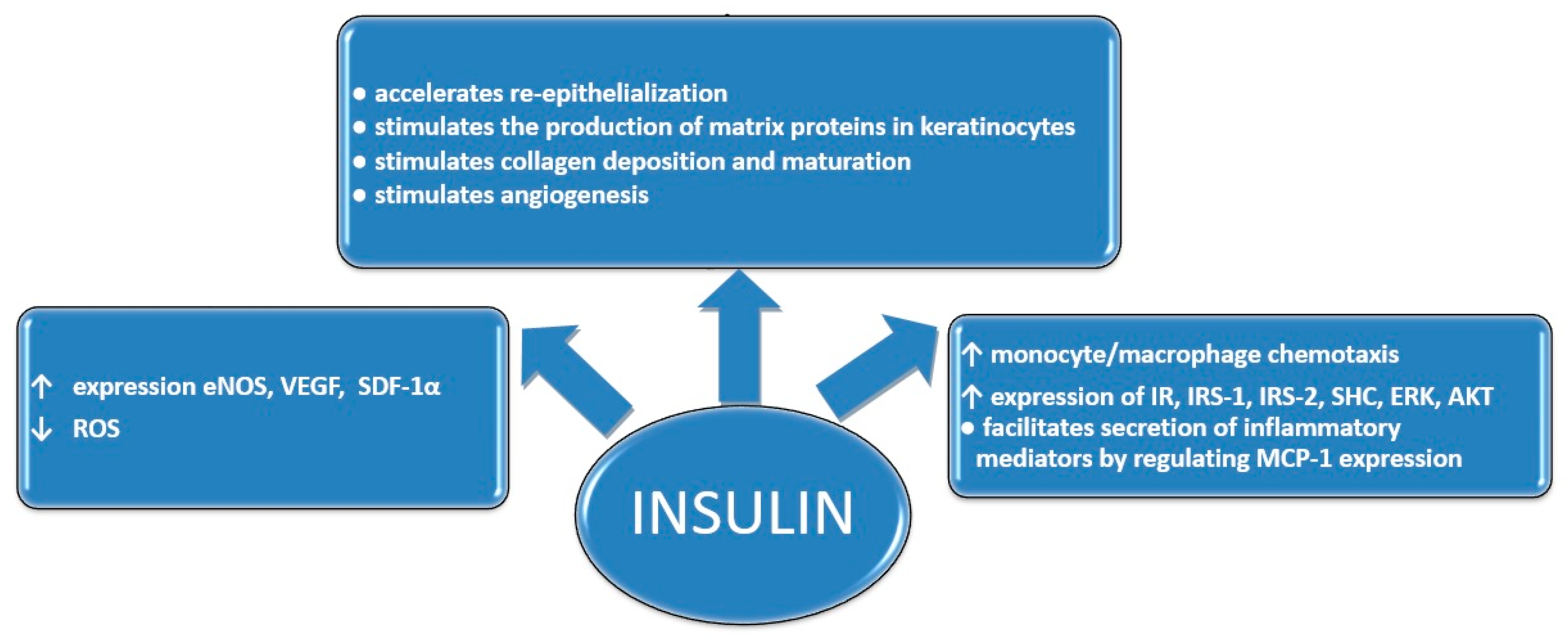
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quitério, M.; Simões, S.; Ascenso, A.; Carvalheiro, M.; Leandro, A.P.; Correia, I.; Viana, A.S.; Faísca, P.; Ascensão, L.; Molpeceres, J.; et al. Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach. Int. J. Mol. Sci. 2021, 22, 4087. [Google Scholar] [CrossRef] [PubMed]
- Madibally, S.V.; Solomon, V.; Mitchell, R.N.; Van De Water, L.; Yarmush, M.L.; Toner, M. Influence of insulin therapy on burn wound healing in rats. J. Surg. Res. 2003, 109, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, D.H.; Osman, M.A.; El-Gizawy, S.A.; Hawthorne, S.J.; Faheem, A.M.; McCarron, P.A. Effect of poly(ethylene glycol) on insulin stability and cutaneous cell proliferation in vitro following cytoplasmic delivery of insulin-loaded nanoparticulate carriers—A potential topical wound management approach. Eur. J. Pharm. Sci. 2018, 114, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.J.; McCaig, C.D.; Forrester, J.V.; Zhao, M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Mendes, F.; Filipe, P.; Reis, S.; Fonte, P. Nanocarrier-Mediated Topical Insulin Delivery for Wound Healing. Materials 2021, 14, 4257. [Google Scholar] [CrossRef]
- AbdelKader, D.H.; Tambuwala, M.M.; Mitchell, C.A.; Osman, M.A.; El-Gizawy, S.A.; Faheem, A.M.; El-Tanani, M.; McCarron, P.A. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly(vinyl alcohol)-borate hydrogels. Drug Deliv. Transl. Res. 2018, 8, 1053–1065. [Google Scholar] [CrossRef]
- Benoliel, A.M.; Kahn-Perles, B.; Imbert, J.; Verrando, P. Insulin stimulates haptotactic migration of human epidermal keratinocytes through activation of NF-kappa B transcription factor. J. Cell Sci. 1997, 110, 2089–2097. [Google Scholar] [CrossRef]
- Hermann, C.; Assmus, B.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Insulin-mediated stimulation of protein kinase Akt: A potent survival signaling cascade for endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 402–409. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhang, X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair. Regen. 2012, 20, 425–434. [Google Scholar] [CrossRef]
- Benkő, B.M.; Sebe, I.; Szabó, Z.I. Insulin for topical use in wound healing: Opportunities and limitations. Acta Pharm. Hung. 2022, 92, 3–19. [Google Scholar] [CrossRef]
- Besson, J.C.F.; Hernandes, L.; Campos, J.M.; Morikawa, K.A.; Bersani-Amado, C.A.; Matioli, G. Insulin complexed with cyclodextrins stimulates epithelialization and neovascularization of skin wound healing in rats. Injury 2017, 48, 2417–2425. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J. Effects of Topical Insulin on Wound Healing: A Review of Animal and Human Evidences. Diabetes Metab. Syndr. Obes. 2020, 13, 719–727. [Google Scholar] [CrossRef]
- Dhall, S.; Silva, J.P.; Liu, Y.; Hrynyk, M.; Garcia, M.; Chan, A.; Lyubovitsky, J.; Neufeld, R.J.; Martins-Green, M. Release of insulin from PLGA-alginate dressing stimulates regenerative healing of burn wounds in rats. Clin. Sci. 2015, 129, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Liu, Y. Effect of topical insulin application on wound neutrophil function. Wounds 2012, 24, 178–184. [Google Scholar]
- Liu, Y.; Dhall, S.; Castro, A.; Chan, A.; Alamat, R.; Martins-Green, M. Insulin regulates multiple signaling pathways leading to monocyte/macrophage chemotaxis into the wound tissue. Biol. Open. 2018, 7, bio026187. [Google Scholar] [CrossRef]
- Lima, M.H.M.; Caricilli, A.M.; de Abreu, L.L.; Araújo, E.P.; Pelegrinelli, F.F.; Thirone, A.C.P.; Tsukumo, D.M.; Pessoa, A.F.M.; dos Santos, M.F.; de Moraes, M.A.; et al. Topical Insulin Accelerates Wound Healing in Diabetes by Enhancing the AKT and ERK Pathways: A Double-Blind Placebo-Controlled Clinical Trial. PLoS ONE 2012, 7, e36974. [Google Scholar] [CrossRef]
- Barker, J.N.; Jones, M.L.; Mitra, R.S.; Crockett-Torabe, E.; Fantone, J.C.; Kunkel, S.L.; Warren, J.S.; Dixit, V.M.; Nickoloff, B.J. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am. J. Pathol. 1991, 139, 869–876. [Google Scholar]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000905. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Hong, W.; Zhang, Y.; Xu, B.; Song, G.; Liu, T.; Hong, C.; Ruan, L. Rapid gelation of oxidized hyaluronic acid and succinyl chitosan for integration with insulin-loaded micelles and epidermal growth factor on diabetic wound healing. Mater Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111273. [Google Scholar] [CrossRef]
- Cai, Y.; Che, J.; Yuan, M.; Shi, X.; Chen, W.; Yuan, W.E. Effect of glycerol on sustained insulin release from PVA hydrogels and its application in diabetes therapy. Exp. Ther. Med. 2016, 12, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, M.H.S.; Yassin, G.E.; Ghorab, D.M.; Morsi, N.M. Insulin Mucoadhesive Liposomal Gel for Wound Healing: A Formulation with Sustained Release and Extended Stability Using Quality by Design Approach. AAPS PharmSciTech 2019, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, F.; Kan, J.; Deng, J.; Wang, B.; Hao, S. Synthesis and fabrication of a keratin-conjugated insulin hydrogel for the enhancement of wound healing. Colloids Surf. B Biointerfaces 2019, 175, 436–444. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, A.K.; Nag, D.; Das, A.; Datta, S.; Ganguli, A.; Goel, V.; Rajput, S.; Chakrabarti, G.; Basu, B.; et al. Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing. Nanomedicine 2019, 15, 47–57. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Correa, V.L.R.; Silva, F.K.L.D.; Casas, A.A.; Chagas, A.L.D.; Oliveira, L.P.; Miguel, M.P.; Diniz, D.G.A.; Amaral, A.C.; Menezes, L.B. Wound healing treatment using insulin within polymeric nanoparticles in the diabetes animal model. Eur. J. Pharm. Sci. 2020, 150, 105330. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Maciążek-Jurczyk, M.; Pożycka, J.; Dolińska, B. Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Gupta, S.; Nair, A.; Chauhan, S.; Saini, V. Wound healing potential of insulin-loaded nanoemulsion with Aloe vera gel in diabetic rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102601. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of poly (vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Bardill, J.R.; Laughter, M.R.; Stager, M.; Liechty, K.W.; Krebs, M.D.; Zgheib, C. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2022, 138, 73–91. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Kumar, H.; He, X.; Lu, Q.; Bai, H.; Kim, K.; Hu, J. The Effect of Crosslinking Degree of Hydrogels on Hydrogel Adhesion. Gels 2022, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe vera hydrogel films for biomedical applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A. The Potential of Pharmaceutical Hydrogels in the Formulation of Topical Administration Hormone Drugs. Polymers 2022, 14, 3307. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Wu, P.-C.; Minhas, M.U. Using Carbomer-Based Hydrogels for Control the Release Rate of Diclofenac Sodium: Preparation and In Vitro Evaluation. Pharmaceuticals 2020, 13, 399. [Google Scholar] [CrossRef]
- Sarfraz, M.; Iqbal, R.; Khan, K.U.; Minhas, M.U. Carbopol Based Hydrogels for ITOPRIDE Hydrochloride Delivery; Synthesis, Characterization and Comparative Assessment with Various Monomers. J. Funct. Biomater. 2022, 13, 295. [Google Scholar] [CrossRef]
| Author, Year of Publication | Dosage Insulin | Hydrogel Carrier/Insulin Form | Research Model | Effects of the Insulin Preparation |
|---|---|---|---|---|
| Dhall et al. [13] | 0.04 mg/cm2 | Alginate gels; insulin-loaded PLGA microparticles | Female adult Sprague–Dawley rats; burn wound model | Accelerated healing via a decrease in oxidative stress and tissue damage, early recruitment of neutrophils, management of inflammatory cells, enhanced angiogenesis, and proper collagen deposition and maturation |
| Cai et al. [22] | 14.2 mg | Glycerol/PVA hydrogel | In vitro: 6-well plate; in vivo: male Wistar diabetic rats; | Addition of glycerol reduced the swelling ratio and hardness of the hydrogel, and enhanced the release of insulin in vitro and in vivo; glycerol disrupted the crystallite structure of PVA molecules while forming crosslinked structures between them, thereby promoting insulin release; insulin-loaded PVA hydrogel film exhibited a hypoglycemic effect in diabetic rats over 10 days |
| Besson et al. [11] | 50 IU | Carbopol 940 gel; insulin complexed with 2-hydroxypropyl-β-cyclodextrin (HPβCD-INS) | Excisional wounds in the skin of rats; chronic wound | Formulations: showed no cytotoxic or irritative effects; prolonged proliferation and migration of keratinocytes; increased deposition of type I and III collagen fibers |
| Abdelkader et al. [6] | 33.86 μg/mg | PVA-borate hydrogel; Insulin-loaded PLGA nanoparticles | Excisional wounds in the skin of rats; diabetic and healthy rats | In non-diabetic rats, there was no significant difference between healing observed in control and wounds treated with free insulin; in diabetic rats, insulin induced significant improvement in wound healing; histological images of diabetic wounds: reduction in the inflammatory process, increased angiogenesis, formation of granulation tissue, and completely reconstructed epidermis and collagen deposition |
| Dawoud et al. [23] | 20 mg/g (2% w/w) | chitosan gel; insulin-loaded liposomes | In vitro: franz diffusion cells; cellophane membrane; in vivo: patients with chronic wounds | Release was sustained up to 24 h; release rate of 91.521 μg/cm2/h; improvement in the wound healing rate; reduction in the erythema of the ulcer and no signs of hypoglycemia |
| Li et al. [24] | 5 mg | Keratin-conjugated insulin hydrogel (Ins-K) | Hairless rat skin | Promoted wound healing by stimulating cellular migration; Ins-K hydrogel shows a stronger hemostatic ability than keratin hydrogel; stronger wound healing effect of Ins-K was found in the early regeneration stage; more smooth skin tissues at excision section were obtained treatment with Ins-K hydrogel |
| Kaur et al. [25] | 150 μM to 15 mM | Carbopol 980 gel; insulin-loaded silver nanoparticles (AgNPs) | In vitro: HEKa cells; in vivo: male Wistar rats; diabetic and healthy rats | Higher wound healing activity in higher hyperglycemic condition; improvement in collagen deposition; insulin regulates the early inflammatory phase; rapid decrease in pro-inflammatory cytokines and an increase in anti-inflammatory cytokine antibacterial activity |
| Ribeiro et al. [26] | 0.5 IU | Chitosan gel; insulin-loaded chitosan nanoparticles | Diabetes mellitus animal model using Wister rats | Stimulate inflammatory cell and angiogenesis; improve wound maturation in diabetic rats |
| Zhu et al. [21] | 10 mg/mL | Oxidized hyaluronic acid/succinyl chitosan gel; insulin-loaded micelles | In vitro: 24-well plates; in vivo: Type 1 diabetes male Sprague-Dawley rats | The rate of insulin release depends on the glucose concentration in the wounded tissue; high biocompatibility and low cytotoxicity; promotion of fibroblast proliferation and tissue internal structure integrity, as well as the deposition of collagen and myofibrils; combining insulin with epidermal growth factor resulted in even more effective wound healing |
| Ostróżka-Cieślik et al. [27] | 1 mg/g (0.1% w/w) | Carbopol Ultrez 10, Carbopol Ultrez 30, methyl cellulose, glycerol ointment | In vitro: enhancer cel; cellulose dialysis membrane | Insulin release from the formulations occurs in a prolonged manner; methyl cellulose-based hydrogel released API, reaching 75% after 9 h |
| Chakraborty et al. [28] | 0.2 IU/g | Aloe vera gel; insulin-loaded nanoemulsion | Diabetic rats | Greater wound contraction (75% in 15 days); improvement in the skin histological architecture; gel is non-irritant and is safe for topical use; aloe vera with insulin-loaded nanoemulsion showed synergistic effect |
| Quitério et al. [1] | 10 mg/g (1% w/w) | Pluronic ® F 127 gel; insulin-loaded PLGA nanoparticles | Human keratinocytes cells, female mice, | Insulin was completely released from NPs and its structure was preserved; in vitro release studies suggested a controlled release profile (5 µg/cm2/8 h); improves wound healing without causing side effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A.; Przybyła, M.; Wójcik, W.; Birówka, K.; Majczyna, M.; Dolińska, B. Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing. Med. Sci. Forum 2023, 21, 17. https://doi.org/10.3390/ECB2023-14290
Ostróżka-Cieślik A, Przybyła M, Wójcik W, Birówka K, Majczyna M, Dolińska B. Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing. Medical Sciences Forum. 2023; 21(1):17. https://doi.org/10.3390/ECB2023-14290
Chicago/Turabian StyleOstróżka-Cieślik, Aneta, Marcin Przybyła, Weronika Wójcik, Klaudia Birówka, Marta Majczyna, and Barbara Dolińska. 2023. "Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing" Medical Sciences Forum 21, no. 1: 17. https://doi.org/10.3390/ECB2023-14290
APA StyleOstróżka-Cieślik, A., Przybyła, M., Wójcik, W., Birówka, K., Majczyna, M., & Dolińska, B. (2023). Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing. Medical Sciences Forum, 21(1), 17. https://doi.org/10.3390/ECB2023-14290








