Abstract
Muskellunge (Esox masquinongy) are a recreationally and ecologically important apex predator found throughout North America. In West Virginia, the genetic structuring and diversity of native muskellunge is poorly understood. The supplementary stocking of non-native muskellunge has further complicated the issue, as the introgression of non-native alleles and prevalence of non-native muskellunge post stocking remains unclear as well. Using ddRAD sequencing, several datasets were generated to investigate the population structure and genomic diversity of muskellunge in West Virginia. Populations stocked with New York-strain muskellunge exhibited significant introgression, with genetic composition diverging from unstocked native West Virginia populations. However, one population showed greater genetic similarity to native and unstocked populations despite New York-strain prevalence, suggesting resilience against genetic alteration. Fixed SNPs between the New York and West Virginia strains were identified that can be used for broodstock screening and the enhancement of native populations. A genetically distinct population was identified in the Little Kanawha River system, with this population having the highest levels of genomic diversity among native populations as well as a high number of private alleles. However, elevated inbreeding coefficients highlight potential conservation concerns for this unique population. This study establishes a genomic baseline for muskellunge in West Virginia and underscores the importance of preserving native genomic diversity while balancing the demands of recreational fishing programs.
1. Introduction
Muskellunge (Esox masquinongy) are native to central North America with an original distribution found throughout the upper Mississippi River basin, major tributaries of the Ohio, Tennessee, and Cumberland Rivers, and the Great Lakes, extending northwards through the southern reaches of the Hudson Bay watershed [1,2,3]. While originally designated as three separate sub-species based on color pattern and distribution, the muskellunge has since been identified as a single species as the color patterns were not limited to certain ranges, and widespread introductions have occurred throughout North America [4,5,6]. Throughout both their native and introduced range, muskellunge serve as an important recreational and ecological species. Recreationally, muskellunge are often managed as a trophy fish due to their large size potential, occurrence in low densities, and the difficulty associated with its elusive nature for recreational anglers, earning it the nickname “the fish of 10,000 casts” [7,8,9]. Ecologically, muskellunge can often serve as a bioindicator species due to their sensitivity to certain environmental disturbances on spawning habitats [10]. Muskellunge also serve as an apex predator within local ecosystems and are primarily piscivorous predators upon reaching adulthood [11].
Genetic techniques to enhance fishery conservation and assist management directives have been a commonly used method for decades. With the onset of next-generation sequencing (NGS) techniques and their more recent reductions in cost, the utilization of reduced genome representation and whole-genome sequencing techniques have become more prevalent in fishery research [12,13,14]. Genetic and genomic techniques often assist fishery management directives by combining genetic data with ecological and fishery datasets to create the best management practices to aid in the conservation of imperiled species and to bolster recreational opportunities [15,16]. Data resulting from these investigations can help in establishing genetic baselines to assist management directives, create single-nucleotide polymorphism (SNP) panels to identify the introgression of non-native species or genetic strains, improve the identification of unique genetic populations and stocks, identify populations with low levels of diversity or high rates of inbreeding, and assist in understanding the impacts of stocking on native populations [17,18,19,20,21].
Genetic investigations into muskellunge populations have largely been focused in the central and northern distribution of its range, with comparatively little research having been conducted in the eastern extent of the muskellunge range, including West Virginia. Initial genetic investigations of muskellunge in the state of West Virginia found limited gene flow from Ohio populations, where the Ohio River likely acts a barrier to gene flow, with muskellunge populations in Ohio and West Virginia exhibiting strong genetic differentiation [2]. The limited introgression and gene flow of introduced New York-strain hatchery muskellunge that were stocked into West Virginia to support management directives was also observed in the same study, with the New York strain and native West Virginia populations showing clear genetic distinction and a lack of New York ancestry in West Virginia populations [2]. Native muskellunge throughout the state of West Virginia would belong to the Ohio River strain, except for the far-eastern side of the state that sits in the Atlantic slope, where the Potomac River serves as the primary watershed where muskellunge are introduced, rather than the Ohio River [3].
Outside of West Virginia, genetics research has shown that, due to their philopatric nature, muskellunge show strong patterns of population structuring among spawning sites and low levels of gene flow and dispersal [22]. Across their range, a high degree of differentiation was observed, with multiple regions of the genome showing fixation statistics (FST) above 0.9 when comparing muskellunge hatchery broodstock from Iowa to the St. Lawrence River, Canada, with the Canadian population, showing increased levels of inbreeding [23]. The St. Lawrence River population has been found to be genetically differentiated from stocked sources, tributaries, and inland lakes, with stocking resulting in little admixture, likely due to high effective population sizes within the St. Lawrence River mitigating the impacts of introgression [24].
Insights into genetic structuring by spawning site, the variable impacts of stocking on native genetics depending on the study area, and the known differentiation between the New York and West Virginia strains highlight a clear knowledge gap in West Virginia. Further research is needed to assess the impacts of stocking and identify potential sub-structuring within the state. Given the known introduction of New York-strain muskellunge to assist in bolstering West Virginia muskellunge populations, limited genetic research on populations throughout the state, and the role of muskellunge as an apex predator in ecosystems throughout the state, it is crucial to quantify genetic structuring and assess the genetic diversity of muskellunge populations in the state to assist in the conservation of native genetic diversity and identify populations with depressed genomic diversity. Several study objectives are set forth to assist in establishing genomic baselines of muskellunge in West Virginia: 1. Assess the genetic structure of muskellunge populations across the state, including the potential introgression of New York-strain alleles. 2. Quantify the genomic diversity and assess inbreeding levels of muskellunge populations throughout the state to identify populations of conservation concern. 3. Identify diagnostic differences between West Virginia and New York-strain muskellunge that could be used for genomic screening and conservation strategies.
2. Materials and Methods
2.1. Sample Collection
Muskellunge broodstock were collected throughout the state of West Virginia between the years 2019 and 2023. Broodstock were sampled during their annual spawning season when water temperatures began to reach 9–15 °C [25]. All sampling was performed by the West Virginia Division of Natural Resources (WVDNR) following a standard protocol. Fin clips were collected using pulsed-DC (square waveform) electrofishing utilizing a Smith Root Apex box set at a 20–30% duty cycle, 60 Hz, and 300–450 volts. Voltage settings were set to achieve an approximately 5000-watt output and varied by water conductivity. Two netters were used on each boat, and boats were driven at approximately 4–6 kph both upstream and downstream. If necessary, the boat driver would maneuver the vessel to “chase” muskellunge that were encountered. Fin clips were collected in a few targeted areas that were inaccessible by electrofishing boats through angler submission (i.e., Sandy Creek). A total of 346 muskellunge from 9 locations were sampled for genomic investigations. Fin clips were taken from individual muskellunge, stored in individual coin envelopes, and sent to the Wild Genomics Lab at West Virginia University for genetic analysis.
2.2. Study Area
All sample sites were located within the state of West Virginia, United States of America, from a total of 9 sampling locations (Figure 1). These sampling locations were chosen due to being prominent muskellunge fisheries in the state, are sources of broodstock used for ongoing supplementary stocking regimes, are actively utilized by recreational anglers, and are actively managed by the WVDNR. Some populations have also been recently stocked with New York-strain muskellunge include the Buckhannon, West Fork, and Monongahela Rivers, Kimsey Run Lake, East Lynn Lake, and Stonewall Jackson Lake. The Buckhannon River is a 72 km long tributary to the Tygart Valley River, which flows northwards and eventually joins the West Fork River. Upstream within the West Fork River, Stonewall Jackson Lake was created by the United States Army Corps of Engineers in 1990 by impounding the West Fork River as a flood control measure. Stonewall Jackson Lake is a 1064-hectare impoundment and is one of the largest lakes in the state of West Virginia. At the confluence of the Tygart Valley and West Fork Rivers, the Monongahela River is formed and flows north for 130 km before joining the Allegheny River to form the Ohio River.
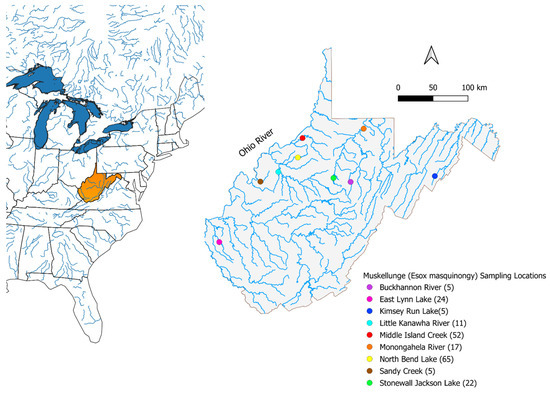
Figure 1.
The state of West Virginia is highlighted in orange on the left showing the geographic extent of the United States, with muskellunge (Esox masquinongy) sampling locations where fin clips for genetic analysis were collected within West Virginia shown on the right with the sample size for each location given in the parenthesis. These sites were chosen due to their importance as prominent muskellunge populations in the state, their utilization as recreational angling opportunities, and being the most utilized and targeted populations in the state.
Several tributaries of the Ohio River were sampled during the current study including Middle Island Creek, Little Kanawha River, Sandy Creek, and East Lynn Lake, a dammed reservoir within the Twelvepole Creek system. Middle Island Creek is a 124 km long river that flows through north-western West Virginia before emptying into the Ohio River. North Bend Lake is a 123-hectare reservoir located in the North Fork Hughes River and was developed in 2002 following the damming of the river. The North Fork Hughes River joins the South Fork Hughes River to form the Hughes River, the largest tributary of the Little Kanawha River. The Little Kanawha River flows west-northwestwardly for 269 km before the mouth of the Little Kanawha River empties into the Ohio River. Sandy Creek is a 35 km long river formed at the confluence of the Left and Right Fork Sandy Creek in western West Virginia before draining into the Ohio River. East Lynn Lake is a 406-hectare reservoir of the East Fork Twelvepole Creek in southeast West Virginia, created in 1971 as a flood control measure for the surrounding region. The only sampling location in the current study not found in the Ohio River watershed is Kimsey Run Lake, a 19-hectare reservoir of the Lost River, a stream in eastern West Virginia that is the headwaters of the greater Cacapon River. The Cacapon River flows for 130 km before the mouth of the river meets the Potomac River, which deposits into the Chesapeake Bay.
2.3. DNA Extraction, Library Preparation, and Sequencing
DNA was extracted from fin clips using the QIAcube HT® automated nucleic acid purification instrument using the QIAamp 96 DNA QIAcube HT Kit (©Qiagen, Hilden, Germany). DNA extracts were quantified on a Nanodrop spectrophotometer (©ThermoScientific, Wilmington, DE, USA) and standardized to a concentration of 20 ng/µL for downstream protocols.
Standardized genomic DNA was processed using a double digest restriction site associated DNA sequencing (ddRAD-seq) genotype-by-sequencing (GBS) modified protocol [26]. In total, 10 μL of standardized genomic DNA was added to a master mix containing 16.2 μL of nuclease-free water, 3.0 μL of 10× NEBuffer4 (New England Biolabs, Ipswich, MA, USA), and 0.4 μL of PstI and MspI restriction enzymes (New England Biolabs #R3140L and #R0106L, respectively) and run with thermocycler conditions set to 37 °C for 120 min and 80 °C for 20 min with an infinite hold of 8 °C. A master mix containing 8.4 μL of nanopure water, 5.0 μL of 10× T4 DNA Ligase Reaction Buffer, and 1.6 μL of T4 DNA Ligase (New England Biolabs #B0202S and #M0202L, respectively) per sample and 5 μL of unique individual barcodes was added to each well and ligated to the resulting DNA fragments (thermocycler conditions set to 22 °C for 120 min and 65 °C for 20 min with an infinite 8 °C hold). Samples were then pooled and purified using the GeneJET PCR Purification Kit (©ThermoScientific, Wilmington, DE, USA) following manufacturer protocol (#K0701), with the purified samples run on a Pippin Prep (Sage Science, Beverly, MA, USA) to collect fragments between 250 and 450 base pairs in length. In total, 2 μL of the resulting product was added to a master mix containing 22.5 μL of nanopure water, 10 μL of 5× Q5 buffer, 10 μL 5× Q5 High GC Enhancer, 0.50 μL Q5 High-Fidelity DNA Polymerase (2000 units/mL) (New England Biolabs MO491L), and 2 μL of both forward and reverse Illumina primers at 10 uM, and amplified using the following parameters: 5 min at 72 °C, 30 s at 98 °C, 21 cycles (10 s at 98 °C, 30 s at 65 °C, and 30 s at 72 °C), a final extension of 5 min at 72 °C, and an infinite hold of 4 °C. The resulting PCR product was then purified again using the GeneJET PCR Purification Kit (©ThermoScientific, Wilmington, DE, USA) and eluted to a final volume of 50 μL. The quality and quantity of the generated libraries were assessed using an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) at the West Virginia University Genomics Core Facility. The generated and quality-control-validated libraries were then sequenced on an Illumina NextSeq200 (Illumina, San Diego, CA, USA) at the Marshall University Genomics Core Facility.
2.4. Processing of Generated ddRAD Sequences
Generated multiplexed sequence libraries were first processed using the CUTADAPT program ver. 5.0 [27] to remove adaptor sequences and discard reads that were shorter than 50 bp in length. The filtered libraries were then processed using the STACKS software program ver. 2.65 [28]. Process_radtags was used to demultiplex the data and separate individuals by barcode sequences. Sequences were cleaned (-c) and low-quality reads were discarded (-q), with low-quality reads being discarded if bases were uncalled or if the raw phred score dropped below 10 using a sliding window of 15%.
Paired-end sequences for each individual were aligned to the muskellunge reference genome (NCBI WGS accession: JACXGI01) using Bowtie 2 software ver. 2.5.0 [29], and the resulting SAM files were then converted to BAM files using SAMtools software ver. 1.21 [30]. Aligned sequences were then processed using STACKS ref_map pipeline. The number of reads generated, mean coverage, and the number of loci generated for each individual were assessed using the gstacks.log.distribs file generated following the ref_map pipeline. Individuals with low coverage (<3×) and a low number of loci generated (60,000 loci) were removed from downstream analysis. The resulting catalogs were then processed and filtered using the populations program with the following parameters: to be filtered to the final dataset, a locus had to be found in every population and be present in 80% of the individuals within each of the populations (-p 9, -r 0.80); only the first SNP for each locus was used for population genetics investigations (--write-single-snp) to mitigate the influence of linked SNPs and generate independent SNP observations in genomic diversity assessments. A minor allele count (MAC) of 3 was used to filter out singletons and doubletons, and to exclude any errant genotyping calls. Hardy–Weinberg equilibrium and F statistics were assessed for each SNP (--hwe, --fstats), and the final genomic dataset was exported into genepop and PLINK formats for genomic analysis [31,32].
2.5. Population Structuring and Genomic Diversity
The exported SNP dataset was converted to binary file formats using PLINK ver. 1.9 [32] and SNP data management, and analyses were performed using wrapper functions within the R package SambaR ver. 1.10 [33] (Github page: https://github.com/mennodejong1986/SambaR accessed on 14 November 2024). Initial population structuring inferences were performed by using principal component analysis (PCA) using PLINK (--pca) [32] and plotting the resulting eigenvectors for each individual using ggplot2 ver. 3.5.1 [34]. Population structuring was assessed using the discriminant analysis of principal components (DAPC) in the R package adegenet ver. 2.1.10 and the software program ADMIXTURE ver. 1.3.0 [35,36]. The optimal number of clusters (K) was determined from each model, wherein k-means clustering was performed on the data after being transformed using PCA, with an optimal K chosen where the resulting Bayesian Information Criterion (BIC) was lowest among tested K’s. The resulting K was then used for the defined K within the DAPC analysis. Ten-fold cross-validation within ADMIXTURE was used to determine the optimal K within the ADMIXTURE model, with the resulting cross-validation error estimates plotted and the lowest cross-validation error estimate used as the inferred optimal K. The PCA plot and the optimal K values generated across the three models were used to infer the number of unique genetic populations of muskellunge within West Virginia. Pairwise genetic differentiation FST was assessed among all sample sites and populations using the gl.fst.pop function in the R package dartR ver. 2.9.7, with the resulting p-values adjusted using the false discovery rate method built within base R to correct for Type I errors [37,38]. Heatmaps of pairwise comparisons were then plotted using ggplot2.
Genomic diversity estimates for observed and expected heterozygosity (HO and HE), nucleotide diversity (π), number and proportion of SNPs out of Hardy–Weinberg equilibrium (HWE), proportion of polymorphic loci, mean frequency of the most frequent allele at each locus (P), and inbreeding coefficients (FIS) for each population were generated from the STACKS populations output [28]. The number of private alleles within each population, and Watterson’s Θ [39] and Tajima’s D [40] per nucleotide, were assessed using the calcdiversity function within SambaR [33]. Selection analysis was performed using OutFLANK ver. 0.2 [41] and pcadapt ver. 4.3.5 [42], with only SNPs that were identified in both approaches categorized as potentially undergoing selection. Effective population sizes (Ne) for all sampled populations were calculated using the R package dartR ver. 2.9.7 using the gl.LDNe function, which utilizes the NeEstimator software ver. 2.1 [37,43]. Fixed SNP differences between identified New York-strain muskellunge and native West Virginia muskellunge were assessed using the gl.fixed.diff function within dartR [37]. SNPs were tested for fixed differences between the two strains using both true fixation (tloc = 0) and a 95:5 threshold (tloc = 0.05) to identify potential broodstock screening SNPs and to assist in the conservation of the native strain.
3. Results
3.1. Dataset Generation
After removing individuals with low-quality sequencing, a total of 206 muskellunge from nine sampling locations were retained. A dataset containing 203,946,414 total reads with an average of 990,031 reads per individual was retained for the genomic evaluation of West Virginia muskellunge. The average coverage was 11.34× with a range of 3.96–51.52×, with an average of 100,795 loci per individual. A total of 3148 SNPs were retained with a genotyping rate of 96.63%. Following the identification and removal of New York-strain muskellunge and highly introgressed individuals, 158 individuals were retained from eight sampling locations with a total of 2017 SNPs and a genotyping rate of 94.90%. A final dataset containing unmixed West Virginia-strain muskellunge unimpacted from stocking was retained after additional filtering, with a total of 147 muskellunge from five populations generating a dataset of 3083 SNPs, and a genotyping rate of 93.73% was retained to assess genomic diversity and population structuring insights within the West Virginia strain.
3.2. Population Structuring and Strain Identification
Initial population structuring inferences using PCA (Figure 2, top) with all retained muskellunge shows the separation of the native West Virginia muskellunge and individuals from populations stocked with New York-strain muskellunge including the Buckhannon and Monongahela Rivers, East Lynn Lake, Kimsey Run Lake, and Stonewall Jackson Lake on the first principal component, explaining 19.50% of the total variance in the dataset. Along the second principal component, explaining 8.86% of the total variance, muskellunge from the Little Kanawha River, Monongahela River, Kimsey Run Lake, and one individual from North Bend and Stonewall Jackson Lake were found to cluster away from the main West Virginia cluster. DAPC results (Figure 2, bottom) highlight a similar trend, where an optimal K of three was found using k-means clustering (Supplementary Figure S1). The main cluster consists of West Virginia-strain muskellunge from North Bend, East Lynn, Middle Island Creek, and Sandy Creek. A second cluster consisting of muskellunge from the Little Kanawha River as well as individuals from the Monongahela River, Middle Island Creek, Stonewall Jackson Lake, and three individuals from Kimsey Run Lake is apparent. A third cluster consisting of assumed New York-strain muskellunge from Stonewall Jackson and Kimsey Run Lakes as well as the Monongahela and Buckhannon Rivers is also observed. ADMIXTURE cross-validation found an optimal K of five (Supplementary Figure S2), where, at K = 2, ADMIXTURE first clusters out individuals of New York-strain influence, predominantly from the Monongahela and Buckhannon Rivers, and Stonewall Jackson, East Lynn, and Kimsey Run Lakes (Figure 3A). At K = 3, a genetic strain found most prominently in Kimsey Run Lake, the Monongahela River, Stonewall Jackson, and East Lynn Lake becomes apparent. At K = 4, this ancestry remains prominent in the four populations stocked with New York-strain muskellunge, and a prominent unique ancestry is found in the Little Kanawha River and North Bend Lake. At K = 5, a lineage is identified that is found in all populations to some extent, but is not prominent in any specific population.
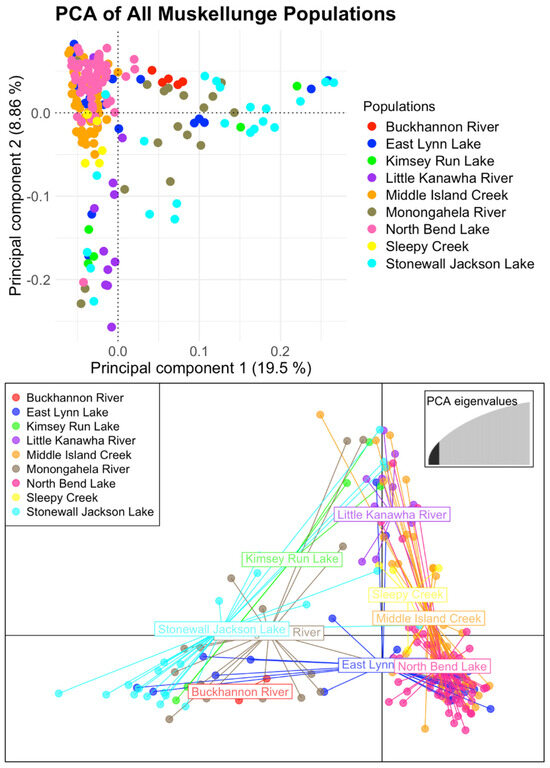
Figure 2.
Resulting PCA (top) and DAPC (bottom) plots for all sampled muskellunge using a total of 3148 SNPs. PCA inference shows that the individuals of assumed New York ancestry from Stonewall Jackson Lake, East Lynn Lake, Kimsey Run Lake, and the Monongahela River cluster along the first principal component, while muskellunge from the Little Kanawha River and a few individuals from Stonewall Jackson and East Lynn Lake cluster together along the second principal component. DAPC reveals a similar trend, with the vast majority of sampled muskellunge forming the North Bend Lake cluster, individuals of New York ancestry forming the Stonewall Jackson cluster, and a third cluster being composed primarily of Little Kanawha River muskellunge.
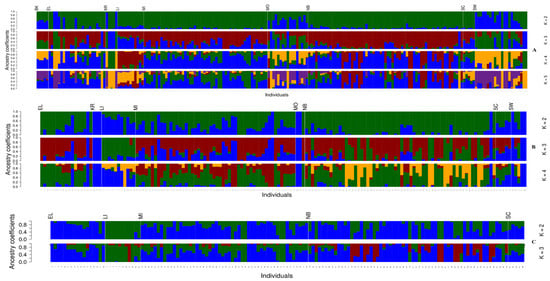
Figure 3.
ADMIXTURE ancestry coefficient plots for all sampled muskellunge (A), West Virginia muskellunge (B), and pure West Virginia muskellunge (C) using 3148, 2017, and 3083 SNPs, respectively. From left to right: BK—Buckhannon River, EL—East Lynn Lake, KR—Kimsey Run Lake, LI—Little Kanawha River, MI—Middle Island Creek, MO—Monongahela River, NB—North Bend Lake, SC—Sandy Creek, SW—Stonewall Jackson Lake. Different colors in each graph represent a unique genetic ancestry, with the number of unique colors in each plot being defined by the value of K tested.
After the removal of individuals of New York-strain ancestry and highly introgressed individuals, West Virginia-strain muskellunge from Stonewall Jackson and Kimsey Run Lakes and the Monongahela River that had been stocked with New York-strain muskellunge show genetic differentiation from unstocked populations such as Middle Island Creek and North Bend Lake. PCA plot inferences show that individuals from these stocked populations cluster apart from the main West Virginia cluster on the second principal component, while muskellunge from the Little Kanawha River cluster apart on the first principal component (Figure 4, top). An optimal K of two (Supplementary Figure S3) was found using k-means clustering, and, in the resulting DAPC plot, Stonewall Jackson, Kimsey Run, the Monongahela River, and Little Kanawha River populations cluster apart from the main West Virginia muskellunge cluster (Figure 4, bottom). ADMIXTURE ancestry inference found an optimal K of four (Supplementary Figure S4) and, at K = 2, stocked populations cluster out, with this ancestry also found prominently in the Little Kanawha River and Middle Island Creek. At K = 3, a unique ancestry is found in the Little Kanawha River and North Bend Lake; at K = 4, the North Bend Lake ancestry remains the same, while all but one individual within the Little Kanawha River display the ancestry found in stocked populations, with the North Bend Lake ancestry still featuring in all Little Kanawha River individuals (Figure 3B).
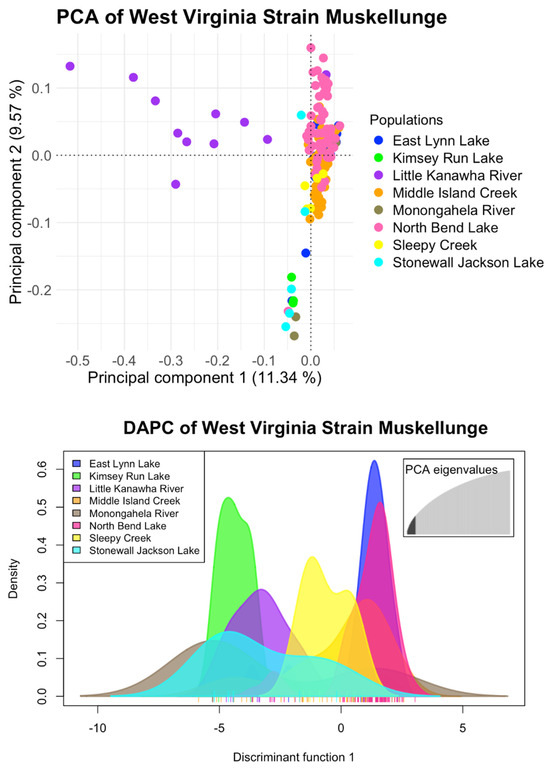
Figure 4.
Resulting principal component analysis (PCA) plot (top) and discriminant analysis of principal components (DAPC, bottom) plots of highly introgressed West Virginia−strain muskellunge populations and New York−strain individuals removed using a total of 2017 SNPs. In the PCA plot, muskellunge from the Little Kanawha River cluster apart from the main cluster along the first principal component, while muskellunge from populations stocked with New York-strain ancestry cluster together along the second principal component including Stonewall Jackson, Kimsey Run Lake, and the Monongahela River.
Following final filtration and removing all muskellunge from the Monongahela River, Stonewall Jackson, and Kimsey Run Lake, the population structure of native West Virginia muskellunge could be resolved. Initial inference using PCA (Figure 5) showed the same trend of the Little Kanawha River population clustering apart from the main cluster on the first principal component, which explained 11.28% of the total variance. Some individuals from North Bend Lake and one individual from the Little Kanawha clustered apart on the second principal component, which explained 8.4% of the total variance. K-means clustering, however, found K = 1 as the optimal K (Supplementary Figure S5), and ADMIXTURE found the lowest cross-validation error at K = 3 (Supplementary Figure S6). At K = 2, ADMIXTURE first separates the Little Kanawha River from the other populations, with the Little Kanawha ancestry found in the other four populations, with the highest prevalence in North Bend Lake. At K = 3, muskellunge from North Bend Lake and one individual from the Little Kanawha show a unique ancestry, a second ancestry found most prominently in the Little Kanawha River is displayed, and a mix of a third ancestry and the Little Kanawha River ancestry is found throughout the remaining populations (Figure 3C).
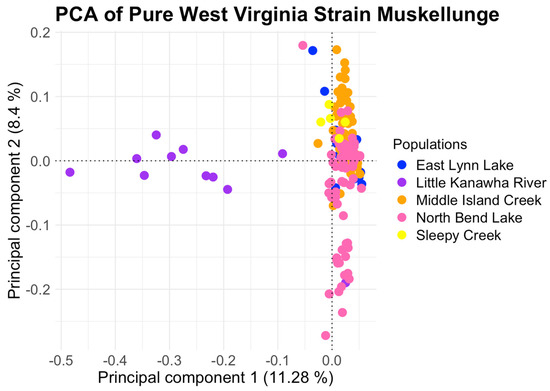
Figure 5.
Resulting principal component analysis (PCA) of pure West Virginia−strain muskellunge populations using a total of 3083 SNPs. Muskellunge from the Little Kanawha River form a distinct cluster on the first principal component, while muskellunge from North Bend Lake and one individual from the Little Kanawha River cluster together along the second principal component.
Pairwise genetic differentiation FST comparisons (Figure 6) between all sampled populations were found to be statistically significant for each comparison following a false discover rate correction, except for Kimsey Run and Stonewall Jackson Lakes in the West Virginia-strain dataset (Figure 6B). An overall trend can be observed that populations that have been previously stocked with New York-strain muskellunge including Stonewall Jackson, Kimsey Run Lake, the Monongahela River, and the Buckhannon River display a lower FST to each other and a higher FST to those that are dominated by the native strain such as Middle Island Creek and North Bend Lake. Despite this trend, East Lynn Lake remains more genetically similar to West Virginia-strain populations than New York-strain-dominated populations, despite having individuals of New York-strain origin within the population (Figure 3 and Figure 6). The Little Kanawha River population was the most genetically distinct population regardless of New York influence, showing a moderately high FST when compared to every population, with the most similar population to it being Sandy Creek (FST = 0.061–0.065).
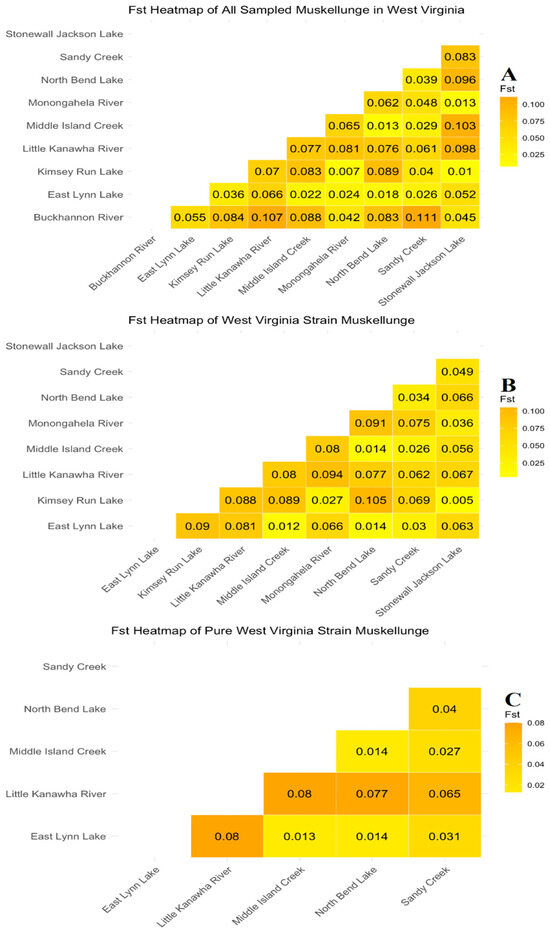
Figure 6.
Genetic differentiation (FST) among all sampled muskellunge populations (A), only assumed West Virginia-strain muskellunge (B), and pure West Virginia-strain populations (C) using a total of 3148, 2017, and 3083 SNPs, respectively.
Fixed SNP differences between West Virginia-strain and New York-strain muskellunge were investigated for genomic screening using 10 identified New York-strain muskellunge from East Lynn Lake, Kimsey Run Lake, and Stonewall Jackson Lake, and 15 assumed pure West Virginia muskellunge from five different populations including East Lynn Lake, Middle Island Creek, Little Kanawha River, North Bend Lake, and Sandy Creek. They displayed 99.99% assignment to the West Virginia strain at K = 2 with all muskellunge in the dataset. One SNP was found to be fixed between the two strains, and an additional nine were found to be fixed with a 95% threshold (Table 1).

Table 1.
Fixed single-nucleotide polymorphism (SNP) differences between the West Virginia-strain and New York-strain muskellunge with the corresponding locus, scaffold, and base pair (BP) position of the fixed SNP within the scaffold of the muskellunge reference genome. The fixed SNP difference is bolded within each sequence. Locus 871013 was found to be completely fixed, while the remaining 9 were found to be fixed at a 95:5 threshold limit.
3.3. Genomic Diversity
Genomic diversity for sampled populations with all samples included (Table 2) and only pure West Virginia-strain muskellunge (Table 3) highlights that the Little Kanawha River population has a high number of private alleles and genomic diversity compared to other populations, but also shows an increased FIS value compared to all other populations. Among all sampled locations, New York-strain-dominated populations such as the Monongahela River, Stonewall Jackson, and Kimsey Run Lake had the highest levels of genomic diversity, while the Buckhannon River was the only population to have a higher heterozygosity than expected under Hardy–Weinberg equilibrium. East Lynn and Stonewall Jackson had the lowest Ne among all sampled populations, with North Bend Lake and the Monongahela River showing intermediate Ne, and Little Kanawha River and Middle Island Creek displaying the highest Ne. Little Kanawha River, Middle Island Creek, and North Bend Lake had the highest number of private alleles, while Sandy Creek and the Buckhannon River had 0 private alleles.

Table 2.
Estimates of genetic diversity for all sampled muskellunge across nine West Virginia sample sites; the sample size for each population is presented in parenthesis. Genomic diversity estimates were assessed using a total of 3148 single-nucleotide polymorphisms (SNPs) used for analysis. The mean frequency of the most frequent allele at each locus (P), percentage of polymorphic loci (P loci), number of private alleles (Private), nucleotide diversity (π; standard error SE), observed heterozygosity (HO), expected heterozygosity (HE), number and percentage of SNPs found to be out of Hardy–Weinberg equilibrium (HWE), inbreeding coefficient (FIS), Watterson’s Ɵ (Ɵ), Tajima’s D statistic (Tajima), and estimated effective population size (Ne) with corresponding 95% confidence intervals are presented.

Table 3.
Estimates of genetic diversity for pure West Virginia-strain muskellunge from five sampling locations; the sample size for each population is presented in parenthesis. Genomic diversity estimates were assessed using a total of 3083 single-nucleotide polymorphisms (SNPs) used for analysis. The mean frequency of the most frequent allele at each locus (P), percentage of polymorphic loci (P loci), number of private alleles (Private), nucleotide diversity (π; standard error SE), observed heterozygosity (HO), expected heterozygosity (HE), number and percentage of SNPs found to be out of Hardy–Weinberg equilibrium (HWE), inbreeding coefficient (FIS), Watterson’s Ɵ (Ɵ), Tajima’s D statistic (Tajima), and estimated effective population size (Ne) with corresponding 95% confidence intervals are presented.
Among pure West Virginia muskellunge populations, the Little Kanawha River had the highest genomic diversity using both Ɵ and π, and, despite only having 11 samples, it had the second-highest number of private alleles, while North Bend Lake, with 62 samples, had only four more private alleles. East Lynn Lake had the lowest Ne, Middle Island Creek had the highest, and North Bend Lake and the Little Kanawha River displayed intermediate values. Sandy Creek Ne could not be assessed due to a small sample size. Despite a high level of genomic diversity compared to other West Virginia populations, the Little Kanawha River displayed the highest FIS, and Middle Island Creek displayed a moderate FIS, while the remaining populations showed lower FIS values. The Little Kanawha River, Sandy Creek, and Easy Lynn Lake showed the lowest Tajima’s D values, indicating an excess of rare alleles in these populations compared to North Bend Lake and Middle Island Creek.
Only one SNP was found to be potentially undergoing selection in the pure West Virginia muskellunge dataset by both pcadapt and OutFLANK, with this SNP showing the highest frequency in the Little Kanawha River (0.61). This allele was absent from East Lynn Lake and Middle Island Creek and found in one individual in North Bend Lake and two individuals in Sandy Creek. The SNP was found in scaffold JACXGI010039364.1 in position 2672, but was unable to be traced to a specific chromosome in the annotated reference genome of the closely related Northern Pike (Esox lucius).
4. Discussion
Genomic investigations reveal that stocking non-native New York-strain muskellunge has likely altered the genetic composition of recipient West Virginia populations. All populations that have been stocked with New York-strain muskellunge, except for East Lynn Lake, showed that, even after the removal of highly introgressed individuals, these populations were genetically distinct from populations containing only native-strain muskellunge. Additionally, populations stocked with New York-ancestry muskellunge are more genetically similar to each other than to the unstocked populations, while the Little Kanawha River remains genetically distinct to all populations regardless of stocking influence, containing an unknown but distinct ancestry.
The persistence of New York-strain muskellunge in all previously stocked populations is observed in the current study with the introduced strain comprising a significant proportion of the sampled muskellunge from these populations. Previous research on West Virginia muskellunge genetics using five microsatellite loci found that there was a clear distinction of New York-hatchery muskellunge from those sampled from West Virginia with limited introgression observed in any of the sampled populations including the Buckhannon River and Middle Island Creek [2]. Historically, muskellunge from Kentucky, Ohio, West Virginia, and New York were stocked throughout West Virginia waters; in 2000, WVDNR shifted their broodstock sources to those from within West Virginia and muskellunge from the Chautauqua State Fish Hatchery in New York [2]. Middle Island Creek was stocked with New York-hatchery muskellunge extensively prior to 2000 and is now a self-sustaining unstocked population that shows clear distinction from populations with recent stocking of New York-strain muskellunge. The Buckhannon River was also previously found to have limited influence of the New York strain within the population, with population structuring inferences showing clear distinction between the two strains and a lack of New York-strain influence in the Buckhannon River [2].
The present study shows clear influence of the introduced strain on the genetic composition of the sampled Buckhannon River muskellunge, contradicting previous results. This contradiction could be explained by the limited number of microsatellite loci used in the previous study (5) compared to the 3148 SNPs employed in the present study, which could better elucidate the introgression of the New York strain. The increased resolution of SNP data compared to microsatellite loci likely accounts for the observed differences, as SNPs provide finer-scale insights into introgression and population structure [44,45,46]. The observed difference in the genetic composition and influence of the New York strain could also potentially be explained by a difference in demographic shifts in the population between the timelines of the two studies, where the number of stocked fish, size of the stocked population, size at stocking, origin of brood fish used for supplemental stocking, and successful reproduction of the stocked fish could all impact the discrepancy between current and previous results [47,48,49,50].
Regardless of the various number of influences that could impact the genetic composition of a stocked population, East Lynn Lake remains more genetically similar to native West Virginia-strain populations, despite the previous stocking of New York-strain muskellunge. All other stocked populations show divergence from the unstocked populations following the removal of New York-strain muskellunge. This genetic similarity of East Lynn Lake could be explained by a higher census population of the Ohio River/West Virginia-strain muskellunge within East Lynn Lake as it had the lowest proportion of New York-strain influence compared to the other stocked populations. This could also be explained by only two known introductions of New York-strain muskellunge into East Lynn Lake, with current stocking regimes utilizing muskellunge from the Kanawha River system. The Kanawha River system has previously been stocked with and is likely derived from New York-strain muskellunge, also potentially explaining the observed New York-strain influence in East Lynn Lake [2]. The stocking of non-native-strain individuals into populations experiencing a decline from historical benchmarks has been observed to lead to genetic divergence, a pattern observed in all stocked populations except East Lynn Lake [51,52,53]. While the ongoing stocking of New York-strain muskellunge may enhance population persistence, support recreational fishing, and benefit ecological stability, continued stocking in East Lynn Lake should be reassessed to prevent future potential genetic divergence and conserve native genetic diversity.
A genetically distinct population was identified within the Little Kanawha River system, including the South Fork Hughes River, an upstream first-order tributary to the Little Kanawha River. The Little Kanawha River system represents the only genetically distinct population identified in the present study and remains genetically distinct from both native and introduced populations. The Little Kanawha River contains a high level of genomic diversity within the population, showing the highest nucleotide diversity and Watterson’s Θ, while also containing the second-highest number of private alleles within pure West Virginia populations, despite having the second-lowest number of samples. However, the Little Kanawha River also shows the highest FIS value among all populations, highlighting the urgency needed to develop conservation strategies to alleviate this potential inbreeding. FIS is a comparison of expected and observed heterozygosity under Hardy–Weinberg equilibrium and could be found to be higher in populations with higher rates of missing data and not be directly due to inbreeding [54,55]. The Little Kanawha River had the highest rate of missing data across all populations (9.75%), which may partially explain the high observed FIS; however, Sandy Creek exhibited the second-highest rate of missing data (7.88%) and had among the lowest FIS values, suggesting that inbreeding could be a contributing factor in the Little Kanawha River system and should not be ruled out. There is growing evidence that muskellunge exhibit spawning site fidelity despite large seasonal movements; however, whether this behavior is a natal trait or develops later in adulthood remains unclear [2,22,56,57]. The identification of distinct spawning sites within the Little Kanawha River and its tributaries would serve as a first step to aid in conserving this genetically distinct population. The utilization of unrelated individuals from different spawning grounds in an artificial propagation system would help alleviate potentially observed inbreeding and support long-term population stability.
The identification of distinct spawning sites within the Little Kanawha River system should also extend to North Bend Lake, a reservoir built in the North Fork Hughes River, which flows into the Hughes River, the major tributary of the Little Kanawha River. A potentially distinct genetic population was found within North Bend Lake that one individual sampled from the Little Kanawha River also clustered with. This genetically distinct population within North Bend Lake may be indicative of a remnant population native to the North Fork Hughes River, with the remaining North Bend Lake muskellunge being introduced from the historical and ongoing stocking of West Virginia-strain fish. This is supported by the clustering of the remaining North Bend Lake muskellunge with other West Virginia-strain populations. The one individual from the Little Kanawha River that clustered with the proposed remnant North Bend Lake population may have originated from the North Fork Hughes River and was simply sampled in the Little Kanawha River due to the complex movement patterns of muskellunge [58,59,60].
Previous acoustic telemetry research has been conducted in North Bend Lake muskellunge, where 7 of 24 sampled muskellunge escaped North Bend Lake through the dam escapement over a four-year time span [61]. This demonstrated escapement of muskellunge supports the movement of the North Bend Lake muskellunge into the Little Kanawha River system, highlighting the need to further research into spawning site fidelity and spawning behavior in this interconnected system. While muskellunge in the Potomac River system exhibit an average movement of just under 140 m per day, home range size can vary significantly, with homing range behavior observed to have a range of 290–25,000 m in a riverine system and 17–1309 hectares in a lake system [60]. Given this wide range of movement and home range behavior, additional research is needed to better understand muskellunge movement in the Little Kanawha River, North Bend Lake, and connected tributaries including North Fork, South Fork, and Hughes Rivers. Combining spatial understanding with genomic data would enhance the ability to conserve the native genetic diversity of a population found to have the highest genomic diversity among native West Virginia muskellunge populations.
Despite the observed population structuring of muskellunge throughout their entire range [2,22,49,50,56], very limited structuring was found within West Virginia. The dominant factor influencing population structuring in West Virginia waters is the stocking of the New York strain, with the only sub-structuring within native populations occurring in the Little Kanawha River system. This lack of observed genetic differentiation within West Virginia could be a result of both contemporary management and historical contexts. Native muskellunge within West Virginia would all be of Ohio River-strain origin [3], where this strain may naturally exhibit low population structuring, as observed in the current results outside of the Little Kanawha River.
Historically, West Virginia muskellunge populations were supplemented with non-native muskellunge sourced from Kentucky, New York, and Ohio [2]. Despite historic stocking, the remnant native Ohio River-strain muskellunge in West Virginia may have been prominent enough to retain native diversity and dilute non-native alleles. Contemporarily, the main driver of non-native-strain alleles is due to stocking New York-strain muskellunge [2]. It could also be hypothesized that the stocking of muskellunge from non-native sources could have also resulted in a uniform genetic composition following historical reduction in population sizes, resulting in the contemporary native West Virginia strain being an admixture of several introduced and native ancestries. The observed genetic homogeneity across most sampled West Virginia populations may reflect historical reductions in population sizes combined with extensive interbreeding between introduced and native strains, eroding fine-scale genetic differentiation.
Genomic diversity was similar among all native West Virginia populations except for the Little Kanawha River system, which boasted higher genomic diversity metrics than all other native populations. While genomic diversity was generally similar among native populations, populations stocked with New York-strain muskellunge had observably higher genomic diversity. This artificial inflation of genomic diversity metrics in stocked populations is likely a consequence of the presence of New York-strain alleles that are not found in native populations. As a result of this, true estimates of native genomic diversity in stocked populations is complicated by previous stocking practices. Sample size variation among populations may also have influenced results, as Middle Island Creek and North Bend Lake expectedly have a higher number of private alleles than most other sampled locations, given their increased sample size. However, the Little Kanawha River boasted the second-highest number of private alleles in native populations with the second-fewest number of samples, reinforcing its genetic distinctiveness. While the present study highlights genetic structuring and diversity metrics, environmental and ecological variables such as movement patterns, habitat availability, habitat conductivity, and census population sizes may be key factors driving the current results. Future research should integrate genomic, ecological, habitat, and movement patterns to provide a comprehensive framework for understanding the population dynamics of muskellunge.
The results of the current study could be utilized for future management directives in assisting the conservation of native genomic diversity. Based on the current results, management directives should be aimed at the conservation of the genetically distinct Little Kanawha River system through the identification of spawning grounds in both the Little Kanawha River and upstream tributaries to assist in delineating true genetic populations. The alleviation of observed potential inbreeding should also be implemented through the propagated spawning of unrelated individuals found within the Little Kanawha River system to conserve this previously unknown hotspot of genomic diversity. Secondly, the utilization of the identified fixed SNPs between the New York-strain and West Virginia-strain muskellunge would be effective for broodstock screening to ensure that ongoing stocking regimes use native-strain individuals and to prevent the introgression of New York-strain alleles into pure West Virginia-strain populations [46,62]. In line with the potential broodstock screening of native muskellunge, East Lynn Lake should only be stocked with West Virginia-strain muskellunge to prevent the potential genetic divergence of this population due to the stocking of New York-strain muskellunge that has been observed in the other stocked systems. Within these potentially diverged systems, two management directives could take place. Management officials could continue to stock New York-strain muskellunge to meet recreational and ecological needs due to the already high levels of observed introgression. Management directives could also try to restore the native genomic diversity of these populations by using broodstock from pure West Virginia populations, but the restoration of these populations to a “pure” native benchmark may require investment, and the cost to achieve this may not be feasible.
This study represents the first genomic analysis of muskellunge in West Virginia, providing a critical baseline for understanding the impact of stocking on native populations. Future research should focus on the long-term monitoring of genetic diversity and evaluating the success of conservation efforts, particularly in maintaining the unique genomic signature of the Little Kanawha River population. Furthermore, research should include the identification of spawning sites within the Little Kanawha River population, the ongoing genomic monitoring of muskellunge populations to elucidate the genetic impact of potential changes in stocking regimes, and the combination of habitat and ecological data with genomic data to identify any variables that may impact the success of stocked individuals in West Virginia waters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/hydrobiology4010007/s1, Figure S1: Optimal number of clusters using k-means clustering on the PCA-transformed data on all sampled muskellunge in the present study using a total of 3148 SNPs; Figure S2: Optimal number of clusters inferred using ADMIXTURE cross-validation data on all sampled muskellunge in the present study using a total of 3148 SNPs; Figure S3: Optimal number of clusters using k-means clustering on the PCA-transformed data of the assumed West Virginia-strain muskellunge in the present study using a total of 2017 SNPs; Figure S4: Optimal number of clusters inferred using ADMIXTURE cross-validation data on West Virginia-strain muskellunge in the present study using a total of 2017 SNPs; Figure S5: Optimal number of clusters using k-means clustering on the PCA-transformed data of the pure West Virginia-strain muskellunge populations in the present study using a total of 3083 SNPs; Figure S6: Optimal number of clusters inferred using ADMIXTURE cross-validation data on pure West Virginia-strain muskellunge populations in the present study using a total of 3083 SNPs.
Author Contributions
Conceptualization, A.J. and A.W.; Validation, N.T.; Formal analysis, A.J.; Investigation, A.J. and N.T.; Resources, N.T. and A.W.; Data curation, A.J.; Writing—original draft, A.J.; Writing—review and editing, N.T. and A.W.; Visualization, A.J.; Supervision, A.W.; Project administration, A.W.; Funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this project was provided by the West Virginia Division of Natural Resources (WVDNR). This work was also supported by the USDA National Institute of Food and Agriculture (NIFA), Hatch project WVA00747, and the West Virginia Agricultural and Forestry Experiment Station. This research was funded by NIH grant U54GM104942 and NSF MRI award 2117043, and partly funded by NSF OAC-1726534.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated in the current manuscript are available online at Figshare to increase open-access data availability and are freely available to download at the following DOIs: Johnson, Andrew (2024). All Muskellunge Dataset. figshare. Dataset. https://doi.org/10.6084/m9.figshare.28112012.v1. Johnson, Andrew (2024). West Virginia Muskellunge. figshare. Dataset. https://doi.org/10.6084/m9.figshare.28112021.v1. Johnson, Andrew (2024). Pure WV Strain Dataset. figshare. Dataset. https://doi.org/10.6084/m9.figshare.28112006.v1. BAM files used in the present study are available on NCBI under BioProject accession PRJNA1229415.
Acknowledgments
We acknowledge the WVU Genomics Core Facility, Morgantown, WV, for their support in making this publication possible, which has been supported by NIH grant U54GM104942 and NSF MRI award 2117043. Computational resources were provided by the WVU Research Computing Thorny Flat HPC cluster, partly funded by NSF OAC-1726534.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kerr, S.J. Distribution and Management of Muskellunge in North America; Fisheries Policy Section; Ontario Ministry of Natural Resources: Peterborough, UK, 2011. [Google Scholar]
- White, M.M.; Kohli, B.A.; Converse, P.E. Historical and Contemporary Gene Flow and the Genetic Structure of Muskellunge in the Ohio River Drainage. Trans. Am. Fish. Soc. 2018, 147, 1067–1077. [Google Scholar] [CrossRef]
- Cincotta, D.A.; Welsh, S.A. An Update of the Ichthyofauna of West Virginia with Notes on Historic Sportfish Stockings. Northeast. Nat. 2024, 31, 1–48. [Google Scholar] [CrossRef]
- Hourston, A.S. A Study of Variations in the Maskinonge from Three Regions in Canada; Royal Ontario Museum: Toronto, ON, Canada, 1955. [Google Scholar]
- Crossman, E.J. Taxonomy and Distribution of North American Esocids. Am. Fish. Soc. Spec. Publ. 1978, 11, 13–26. [Google Scholar]
- Crossman, E.J. The Noble Muskellunge: A Review. In Managing Muskies: A Treatise on the Biology and Propagation of Muskellunge in North America; Special Publication; American Fisheries Society: Bethesda, MD, USA, 1986; pp. 1–13. [Google Scholar]
- Bieber, J.F.; Louison, M.J.; Suski, C.D. Capture Is Predicted by Behavior and Size, Not Metabolism, in Muskellunge. North Am. J. Fish. Manag. 2023, 43, 231–243. [Google Scholar] [CrossRef]
- Faust, M.D.; Isermann, D.A.; Luehring, M.A.; Hansen, M.J. Muskellunge Growth Potential in Northern Wisconsin: Implications for Trophy Management. N. Am. J. Fish. Manag. 2015, 35, 765–774. [Google Scholar] [CrossRef]
- Vinson, M.R.; Angradi, T.R. Muskie Lunacy: Does the Lunar Cycle Influence Angler Catch of Muskellunge (Esox Masquinongy)? PLoS ONE 2014, 9, e98046. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Pinkerton, J.J.; Venturelli, P.A.; Miller, L.M. Muskellunge Spatial Ecology in the St. Louis River Estuary and Southwestern Lake Superior. Trans. Am. Fish. Soc. 2020, 149, 651–663. [Google Scholar] [CrossRef]
- Kapuscinski, K.L.; Crane, D.P.; Gronda, T. Prey Selection and Time to Consumption Differ between Congeneric Muskellunge and Northern Pike. J. Great Lakes Res. 2022, 48, 1087–1092. [Google Scholar] [CrossRef]
- Kumar, G.; Kocour, M. Applications of Next-Generation Sequencing in Fisheries Research: A Review. Fish. Res. 2017, 186, 11–22. [Google Scholar] [CrossRef]
- Swain, P.P.; Sahoo, L.; Kumar, R.; Sundaray, J.K. Applications of Next-Generation Sequencing in Aquaculture and Fisheries. In Advances in Fisheries Biotechnology; Pandey, P.K., Parhi, J., Eds.; Springer Nature: Singapore, 2021; pp. 41–64. ISBN 978-981-16-3215-0. [Google Scholar]
- Li, Y.-H.; Wang, H.-P. Advances of Genotyping-by-Sequencing in Fisheries and Aquaculture. Rev Fish Biol Fish. 2017, 27, 535–559. [Google Scholar] [CrossRef]
- Reiss, H.; Hoarau, G.; Dickey-Collas, M.; Wolff, W.J. Genetic Population Structure of Marine Fish: Mismatch between Biological and Fisheries Management Units. Fish Fish. 2009, 10, 361–395. [Google Scholar] [CrossRef]
- Ovenden, J.R.; Berry, O.; Welch, D.J.; Buckworth, R.C.; Dichmont, C.M. Ocean’s Eleven: A Critical Evaluation of the Role of Population, Evolutionary and Molecular Genetics in the Management of Wild Fisheries. Fish Fish. 2015, 16, 125–159. [Google Scholar] [CrossRef]
- Johnson, A.; Zipfel, K.J.; Hallerman, E.M.; Massure, W.; Euclide, P.; Welsh, A.B. Genomic Evaluation of Native Walleye in the Appalachian Region and the Effects of Stocking. Trans. Am. Fish. Soc. 2023, 152, 346–360. [Google Scholar] [CrossRef]
- Euclide, P.T.; Larson, W.A.; Bootsma, M.; Miller, L.M.; Scribner, K.T.; Stott, W.; Wilson, C.C.; Latch, E.K. A New GTSeq Resource to Facilitate Multijurisdictional Research and Management of Walleye Sander Vitreus. Ecol. Evol. 2022, 12, e9591. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Euclide, P.T.; Ludsin, S.A.; Larson, W.A.; Sovic, M.G.; Gibbs, H.L.; Marschall, E.A. RAD-Seq Refines Previous Estimates of Genetic Structure in Lake Erie Walleye. Trans. Am. Fish. Soc. 2020, 149, 159–173. [Google Scholar] [CrossRef]
- Kazyak, D.C.; Lubinski, B.A.; Rash, J.M.; Johnson, T.C.; King, T.L. Development of Genetic Baseline Information to Support the Conservation and Management of Wild Brook Trout in North Carolina. N. Am. J. Fish. Manag. 2021, 41, 626–638. [Google Scholar] [CrossRef]
- O’Leary, S.J.; Hice, L.A.; Feldheim, K.A.; Frisk, M.G.; McElroy, A.E.; Fast, M.D.; Chapman, D.D. Severe Inbreeding and Small Effective Number of Breeders in a Formerly Abundant Marine Fish. PLoS ONE 2013, 8, e66126. [Google Scholar] [CrossRef]
- Wilson, C.C.; Liskauskas, A.P.; Wozney, K.M. Pronounced Genetic Structure and Site Fidelity among Native Muskellunge Populations in Lake Huron and Georgian Bay. Trans. Am. Fish. Soc. 2016, 145, 1290–1302. [Google Scholar] [CrossRef]
- Chinchilla-Vargas, J.; Meerbeek, J.R.; Rothschild, M.F.; Bertolini, F. Signatures of Selection and Genomic Diversity of Muskellunge (Esox Masquinongy) from Two Populations in North America. Genes 2021, 12, 1021. [Google Scholar] [CrossRef]
- Rougemont, Q.; Carrier, A.; Le Luyer, J.; Ferchaud, A.-L.; Farrell, J.M.; Hatin, D.; Brodeur, P.; Bernatchez, L. Combining Population Genomics and Forward Simulations to Investigate Stocking Impacts: A Case Study of Muskellunge (Esox Masquinongy) from the St. Lawrence River Basin. Evol. Appl. 2019, 12, 902–922. [Google Scholar] [CrossRef]
- Scott, W.B.; Crossman, E.J. Freshwater Fishes of Canada. Fish. Res. Board Can. 1973, 184, 1–966. [Google Scholar]
- Poland, J.; Brown, P.; Sorrells, M.; Jannink, J.-L. Development of High-Density Genetic Maps for Barley and Wheat Using a Novel Two-Enzyme Genotyping-by-Sequencing Approach. PLoS ONE 2012, 7, e32253. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An Analysis Tool Set for Population Genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A Complete Re-Implementation of the Genepop Software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- de Jong, M.J.; de Jong, J.F.; Hoelzel, A.R.; Janke, A. SambaR: An R Package for Fast, Easy and Reproducible Population-Genetic Analyses of Biallelic SNP Data Sets. Mol. Ecol. Resour. 2021, 21, 1369–1379. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE Algorithm for Individual Ancestry Estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. Dartr: An r Package to Facilitate Analysis of SNP Data Generated from Reduced Representation Genome Sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Watterson, G.A. On the Number of Segregating Sites in Genetical Models without Recombination. Theor. Popul. Biol. 1975, 7, 256–276. [Google Scholar] [CrossRef]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Whitlock, M.C.; Lotterhos, K.E. Reliable Detection of Loci Responsible for Local Adaptation: Inference of a Null Model through Trimming the Distribution of FST. Am. Nat. 2015, 186, S24–S36. [Google Scholar] [CrossRef]
- Luu, K.; Bazin, E.; Blum, M.G.B. Pcadapt: An R Package to Perform Genome Scans for Selection Based on Principal Component Analysis. Mol. Ecol. Resour. 2017, 17, 67–77. [Google Scholar] [CrossRef]
- Do, C.; Waples, R.S.; Peel, D.; Macbeth, G.M.; Tillett, B.J.; Ovenden, J.R. NeEstimator v2: Re-Implementation of Software for the Estimation of Contemporary Effective Population Size (Ne) from Genetic Data. Mol. Ecol. Resour. 2014, 14, 209–214. [Google Scholar] [CrossRef]
- Sunde, J.; Yıldırım, Y.; Tibblin, P.; Forsman, A. Comparing the Performance of Microsatellites and RADseq in Population Genetic Studies: Analysis of Data for Pike (Esox Lucius) and a Synthesis of Previous Studies. Front. Genet. 2020, 11, 218. [Google Scholar] [CrossRef]
- Zimmerman, S.J.; Aldridge, C.L.; Oyler-McCance, S.J. An Empirical Comparison of Population Genetic Analyses Using Microsatellite and SNP Data for a Species of Conservation Concern. BMC Genom. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Zipfel, K.; Welsh, A. Advancing Conservation Strategies for Native Eastern Highlands-Strain Walleye Sander Vitreus in West Virginia: Insights from Genomic Investigations and Broodstock Screening. Diversity 2024, 16, 371. [Google Scholar] [CrossRef]
- Wahl, D.H. An Ecological Context for Evaluating the Factors Influencing Muskellunge Stocking Success. N. Am. J. Fish. Manag. 1999, 19, 238–248. [Google Scholar] [CrossRef]
- Meerbeek, J.R. Poststocking Behavior and Survival of Large Yearling Muskellunge (Esox Masquinongy) in Two Northern Iowa Natural Lakes. Fishes 2024, 9, 216. [Google Scholar] [CrossRef]
- Miller, L.M.; Mero, S.W.; Younk, J.A. The Impact of Stocking on the Current Ancestry in Twenty Native and Introduced Muskellunge Populations in Minnesota. Trans. Am. Fish. Soc. 2012, 141, 1411–1423. [Google Scholar] [CrossRef]
- Miller, L.M.; Mero, S.W.; Younk, J.A. The Genetic Legacy of Stocking Muskellunge in a Northern Minnesota Lake. Trans. Am. Fish. Soc. 2009, 138, 602–615. [Google Scholar] [CrossRef]
- Page, K.S.; Zweifel, R.D.; Stott, W. Spatial and Temporal Genetic Analysis of Walleyes in the Ohio River. Trans. Am. Fish. Soc. 2017, 146, 1168–1185. [Google Scholar] [CrossRef]
- Guinand, B.; Page, K.S.; Burnham-Curtis, M.K.; Scribner, K.T. Genetic Signatures of Historical Bottlenecks in Sympatric Lake Trout (Salvelinus Namaycush) Morphotypes in Lake Superior. Env. Biol Fish 2012, 95, 323–334. [Google Scholar] [CrossRef]
- Almodóvar, A.; Leal, S.; Nicola, G.G.; Hórreo, J.L.; García-Vázquez, E.; Elvira, B. Long-Term Stocking Practices Threaten the Original Genetic Diversity of the Southernmost European Populations of Atlantic Salmon Salmo Salar. Endanger. Species Res. 2020, 41, 303–317. [Google Scholar] [CrossRef]
- Schmidt, T.L.; Jasper, M.-E.; Weeks, A.R.; Hoffmann, A.A. Unbiased Population Heterozygosity Estimates from Genome-Wide Sequence Data. Methods Ecol. Evol. 2021, 12, 1888–1898. [Google Scholar] [CrossRef]
- Marandel, F.; Charrier, G.; Lamy, J.-B.; Le Cam, S.; Lorance, P.; Trenkel, V.M. Estimating Effective Population Size Using RADseq: Effects of SNP Selection and Sample Size. Ecol. Evol. 2020, 10, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Kapuscinski, K.L.; Sloss, B.L.; Farrell, J.M. Genetic Population Structure of Muskellunge in the Great Lakes. Trans. Am. Fish. Soc. 2013, 142, 1075–1089. [Google Scholar] [CrossRef]
- Weller, J.D.; Leblanc, J.P.; Liskauskas, A.; Chow-Fraser, P. Spawning Season Distribution in Subpopulations of Muskellunge in Georgian Bay, Lake Huron. Trans. Am. Fish. Soc. 2016, 145, 795–809. [Google Scholar] [CrossRef]
- Crossman, E.J. Displacement, and Home Range Movements of Muskellunge Determined by Ultrasonic Tracking. Env. Biol Fish 1977, 1, 145–158. [Google Scholar] [CrossRef]
- Diana, J.S.; Hanchin, P.; Popoff, N. Movement Patterns and Spawning Sites of Muskellunge Esox Masquinongy in the Antrim Chain of Lakes, Michigan. Env. Biol Fish 2015, 98, 833–844. [Google Scholar] [CrossRef]
- Henesy, J.; Goetz, D.; Mullican, J.E. Seasonal Movement Patterns and Summertime Use of Thermal Refuge Areas by Muskellunge in the Nontidal Potomac River, Maryland. North Am. J. Fish. Manag. 2022, 42, 1144–1154. [Google Scholar] [CrossRef]
- Morrison, S.; Warren, L. Seasonal Movements of Muskellunge in North Bend Lake, West Virginia. J. Southeast. Assoc. Fish Wildl. Agencies 2015, 2, 42–49. [Google Scholar]
- Palmer, G.; Williams, J.; Scott, M.; Finne, K.; Johnson, N.; Dutton, D.; Murphy, B.; Hallerman, E.M. Genetic Marker-Assisted Restoration of the Presumptive Native Walleye Fishery in the New River, Virginia and West Virginia. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 2007, 61, 17–22. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).