Abstract
Systemic habitat destruction over the last 100 years combined with major anthropogenic stressors such as aquatic contaminants, exotic species, and economic endeavors is driving the decline in freshwater unionid species diversity. Global temperatures continue to increase, with January 2024 being the warmest on record according to the latest report by the World Meteorological Organization. Freshwater mussels play a crucial role in aquatic ecosystems, contributing significantly to benthic processes in rivers and streams, yet they remain highly sensitive to environmental changes. This study specifically investigates the thermal tolerance of the Texas pigtoe (Fusconaia askewi) under elevated temperature conditions and explores the implications for developing effective conservation strategies in freshwater ecosystems. Eighty-four individual adult Texas pigtoe mussels were collected from the upper Sabine River near Hawkins, Texas, and taken to the University of Texas at Tyler to evaluate the effects of elevated temperature, a likely factor impacting mussels in East Texas. In the thermal tolerance study presented here, 100% survival occurred at both the control (20 °C) and the 25 °C test points. The 30 °C treatment group had an overall mortality of 14% and the 35 °C treatment group showed a mortality rate of 43% by the end of the trial, suggesting the typical summer temperatures in Texas streams will result in the loss of a portion of an otherwise healthy population.
1. Introduction
The invertebrate phylum Mollusca consists of approximately 100,000 freshwater, marine, and terrestrial species. About one-fifth of the molluscan phylum consists of the class Bivalvia which includes marine and freshwater animals that have bodies enclosed by two hinged shells. Just over 1200 species of freshwater bivalves are recognized, of which the largest order is Unionoida, with nearly 850 species found worldwide [1]. Of those, over 500 species are included on the IUCN Red List of Threatened Species [2]. Overall, this faunal group is rapidly decreasing in numbers not only in North America where they have their greatest diversity with some 300 species, but across the world [3,4,5,6]. The IUCN Red List has 301 species listed as extinct worldwide, with 135 of those in North America. Currently, freshwater mussels make up the largest group, by percentage, of federally listed endangered or threatened invertebrates [7]. Systemic habitat destruction, combined with significant anthropogenic stressors such as aquatic contaminants, invasive species, and economic development, has critically reduced the diversity of freshwater mussels worldwide.
One of the primary threats currently facing mussel assemblages is rising temperatures from climate change [8]. Mussels are considered thermo-conformers whose body temperature, and corresponding physiological processes, are regulated by the external environment. Mussels are limited in their mobility as they are predominately sessile and further limited by physical barriers in freshwater systems [9]. The environmental stress of temperature has been shown to influence mussel mortality through altered behavior and is a critical factor in mussels’ ability to regulate physiological processes [10]. The change in average surface temperature is predicted to increase by 1.5 °C by the end of the century [11]. With this increase, temperatures in surface waters also will rise [12]. Recent studies have shown that the ability of mussels to exhibit a physiological tolerance to changes in temperature explains in part the survival of certain species [13].
The freshwater mussel used in this study, Fusconaia askewi (Texas pigtoe), is a species endemic to east Texas rivers and is recognized as near threatened on the IUCN red list, as special concern by the American Fisheries Society, and as threatened by the Texas Parks and Wildlife Department. While a species of concern, the Texas pigtoe has stable populations and is a good surrogate for less common, threatened species such as the Louisiana pigtoe (Pleurobema riddellii) [14,15]. To assess the effects of environmental temperature stress, we exposed individual F. askewi to increasing temperature over time. Specifically, laboratory experiments were used to determine how temperature affects mortality by asking two key questions: (1) What are the thermal tolerance limits of F. askewi under controlled laboratory conditions? (2) How can these findings inform conservation strategies to protect freshwater mussel populations in Texas?
2. Materials and Methods
The Collection and Laboratory Housing
The Sabine River in east Texas was chosen as the collection site for this study. Texas pigtoe, while listed as threatened in Texas, is still found to be locally abundant within certain watersheds [16]. Recent surveys assessing unionid mussels in northeast Texas rivers found Texas pigtoe to be common in many sites in the upper Sabine River and in a wide range of age classes, suggesting active recruitment [17]. A total of 84 individual adult mussels (mean length, 58.7 ± 13.8 mm ± 1 SD) were collected from the upper Sabine River near Hawkins, Texas in April 2017 and testing began in the summer of 2017. Two separate riffle areas about one-half mile south of FM Road 14 crossing were easily accessible by boat or kayak during the summers and were shallow enough for hand collection. Historical daily discharge of the river is variable between 45 cfs to 18,500 cfs [USGS, 2007]. The river had areas of small cobble where mussels and shells were evident and F. askewi was shown to be abundant in both timed and density surveys [18].
Water chemistry data were gathered at the time of collection (mean field parameters: pH—7.5, dissolved oxygen—6.7 mg/L, temperature—24 °C, and conductivity—181 µm/cm) to allow close approximation of field conditions in the laboratory. Summer temperatures recorded in the Neches River, a similar Texas river to the Sabine, in 2017 were 30 °C throughout June and July and recorded as high as 30 °C in August [19]. Mussels were transported back to the lab at the University of Texas at Tyler within 1 h of collection where they were weighed, measured, and tagged before being placed in flow-through acclimation tanks (custom built, UT Tyler, Tyler, TX, USA) at a holding temperature of 20 °C. Specimens were acclimated to laboratory conditions for at least two weeks before testing and fed 100 mL of phytoplankton mixture containing Nannochloropsis, Tetraselmis, and Isochrysis sp. (Kent Marine PhytoPlex, Central Garden and Pet, Walnut Creek, CA, USA) every other day [13,20].
The thermal tolerance of F. askewi was assessed by exposing 84 individuals (21 individuals per treatment) to water temperatures of 20 °C, 25 °C, 30 °C, and 35 °C over 21 days in controlled laboratory settings. Temperature thresholds were selected based on field observations of typical summer water temperatures in east Texas rivers, which range from 20 °C to 35 °C during peak seasons, to closely simulate realistic environmental stressors [19]. The baseline (control) temperature was 20 °C, which was also the holding temperature. The experimental units were two, large 1500 L continuous-flow fish tanks containing approximately 15 cm of small river rock and coarse sand. Each tank had intake valves and was connected to a 946 L plastic reservoir, so the water could be pumped and recirculated through biological filters. This setup mirrored the flowing water in a riverine system and the cobble (>6–25 cm) substrate of the mussels’ natural environment. Deionized water was used; however, additionally, De*nitrate (Seachem Labs., Madison, GA, USA) was used to filter out nitrates, nitrites, ammonia, and organics, and PhosGuard (Seachem Labs., Madison, GA, USA) was used to remove phosphate and silicate. Filters were replaced before each new trial. Each unit was controlled within 1 °C by using an Arctica Titanium Chiller (Transworld Aquatic Enterprises, Inglewood, CA, USA) and an 1800-watt, 120-volt industrial heater (Process Technology, Willoughby, OH, USA) both with digital temperature control.
Mussels were acclimated to the test temperature by increasing water temperatures ≤ 3 °C per day [21]. Water quality was monitored daily with a Hydro Tech HYDROLAB Compact DS5 (OTT HydroMet, Sterling, VA, USA) for temperature, pH, and dissolved oxygen following the Standard Guide for Conducting Laboratory Toxicology Tests with Freshwater Mussels (ASTM, 2006). Temperature data loggers (iButtons, Alpha Mach, Inc., Mont St-Hilaire, QC, Canada) were used to monitor the consistent temperature every minute for the duration of each trial [14]. Mortality was recorded daily and used to create a Kaplan–Meier survivorship curve for visualizing population decline over time and to determine the lethal range of temperatures for adult F. askewii mussels [22]. Further, the Log-rank (Mantel–Cox) test evaluated the different temperature trials for variance.
3. Results
At the control temperature of 20 °C and the 25 °C test point, all individuals survived the duration of the experiment. However, mortality rates increased significantly at higher temperatures, with 14% of individuals succumbing at 30 °C and 43% at 35 °C (Figure 1). The Log-rank (Mantel–Cox) test shows the 30 °C and 35 °C curves are significantly different (p value < 0.0001).
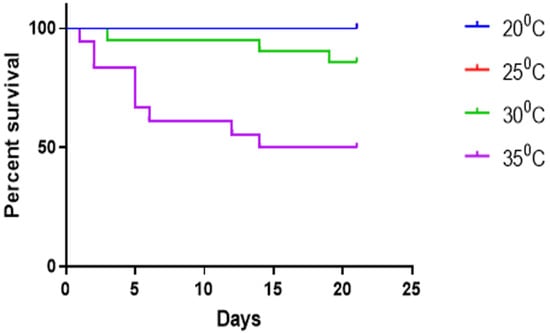
Figure 1.
Kaplan–Meyer survival curve showing mortality in Texas pigtoe at different water temperatures. Note that 25 °C also had 100% survival.
4. Discussion
These findings highlight critical thermal thresholds that may lead to substantial population declines under projected climate warming scenarios, emphasizing the vulnerability of F. askewi to elevated temperature stress.
- Ecosystem Services
The mortality observed in Texas pigtoe mussels exposed to increasing thermal stress highlights a variable which increases this species’ risk of extinction, considering that the 30 °C temperature at which mortality is seen in Texas pigtoe is noted as a summer water temperature for east Texas rivers [19]. The 14% mortality observed at 30 °C over 21 days suggests that the typical summer temperatures in Texas streams could contribute to the loss of a portion of an otherwise healthy population. Mussels are capable of burrowing into sediment to avoid extreme temperatures, but in a relocation study on the Sabine River F. askewi was shown to be less active with slower overall movement in summer months as compared to winter months [23]. Declines in mussel populations diminish essential ecosystem functions, including water filtration, nutrient recycling, and sediment stabilization, which are vital for maintaining the health and stability of aquatic ecosystems [24].
- Conservation Strategies
To mitigate these risks, conservation efforts should prioritize the creation of thermal refugia through riparian shading, river flow regulation to stabilize water temperatures, and the implementation of monitoring programs to detect early signs of thermal stress.
- Sub-lethal Effects
The long-term physiological effects of stress, such as thermal stress, in mussels are strongly related to a switch in metabolic processes from aerobic to anaerobic resulting in decreased function at all levels of biological organization [25]. Physiological evaluations done on Mytilus galloprovincialis, a commonly studied marine species, have shown sub-lethal responses such as reduced enzyme function, increased expression of heat shock proteins, and interrupted cell signaling [26]. Future research should focus on metabolic changes, reproductive impairments, and stress biomarker expression to comprehensively understand population-level impacts beyond immediate mortality. These types of studies can alert conservation managers to potential problems in a population before they become lethal.
5. Conclusions
This study reveals that F. askewi exhibits limited thermal tolerance, with significant mortality occurring at temperatures of 30 °C and above. These results underscore the urgent need for targeted conservation measures, including habitat restoration and thermal regulation, to bolster population resilience under the accelerating impacts of climate change.
Author Contributions
Conceptualization: S.R., M.W., and L.W.; methodology, S.R., M.W., and L.W.; formal analysis, S.R.; investigation, S.R.; resources, L.W.; data curation, S.R.; writing—original draft preparation, S.R.; writing—review and editing, M.W. and L.W.; visualization, S.R.; supervision, L.W.; project administration, L.W.; funding acquisition, S.R., M.W., and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Texas Comptroller of Public Accounts.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets analyzed from the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Neil Ford and Brent Bill for their assistance with design and analysis. Jarod Dickson assisted with field collections and experimental set-up. Marissa Quevedo helped with experimental set-up and data collection. Charlie Halyard designed and built the mussel housing system.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Haag, W.R. North American Freshwater Mussels: Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. 2023. Available online: https://www.iucnredlist.org (accessed on 1 January 2017).
- Lydeard, C.; Mayden, R.L. A diverse and endangered aquatic ecosystem of the southeast United States. Conserv. Biol. 1995, 9, 800–805. [Google Scholar] [CrossRef]
- Neves, R.J. Conservation and commerce: Management of freshwater mussel (Bivalvia: Unionoidea) resources in the United States. Malacologia 1999, 41, 461–474. [Google Scholar]
- Bogan, A.E. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. In Freshwater Animal Diversity Assessment; Springer: Dordrecht, The Netherlands, 2008; pp. 139–147. [Google Scholar] [CrossRef]
- Fritts, A.K.; Fritts, M.W.; Haag, W.R.; DeBoer, J.A.; Casper, A.F. Freshwater mussel shells (Unionidae) chronicle changes in a North American river over the past 1000 years. Sci. Total Environ. 2017, 575, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Haag, W.R.; Williams, H.D. Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 2014, 735, 45–60. [Google Scholar] [CrossRef]
- Ganser, A.M.; Newton, T.J.; Haro, R.J. Effects of elevated water temperature on physiological responses in adult freshwater mussels. Freshwater Biol. 2015, 60, 1705–1716. [Google Scholar] [CrossRef]
- Said, R.M.; Nassar, S.E. Mortality, energy reserves, and oxidative stress responses of three native freshwater mussels to temperature as an indicator of potential impacts of climate change: A laboratory experimental approach. J. Therm. Biol. 2022, 104, 103154. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, T.J.; Kwak, T.J.; Cope, W.G. Thermal tolerances of freshwater mussels and their host fishes: Species interactions in a changing climate. Freshw. Mollusk Biol. Conserv. 2012, 15, 69–82. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Covich, A.; Fritz, S.; Lamb, P.; Marzolf, R.; Matthews, W.; Poiani, K.; Prepas, E.; Richman, M.; Winter, T. Potential effects of climate change on aquatic ecosystems of the Great Plains of North America. Hydrol. Process. 1997, 11, 993–1021. [Google Scholar] [CrossRef]
- Andrade, J.; Cordeiro, N.I.; Montressor, L.C.; Luz, D.M.; Luz, R.C.; Martinez, C.B.; Pinheiro, J.; Paglia, A.P.; Vidigal, T.H. Effect of temperature on behavior, glycogen content, and mortality in Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae). J. Limnol. 2018, 77, 189–198. [Google Scholar] [CrossRef]
- Howells, R.G. Reproductive seasonality of freshwater mussels (Unionidae) in Texas. In Freshwater Mollusk Symposia Proceedings; Ohio Biological Survey: Columbus, OH, USA, 2000; pp. 35–48. [Google Scholar]
- Dickson, J. Habitat Associations and Detectability of Three Unionid Species Along the Upper Sabine River in East Texas. Master’s Thesis, University of Texas at Tyler, Tyler, TX, USA, 2018. [Google Scholar]
- Burlakova, L.E.; Campbell, D.; Karatayev, A.Y.; Barclay, D. Distribution, genetic analysis and conservation priorities for rare Texas freshwater molluscs in the genera Fusconaia and Pleurobema (Bivalvia: Unionidae). Aquat. Biosyst. 2012, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.B.; Gullett, J.; May, M.E. Diversity and abundance of unionid mussels in three sanctuaries on the Sabine River in northeast Texas. Tex. J. Sci. 2009, 61, 279–294. [Google Scholar]
- Ford, N.B.; Heffentrager, K.; Ford, D.F.; Walters, A.D.; Marshall, N. Significant recent records of unionid mussels in northeast Texas rivers. Freshw. Mollusk Biol. Conserv. 2014, 17, 8–15. [Google Scholar] [CrossRef]
- Texas Commission on Environmental Quality. Available online: https://www80.tceq.texas.gov/SwqmisPublic/index.htm (accessed on 1 January 2017).
- ASTM E2455-06; Standard Guide for Conducting Laboratory Toxicity Texts with Freshwater Mussels. ASTM International: West Conshohocken, PA, USA, 2006. [CrossRef]
- Galbraith, H.S.; Blakeslee, C.J.; Lellis, W.A. Recent thermal history influences thermal tolerance in freshwater mussel species (Bivalvia: Unionoida). Freshw. Sci. 2012, 31, 83–92. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Cope, W.G.; Arellano, C.; Bringolf, R.B.; Barnhart, M.C.; Hammer, E. Upper thermal tolerances of early life stages of freshwater mussels. J. N. Am. Benthol. Soc. 2010, 29, 959–969. [Google Scholar] [CrossRef]
- Griffin, L.M. Determing Best Practices for Freshwater Mussel Relocation Using Burrowing and Behavior. Master’s Thesis, University of Texas at Tyler, Tyler, TX, USA, 2015. [Google Scholar]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- De Vooys, C. Adaptation to anaerobic metabolism in two mussel species, Mytilus edulis and Mytilus galloprovincialis, from the tidal zone at Arcachon Bay, France. Neth. J. Sea Res. 1987, 21, 17–23. [Google Scholar] [CrossRef]
- Feidantsis, K.; Giantsis, I.A.; Vratsistas, A.; Makri, S.; Pappa, A.-Z.; Drosopoulou, E.; Anestis, A.; Mavridou, E.; Exadactylos, A.; Vafidis, D. Correlation between intermediary metabolism, Hsp gene expression, and oxidative stress-related proteins in long-term thermal-stressed Mytilus galloprovincialis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, R264–R281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).