Promotion of Dermal Permeation of Bioactive Compounds Using a Microneedle Device †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Skin Permeation Experiments
2.3. Quantification of the Bioactive Compounds
3. Results and Discussion
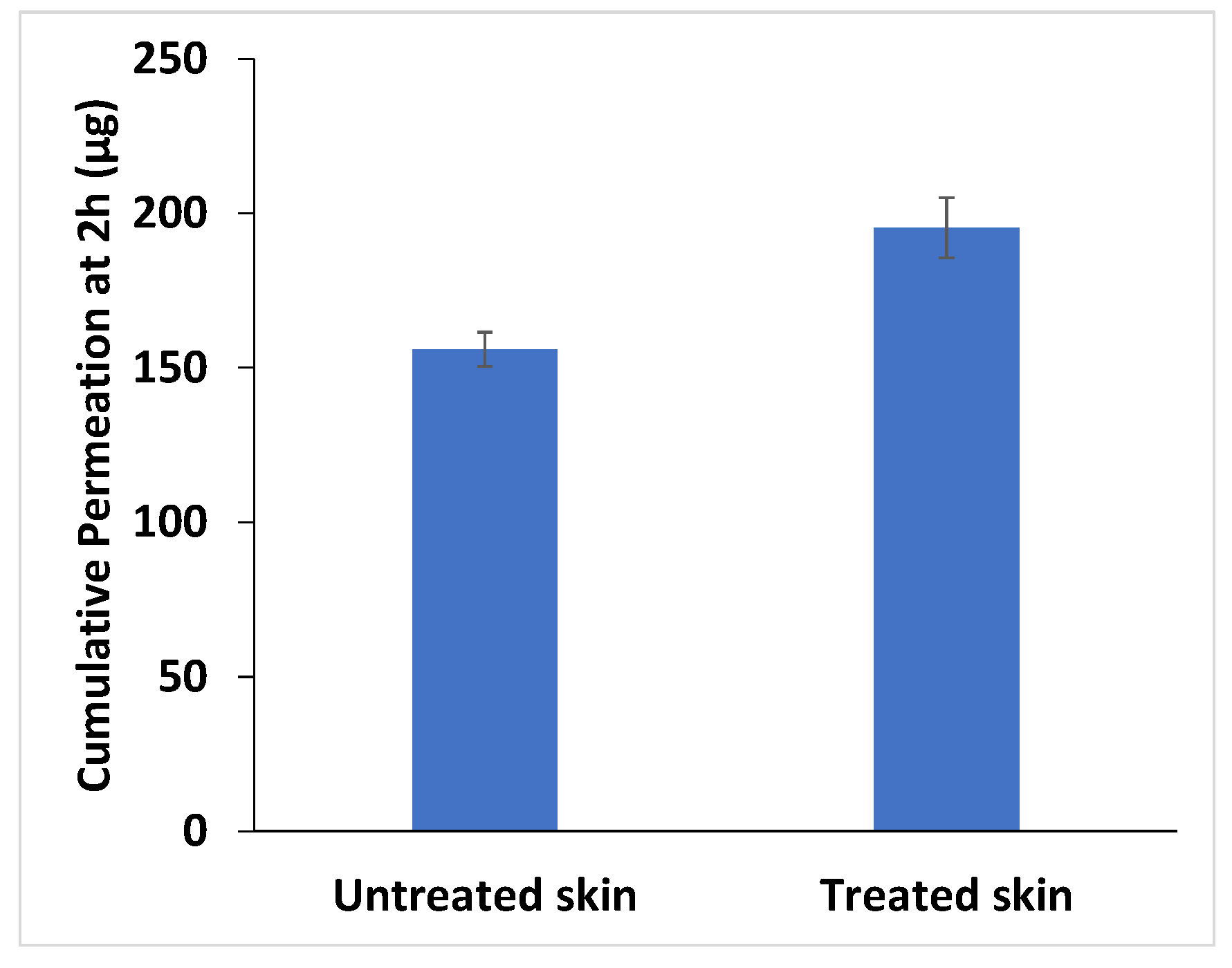
3.1. Effect of Microneedle Pre-Treatment on the Permeation of Caffeine through Pig Skin
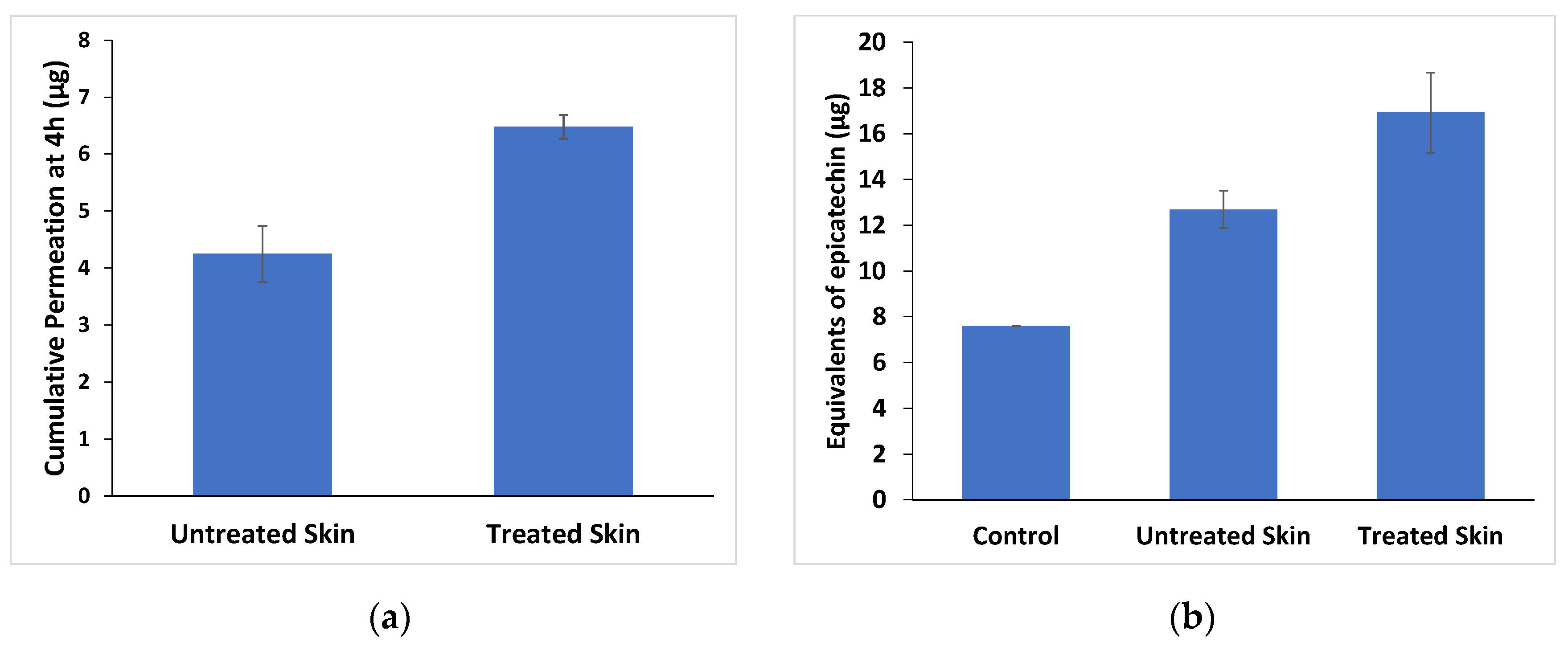
3.2. Effect of Microneedle Pre-Treatment on the Permeation of Epicatechin through Pig Skin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Lou, Y.R.; Xie, J.G.; Peng, Q.Y.; Liao, J.; Yang, C.S.; Huang, M.T.; Conney, A.H. Topical applications of caffeine or (-)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar] [CrossRef] [PubMed]
- Inhibitory Effects of Tea and Caffeine on UV-Induced Carcinogenesis: Relationship to Enhanced Apoptosis and Decreased Tissue Fat—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12570332/ (accessed on 6 July 2022).
- Heinrich, U.; Moore, C.E.; de Spirt, S.; Tronnier, H.; Stahl, W. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Marques-da-Silva, D.; Diniz, M.; Daglia, M.; Bishayee, A. Molecular mechanisms linking environmental toxicants to cancer development: Significance for protective interventions with polyphenols. Semin. Cancer Biol. 2022, 80, 118–144. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Marques-da-Silva, D.; Lagoa, R. Reassessment of the experimental skin permeability coefficients of polycyclic aromatic hydrocarbons and organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2021, 86, 103671. [Google Scholar] [CrossRef] [PubMed]
- Wisuitiprot, W.; Somsiri, A.; Ingkaninan, K.; Waranuch, N. In vitro human skin permeation and cutaneous metabolism of catechins from green tea extract and green tea extract-loaded chitosan microparticles. Int. J. Cosmet. Sci. 2011, 33, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hsieh, M.F.; Ho, Y.N.; Huang, C.M.; Lee, J.S.; Yang, C.Y.; Chang, Y. Enhancement of catechin skin permeation via a newly fabricated mPEG-PCL-graft-2-hydroxycellulose membrane. J. Membr. Sci. 2011, 371, 134–140. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Fang, C.W.; Suhail, M.; Lam Vu, Q.; Chuang, C.H.; Wu, P.C. Improved skin permeability and whitening effect of catechin-loaded transfersomes through topical delivery. Int. J. Pharm. 2021, 607, 121030. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Vanat, P.; Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Metal alginates for polyphenol delivery systems: Studies on crosslinking ions and easy-to-use patches for release of protective flavonoids in skin. Bioact. Mater. 2020, 5, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Mohd Tohit, E.R.; Stanslas, J.; Salim, N.; Tuan Mahmood, T.M. Investigation and Optimization of Hydrogel Microneedles for Transdermal Delivery of Caffeine. In Tissue Engineering Part C: Methods; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2022. [Google Scholar] [CrossRef]
- Hao, Y.; Li, W.; Zhou, X.L.; Yang, F.; Qian, Z.Y. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.O.; Seo, S.; Choy, Y.B.; Prausnitz, M.R. A microneedle roller for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Luu, E.; Ita, K.B.; Morra, M.J.; Popova, I.E. The Influence of Microneedles on the Percutaneous Penetration of Selected Antihypertensive Agents: Diltiazem Hydrochloride and Perindopril Erbumine. Curr. Drug Deliv. 2018, 15, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Ita, K.B.; Morra, M.J.; Popova, I.E. The influence of solid microneedles on the transdermal delivery of selected antiepileptic drugs. Pharmaceutics 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violante, C.; Lagoa, R.; Marques-da-Silva, D. Promotion of Dermal Permeation of Bioactive Compounds Using a Microneedle Device. Med. Sci. Forum 2022, 11, 4. https://doi.org/10.3390/BiTaP-12840
Violante C, Lagoa R, Marques-da-Silva D. Promotion of Dermal Permeation of Bioactive Compounds Using a Microneedle Device. Medical Sciences Forum. 2022; 11(1):4. https://doi.org/10.3390/BiTaP-12840
Chicago/Turabian StyleViolante, Cristiana, Ricardo Lagoa, and Dorinda Marques-da-Silva. 2022. "Promotion of Dermal Permeation of Bioactive Compounds Using a Microneedle Device" Medical Sciences Forum 11, no. 1: 4. https://doi.org/10.3390/BiTaP-12840
APA StyleViolante, C., Lagoa, R., & Marques-da-Silva, D. (2022). Promotion of Dermal Permeation of Bioactive Compounds Using a Microneedle Device. Medical Sciences Forum, 11(1), 4. https://doi.org/10.3390/BiTaP-12840






