1. Introduction
A major peril among infectious diseases, which account for millions of deaths across the globe per year is a bacterial infection [
1]. The use of antibiotics or chemicals is highly recommended as a therapy. However, there are instances of the development of multiple drug-resistant strains and incomplete bioactivity of these chemical drugs. Furthermore these chemical drugs have been reported to have a profound impact on liver, kidney and several other vital organs in the human body, which can have a deleterious cytopathic effect [
2]. Therefore, the search for novel antimicrobials for emerging and reemerging bacterial diseases is urgent. Mangroves coastline forests are well known for their ecological significance. Many bioactive substances from different mangrove species, including steroids, triterpenes, saponins, flavonoids, alkaloids and tannins have been reported to have therapeutic potentials [
3]. Numerous studies have demonstrated the effectiveness of these plants against pathogens that affect people, animals and plants. In comparison to seaweeds and sea grasses, it has also been claimed that these are an excellent source of antiviral compounds [
4]. They were described as having incredible potential against cancer cells by Boopathy and Kathiresan [
5].
The present research study aims to screen the bioactive components from
Ceriops Tagal, Rhizophora Mucronata, Brugeria gymnoriza and
Agicerous corniculatom against the bacteria causing respiratory, soft tissue and gastrointestinal infections.
Ceriops tagal is a small tree with short buttresses and knee-like breathing roots. The bark of
C. tagal has been used for the treatment of infected wounds in Thailand and obstetric and hemorrhagic conditions in the Philippines. The species is also used to treat sores, hemorrhages, malignant ulcers and malaria in Asian countries. In the Philippines, the reddish-brown ground bark of
C. tagal is added to toddy (an alcoholic drink from the inflorescence sap of coconut) as a preservative to delay the fermentation process by controlling spoilage microbes [
6].
Rhizophora mucronata is found in the Indo-Pacific region on the banks of rivers and on the edge of the sea. It has long been traditionally used for the treatment of elephantiasis, hematoma, hepatitis, ulcers and febrifuge. Its leaf is being used in the folk medicine for treating diarrhea or gastric motility disorder [
7].
Bruguiera gymnorrhiza (L.) (Rhizophoraceae) is an evergreen mangrove tree, widely distributed in tropical and subtropical coastlines. This plant has exhibited anti-tumor activity against HepG2 hepatoma cells, antibacterial activity against selected microbial species and germicidal activity.
Aegiceras corniculatum is a mangrove plant grows in the wetland of tropical and subtropical regions of Indus Delta valley of Pakistan, Western coastline of India. It has a potential effect in many diseases such as diabetes, inflammation, rheumatism, cardio vascular diseases, etc.
A. corniculatum has been used as folklore medicine for a long time, but there is not much scientific evidence available to justify its medicinal use
The present research study highlights the screening and quantification of bioactive compound of the four plants under study applying in silico tools basal ADME has also been gauged. The antibacterial potentials have been screened against Klebsiealla Pneumonia, E.coli, S.aureus and Bacillus subtilis.
2. Material and Methods
Four different species of mangroves were collected from a mangrove nursery established at shores of Gorai, Mumbai, India. Collected Plant samples were maintained in the greenhouse of SBBDYPUNM, India, under favorable conditions until further studies.
2.1. Extract Preparation
Plant leaves were sorted (less bruised) and washed under running tap water. Leaves were then ground to a fine powder using a mortar and pestle with the help of liquid nitrogen. Then, the extract was packed in a thimble and was extracted using different solvents such as petroleum ether, chloroform, methanol, etc., for 3 h. Resulting extracts in different solvents were evaporated and concentrated to dryness using the rotary evaporator at 50 °C. Powder was dissolved in the solvents used for extraction [
8].
2.2. Qualitative and Quantitative Estimation of Bioactive Compounds
A preliminary qualitative screening of bioactive compounds from plants under study was followed by their quantitative estimation. The procedures described by Syahidah and N Subekti (2019) [
9] were adopted for the study with some modifications.
2.3. Alkaloid Determination
An amount of 5 g of each dried ground sample was placed in a 250 mL beaker. Then, 200 mL of 10% acetic acid in ethanol was added, and the mixture was covered then allowed to stand for 4 h. This was filtered, and the extract was concentrated in a water bath to 1/4 of the original volume. Concentrated ammonium hydroxide was added dropwise to the content until precipitation was complete. The resulting solution was allowed to settle, and the precipitate was collected and washed with dilute ammonium hydroxide and then filtered. The residue was the alkaloid, which was dried and weighed.
2.4. Tannin Determination
Here, 500 mg of each sample was placed in a 50-plastic bottle. Then, 50 mL of distilled water was added and shaken for 1 h in a mechanical shaker. This was filtered into a 50 mL volumetric flask and made up to the mark. Then, 5 mL of the filtrate was pipetted into a test tube and mixed with 2 mL of 0.1 M ferric chloride in 0.1 N hydrochloric acid and 0.008 M potassium ferrocyanide. The absorbance was measured at 280 mm within 10 min. Tannin contents were expressed as a percentage of the dried faction.
2.5. Flavoniod Determination
At this stage, 1 g of each ground sample was extracted repeatedly with 100 mL of 80% aqueous methanol at room temperature. The whole solution was filtered through Whatman filter paper No. 42 (125 mm). The filtrate was later transferred into a crucible and evaporated to dryness (constant weight) over a water bath. The flavonoid content was calculated as a percentage of the dried faction
2.6. Reducing Sugars Determination
Now, 1 g of each ground sample was diluted with water (10 mL) and titrated with a standard Benedict reagent. The sample was hydrolyzed with standard acid (0.5 NHCl). The hydrolyzed fraction gave the total reducing sugars. The results obtained were calibrated using the standard curve of glucose.
2.7. Estimation of Total Phenolic Content
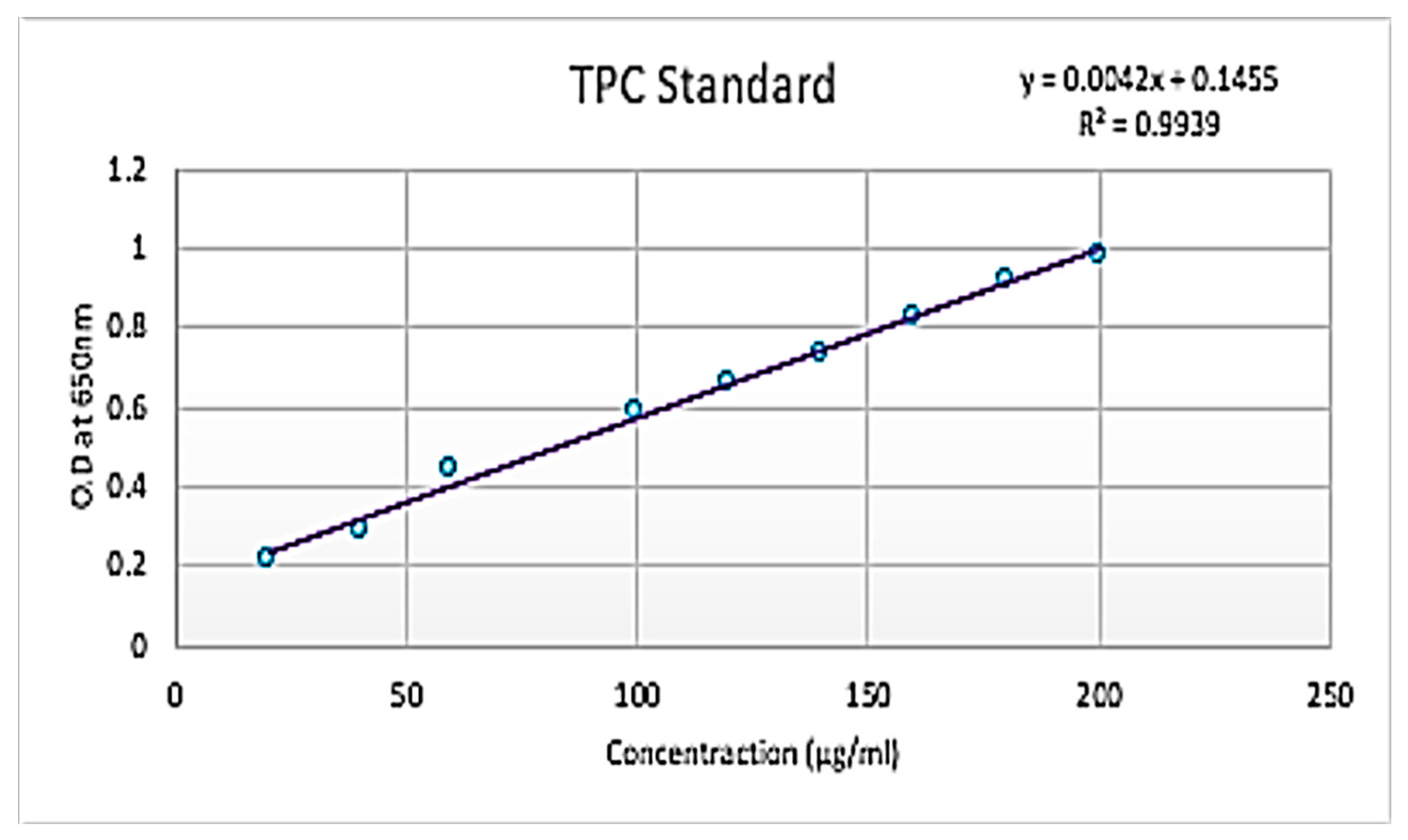
The Folin–Ciocalteu method will be used to estimate the total phenolic content. An amount of 1 mL of extract solution of concentration from 100–500 μg/mL will be added to 2.5 mL of 10% (w/v) Folin–Ciocalteu reagent. Then, 2 mL of 75% Na2CO3 will be mixed into the above solution after 5 min and will be incubated at 500 C for 10 min. Then, the sample will be cooled, and absorbance will be measured at 765 nm by a UV spectrophotometer against the blank solution. The data will be expressed as mg/g of gallic acid equivalents in milligrams per gram of dry extract.
2.8. Estimation of Flavonoids
The mixture of 10 mL solution will be made by mixing 1 mL of plant extract, 3 mL of 70% ethanol, 0.2 mL of 10% aluminum chloride, 0.2 mL of potassium acetate (1 M) and 5.6 mL of distilled water. The solution will be incubated for 30 min at room temperature, and then a UV spectrophotometer at 415 nm will be used to measure the absorbance of the solution against the blank solution. An amount of 5 mg of Quercetin will be mixed with 1 mL of methanol to prepare the stock solution, and then different concentrations (5–200 μg/mL) of standard quercetin solution will be prepared. The concentration of total flavonoid content in the test samples will be calculated from the calibration plot (Y = 0.0162x + 0.0044, R2 = 0.999) and expressed as mg quercetin equivalent (QE)/g of dried plant material. All the determinations were carried out in triplicate.
2.9. Antioxidant Activity (DPPH) Free Radical Scavenging Assay
Here, 1 mL of 0.135 mM DPPH in methanol solution will be added to tubes containing 1 mL of plant extracts, vitamin C and gallic acid at varying doses (0.2–1.0 mg/mL). The mixture will be shaken and then kept at room temperature for 30 min in the dark. After that, the mixture’s absorbance will be measured spectrophotometrically at 517 nm. As benchmarks, vitamin C and gallic acid will be utilized.
The DPPH radical scavenging activity will be calculated by:
where
Abs control is the absorbance of DPPH and methanol, and
Abs sample is the absorbance of DPPH radical + sample extract or standards (Vitamin C and gallic acid).
2.10. Antibacterial Activity
A bacterial culture was prepared by inoculating a fresh colony from an overnight culture into a sterile broth medium and incubating it for 4–6 h at the appropriate temperature with agitation until the bacterial density reaches 0.5 McFarland standard (1–2 × 10
8 CFU/mL). Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae and Bacillus subtilis were the strains used in this assay. Serial dilutions were prepared for the test sample in a suitable broth medium (e.g., Mueller–Hinton broth) in a 96-well plate, starting from the highest concentration. Add the bacterial inoculum to each well, except for the negative control wells (only broth medium). Positive control wells with known antibacterial agents are prepared to ensure the validity of the assay. Cover the plate and incubate it at the appropriate temperature (e.g., 37 °C) for 18–24 h without agitation. After incubation, the MIC (minimum inhibitory concentration) is defined as the lowest concentration of the test sample that completely inhibits the visible growth of bacteria. MIC was determined by visually inspecting the wells or by using a microplate reader to measure the optical density (OD) at 600 nm [
8].
2.11. In Silico Study
Here, we have derived the small chemical structures, from the literature sources and PubChem chemical compound database [
10]. The compounds were initially screened for physiochemical property, drug likeness, ADME properties, PAINS, Brenk alerts and synthetic accessibility using SWISS-ADME online server [
11].
3. Results
Preliminary phytochemical screening revealed the presence of alkaloid, flavonoids, phenolic and tannin in both of the acetone and methanol extracts. On the contrary, terpenoid, steroid and saponin were absent (
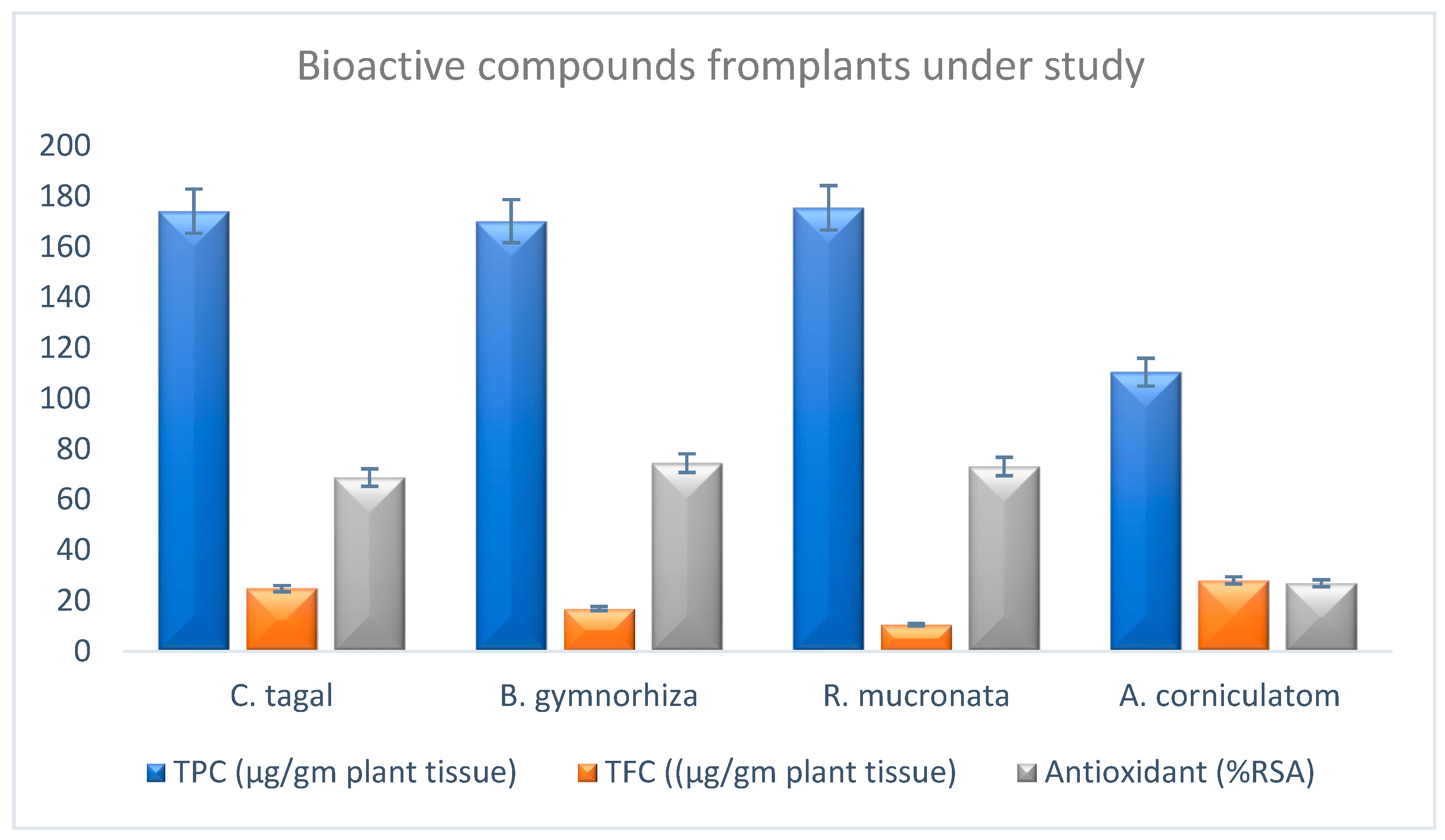
Figure 1). The total phenolic content of four mangrove samples in their crude and 1:10 diluted forms was calculated and found to be 00.07274, 0.87119, 0.83833, 0.851305, 0.8625, 0.8625, 0.87785, 0.536425 and 0.5525 GAE/wet weight (Gallic acid equivalent) for crude (A), 1:10 (A), 1:10 (A), Crude (B), 1:10 (B), Crude (C), 1:10 (C), Crude (D) and 1:10 (D), respectively. The total flavonoids content of four mangrove samples in their crude and 1:10 diluted forms was calculated and found to be −0.57115, 1.24231, 0.53846, 0.848075, 1.33846, −0.52692, 2.10565 and 13.9038 GAE/wet weight (gallic acid equivalent) for crude (A), 1:10 (A), crude (B), 1:10 (B), crude (C), 1:10 (C), Crude (D) and 1:10 (D), respectively. The study revealed that flavonoids, alkaloids and phenol were found to be present in the extract. Several studies reported that flavonoids show anti-allergic, anti-inflammatory, anti-microbial and anti-cancer activity.
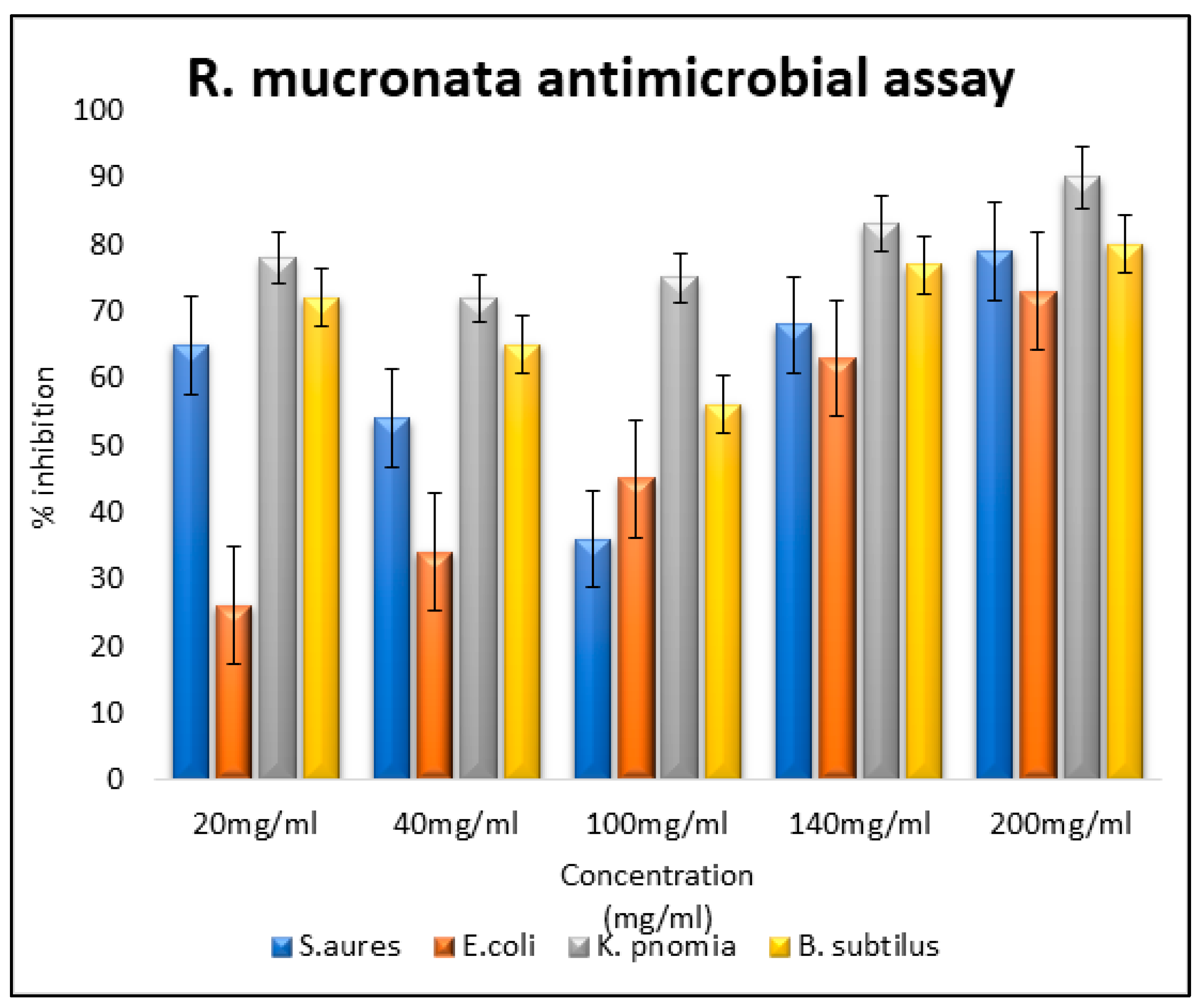
The antioxidant potential (radical scavenging activity) was scored to be 87.8%, 89.5%, 92.07% and 45.8% (DPPH assay was conducted) (
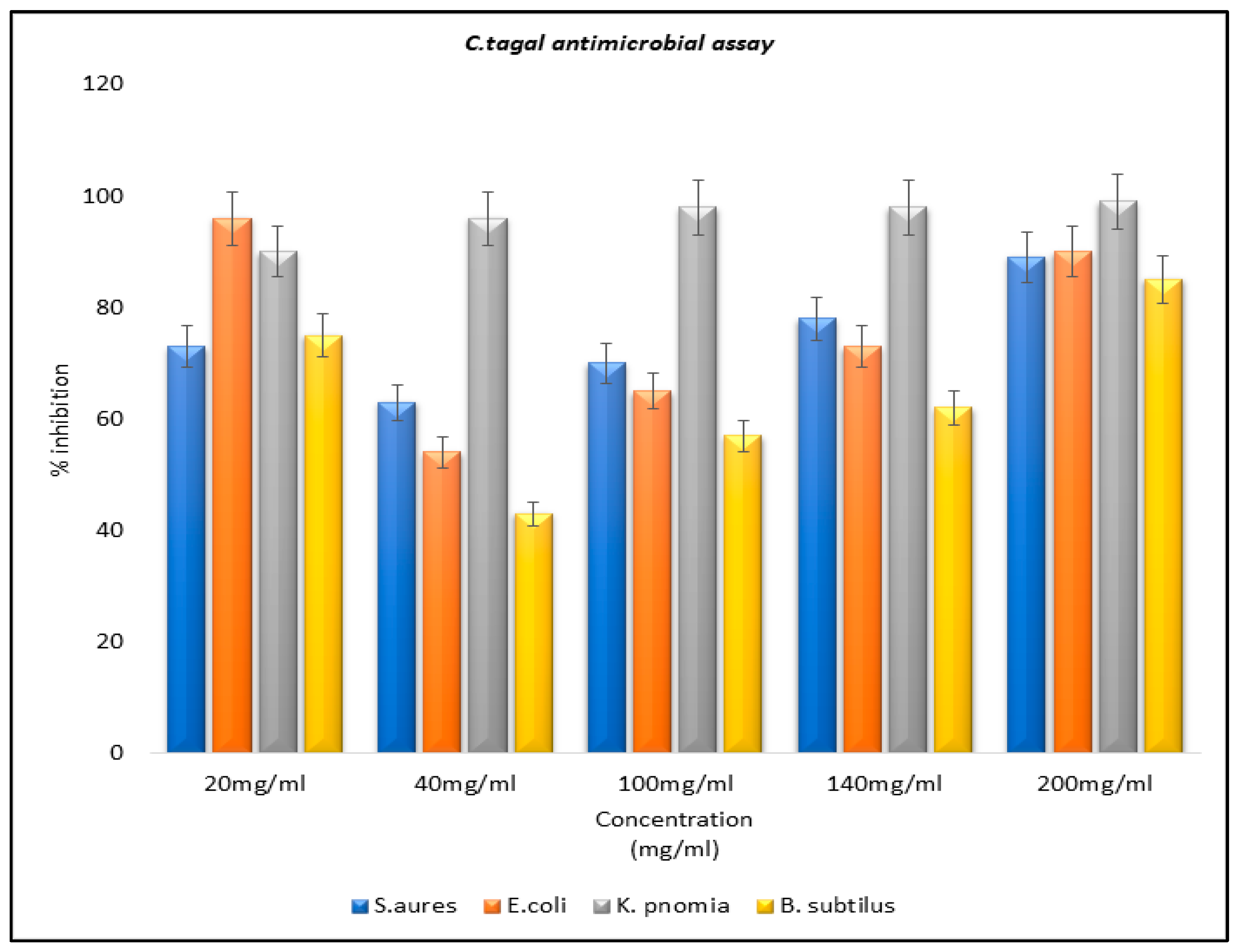
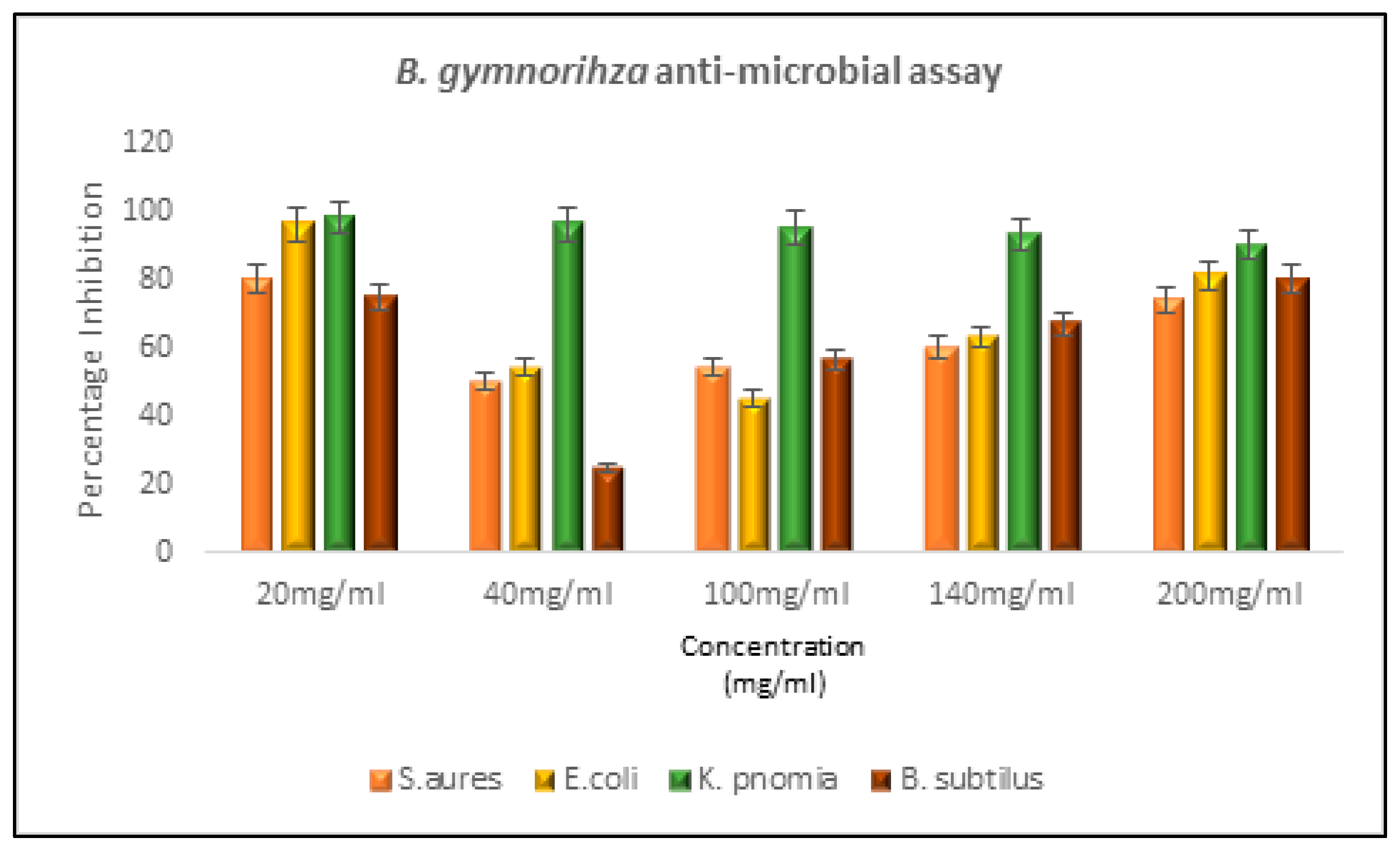
Figure 2). Antibacterial assays for the plant extracts of mangrove plants and for the controls were also carried out. The extracts each exhibited a different percentage of inhibitions for different microbial strains under study (
Figure 3,
Figure 4 and
Figure 5). The antimicrobial analysis was performed on
Staphylococcus aureus, Escherichia coli,
Klebsiella pneumoniae and
Bacillus subtilis. The MIC revealed maximum activity in
Klebsiella pneumonia, while negligible activity was scored for
Staphylococcus aureus and
Escherichia coli.
Riposte of In-Silico Analysis
Here, we have considered 25 molecules to be screened for the drug likeness, ADME properties, metabolizing factor, bioavailability, PAINS, Brenk alert and synthetic accessibility. Molecules 4, 5, 10 and 17 have exhibited acceptable properties with high gastro-intestinal (GI) absorption, being permeable to the CNS; i.e., these compounds are permeant to the BBB (blood–brain barrier). These molecules are predicted to be non-substrate to the p-glycoprotein (No-Pgp substrate); hence, the efflux activity is inhibited against the molecules. As these compounds are predicted for non-inhibitor to the CYP450-class of enzymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6 and CYP3A4); hence, the molecules are known to be the substrate of the CYP45 class of enzymes and are readily metabolized and easily excreted from the system. The molecules exhibiting zero Lipinski violation led to high drug likeness activity. A bioavailability score higher than 0.55 is a good indicator. PAINS reported and Brenk alerted no to the minimum value exhibiting a minimum toxicity, whereas the synthetic accessibility depicts a good value for synthesis (
Table 1).
4. Discussion
Mangroves are one of the most prolific and unmapped ecosystems, roughly covering one fourth of the world’s coastline with a high assortment of thriving organisms. Mangroves support the conservation of biological diversity for a number of endangered species by providing habitats, nurseries, nutrients and spawning grounds [
12]. Mangroves also play a key role in human sustainability and livelihoods, being heavily used for food, timber, fuel and medicine. They offer protection from calamitous events, such as tsunami, tropical cyclones and tidal bores and can dampen shoreline erosion [
13].
Flavonoids are now considered an indispensable component in a variety of nutraceutical, pharmaceutical, medicinal and cosmetic applications. This is attributed to their anti-oxidative, anti-inflammatory, anti-mutagenic and anti-carcinogenic properties coupled with their capacity to modulate key cellular enzyme function [
9]. Alkaloids have been reported as powerful poison, and many alkaloids derived from medicinal plants show biological activities such as anti-inflammatory, antimalarial, antimicrobial, cytotoxicity, antispasmodic and pharmacological effects. Similarly, steroids derived from plants are known to have cardiotonic effect and also possess antibacterial and insecticidal properties. They are very often used in medicines due to their well-known biological activities. Tannins, according to research, are known to have antibacterial, anti-tumor and antiviral activities. These alkaloids show bioactivity against Gram-positive bacteria and cytotoxicity against leukemia and HeLa cell lines. Alkaloids, flavonoids and xanthones are potent inhibitors of various oxidative processes in both in vitro and in vivo systems. These phytochemical compounds identified in the extracts may be responsible for the biological activities of the mangrove leave extract [
14].
Tannin and phenol are necessary for repair and maintenance. Polyphenolic compounds have an aromatic benzene ring with substituted hydroxyl groups, including their functional derivatives. These are able to absorb free radicals and can chelate metal ions that could catalyze the formation of ROS, which promotes lipid peroxidation. Among polyphenols, flavonoids are of great importance because they help the human body to fight against diseases. The ability of flavonoids to act as potent antioxidants depends on their molecular structures, the position of the hydroxyl group and other features in its chemical structure. They are abundantly found in plants as their glycoside. The most abundant flavonol, one which has a good antioxidant property, is quercetin, as it has all the right structural features for free radical scavenging activity [
15].
The findings of our study revealed that the methanol extracts of the mangroves exhibited substantial antibacterial effects against the bacterial strains tested. Notably, the maximum inhibition was observed against Klebsiella pneumoniae, indicating potential effects against bacterial infections of the respiratory and urinary systems. Since these plants are halophytes, which thrive under high salt stress conditions, our results suggest that these mangroves may produce certain bioactive compounds that hold promise for drug development to treat both acute and chronic diseases.
From the in-silico analysis, we can identify that molecules 4, 5, 10 and 17 have exhibited acceptable properties of drug likeness and are within the acceptable toxicity range. Furthermore, further investigations are warranted to evaluate the antimycobacterial, antiviral and antiparasitic activities of these plant extracts. Additionally, other parts of these mangrove species should be studied to assess their potential as sources of novel antimicrobial agents.
5. Conclusions
The research found that the four plant species contain high amounts of organic compounds that have potential medicinal properties, including alkaloids, flavonoids, polyphenols, tannins and total proteins. DNA barcoding was used to identify the four plant species, and their results may have been submitted to NCBI for validation or comparison with other DNA sequences in the public database. The antibacterial activity of the four plant species was tested against three bacterial strains, and it was found that one plant species showed substantial microbial inhibition against Klebsiella, while another plant species showed moderate microbial inhibition against E. coli and Bacillus subtilis. The research suggests that the four plant species may have potential medicinal benefits for respiratory tract infections, based on their observed antibacterial activity against Klebsiella, a respiratory pathogen. However, further research is needed to confirm this hypothesis and investigate the safety and mechanism of the action of these plant compounds.