Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter
Abstract
:1. Introduction
2. Discussion
2.1. Transporter-Conscious Drug Design
2.2. The BBB
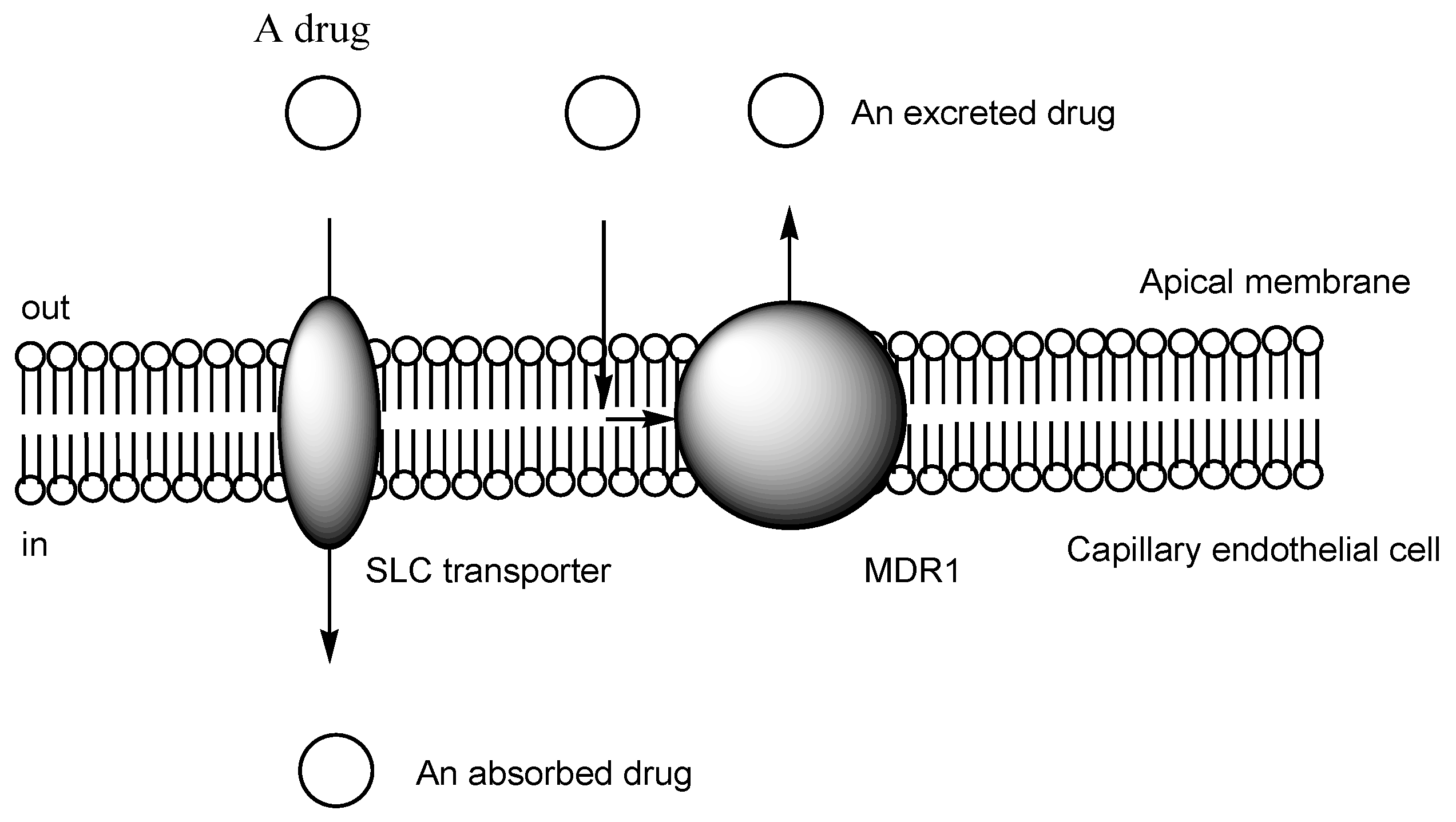
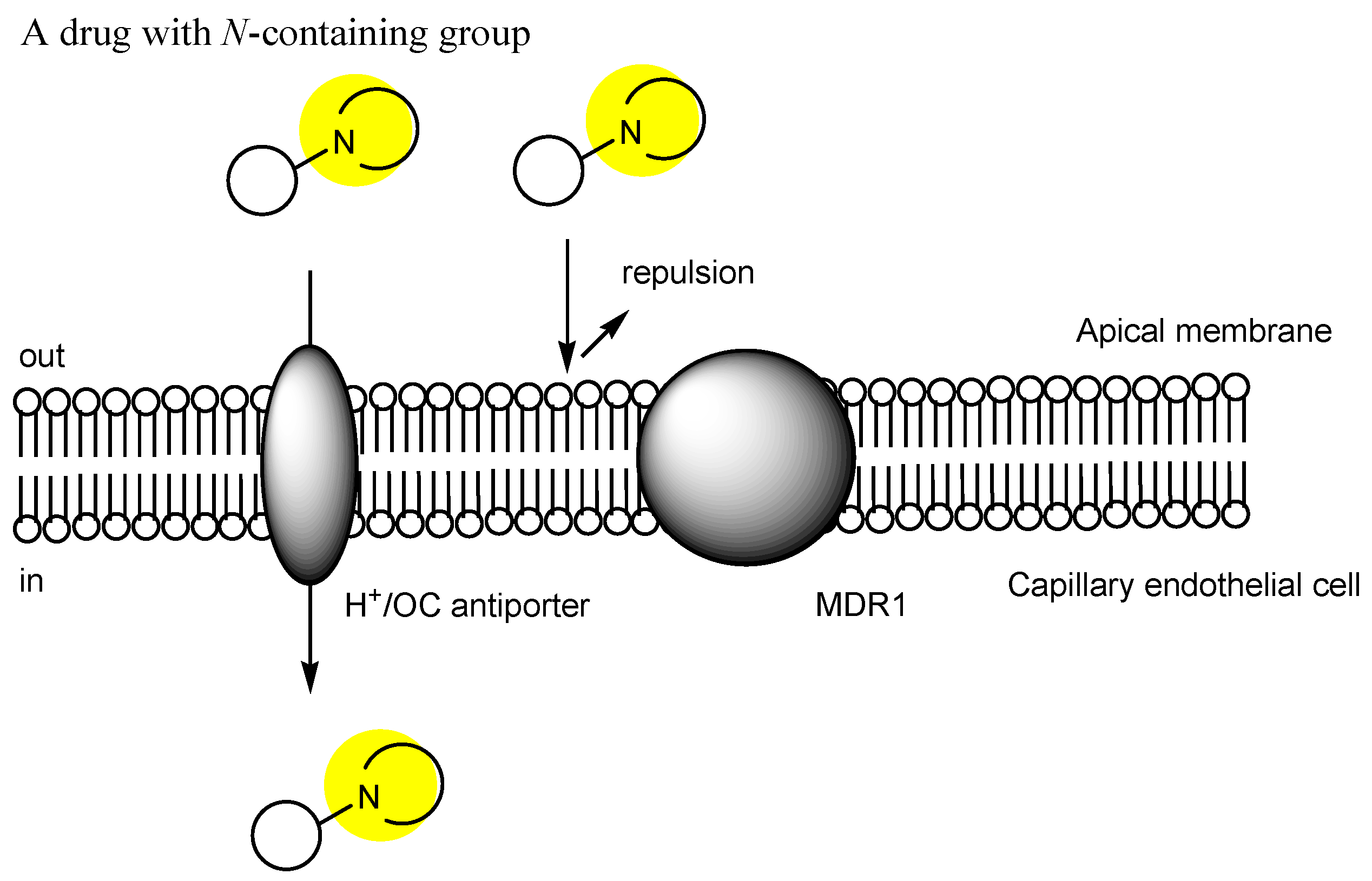
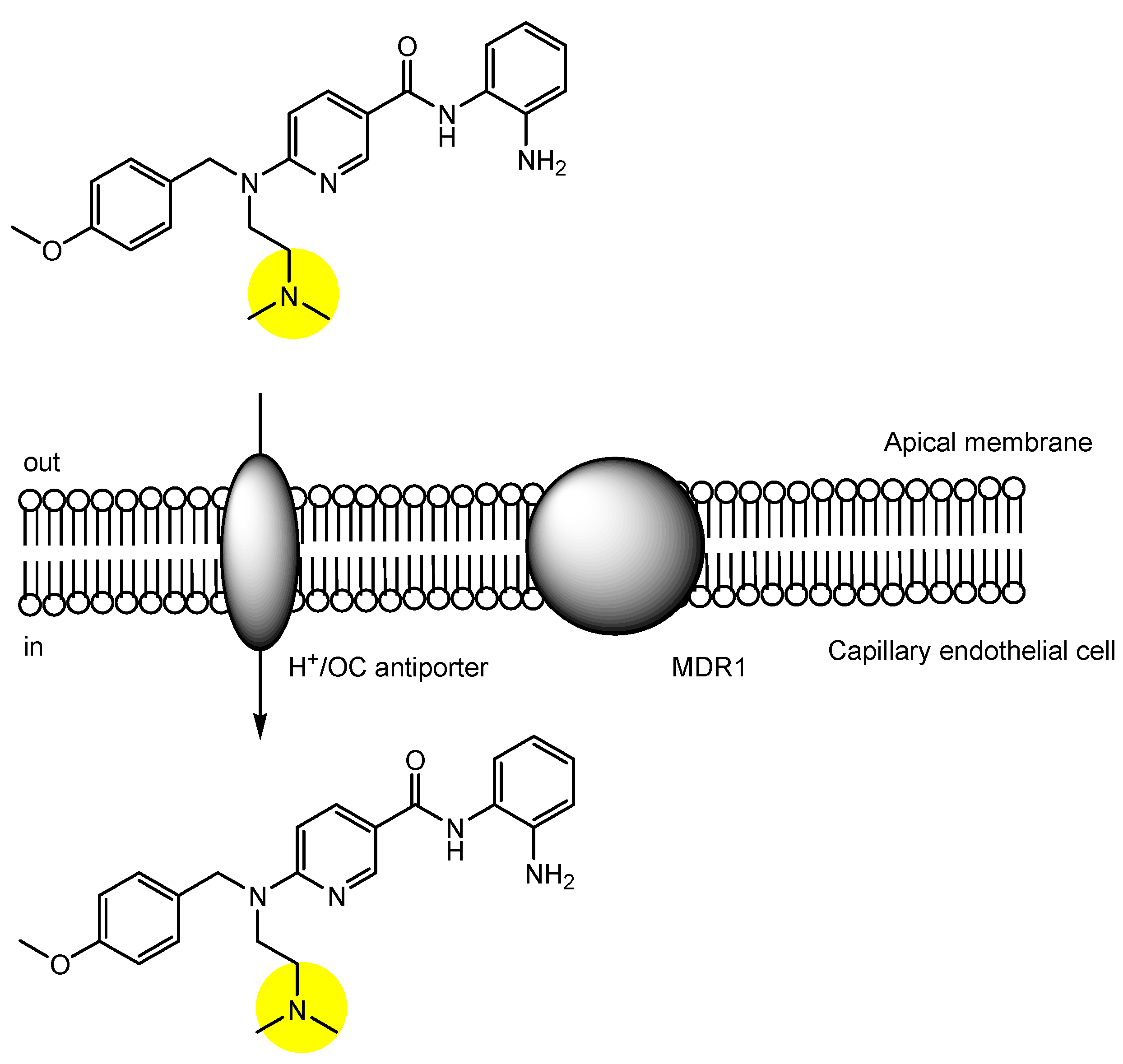
2.3. The Proton-Coupled Organic Cation (H+/OC) Antiporter at the BBB
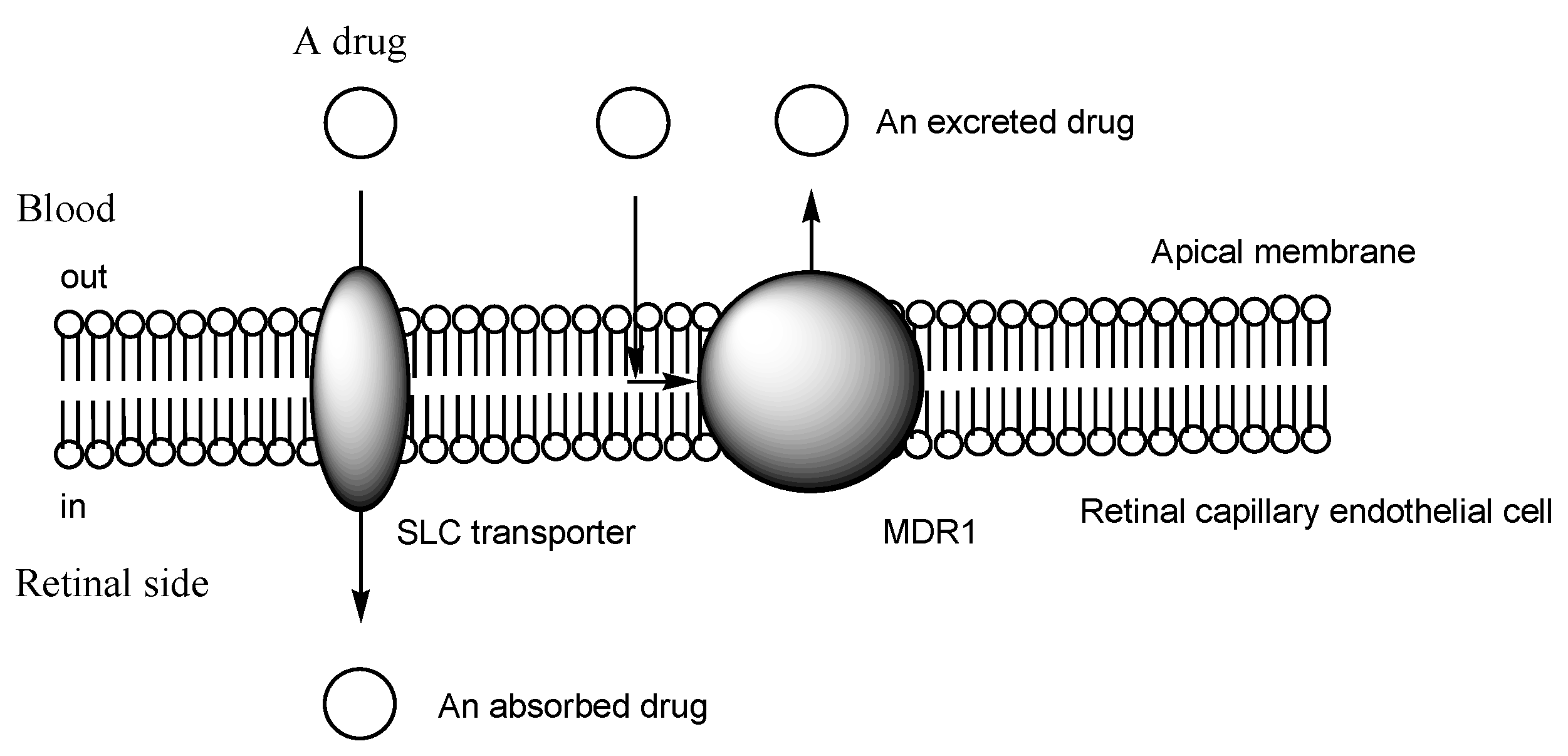
2.4. The BRB
2.5. H+/OC Antiporter at the Inner BRB
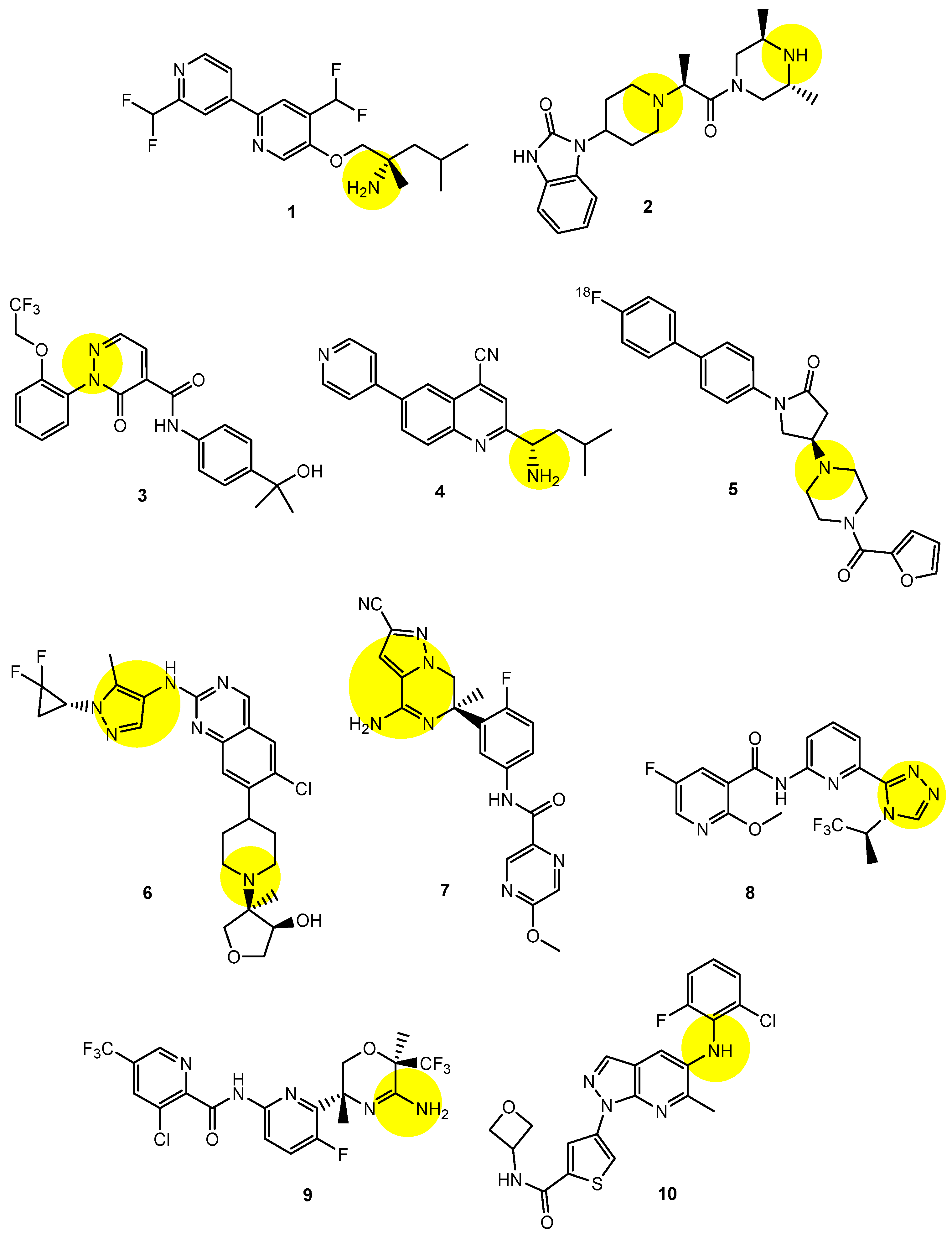
2.6. Implementation of Transporter-Conscious Drug Design with N-Containing Groups
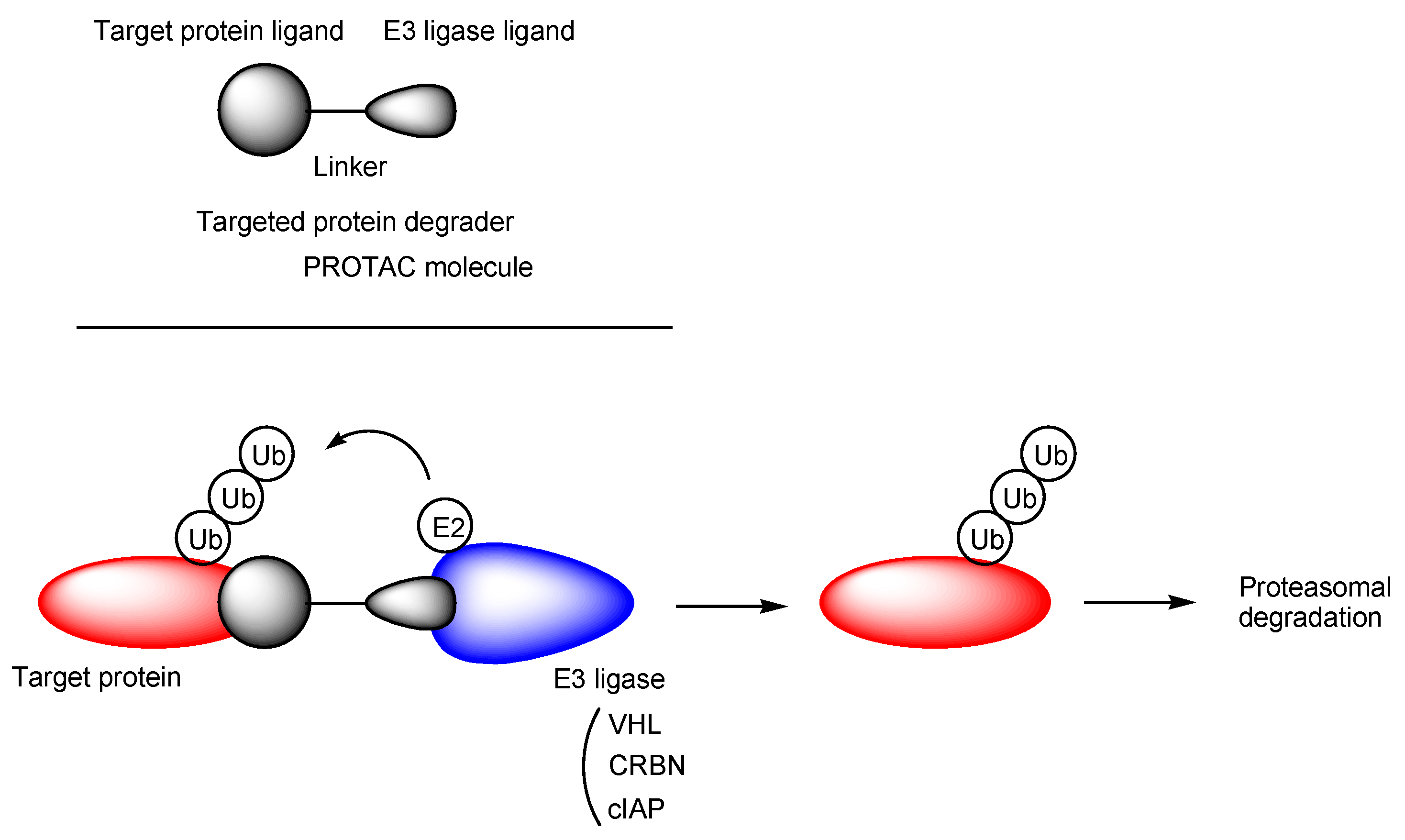
2.7. H+/OC Cation Antiporter-Mediated Transport of PROTACs across the BBB for CNS Diseases
2.8. Eye-Specific Drug Therapy
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stimulus package. Nat. Med. 2018, 24, 247. [CrossRef] [PubMed]
- Angermann, R.; Rauchegger, T.; Nowosielski, Y.; Casazza, M.; Bilgeri, A.; Ulmer, H.; Zehetner, C. Treatment compliance and adherence among patients with diabetic retinopathy and age-related macular degeneration treated by anti-vascular endothelial growth factor under universal health coverage. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood-Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Intriguing possibilities and beneficial aspects of transporter-conscious drug design. Bioorg. Med. Chem. 2015, 23, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Intelligent substance delivery into cells using cell-penetrating peptides. Bioorg. Med. Chem. Lett. 2017, 27, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Effective cancer therapy based on selective drug delivery into cells across their membrane using receptor-mediated endocytosis. Bioorg. Med. Chem. Lett. 2018, 28, 3015–3024. [Google Scholar] [CrossRef]
- Tashima, T. Shortcut Approaches to Substance Delivery into the Brain Based on Intranasal Administration Using Nanodelivery Strategies for Insulin. Molecules 2020, 25, 5188. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Delivery of Intravenously Administered Antibodies Targeting Alzheimer’s Disease-Relevant Tau Species into the Brain Based on Receptor-Mediated Transcytosis. Pharmaceutics 2022, 14, 411. [Google Scholar] [CrossRef]
- Tashima, T. Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates. Biomedicines 2022, 10, 1597. [Google Scholar] [CrossRef]
- Tashima, T. Delivery of Drugs into Cancer Cells Using Antibody–Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect. Antibodies 2022, 11, 78. [Google Scholar] [CrossRef]
- de Mora, F.; Balsa, A.; Cornide-Santos, M.; Carrascosa, J.M.; Marsal, S.; Gisbert, J.P.; Abad, M.-A.; Duarte, R.F.; Wiechmann, M.; Martínez, R. Biosimilar and interchangeable: Inseparable scientific concepts? Br. J. Clin. Pharmacol. 2019, 85, 2460–2463. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Taub, M.E.; Chothe, P.P.; Chu, X.; Giacomini, K.M.; Kim, R.B.; Ray, A.S.; Stocker, S.L.; Unadkat, J.D.; Wittwer, M.B.; et al. Transporters in Drug Development: 2018 ITC Recommendations for Transporters of Emerging Clinical Importance. Clin. Pharmacol. Ther. 2018, 104, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.C.; Saig, F.A.; Cloos, J.; Jansen, G.; Peters, G.J. How to overcome ATP-binding cassette drug efflux transporter-mediated drug resistance? Cancer Drug Resist. 2018, 1, 6–29. [Google Scholar] [CrossRef]
- Hu, C.; Tao, L.; Cao, X.; Chen, L. The solute carrier transporters and the brain: Physiological and pharmacological implications . Asian J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gotfryd, K.; Boesen, T.; Mortensen, J.S.; Khelashvili, G.; Quick, M.; Terry, D.S.; Missel, J.W.; LeVine, M.V.; Gourdon, P.; Blanchard, S.C.; et al. X-ray structure of LeuT in an inward-facing occluded conformation reveals mechanism of substrate release. Nat. Commun. 2020, 11, 1005. [Google Scholar] [CrossRef]
- Kumar, S.; Athreya, A.; Gulati, A.; Nair, R.M.; Mahendran, I.; Ranjan, R.; Penmatsa, A. Structural basis of inhibition of a transporter from Staphylococcus aureus, NorC, through a single-domain camelid antibody. Commun. Biol. 2021, 4, 836. [Google Scholar] [CrossRef]
- Roberts, A.G. The Structure and Mechanism of Drug Transporters. Methods Mol. Biol. 2021, 2342, 193–234. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Liu, S.; Agalliu, D.; Yu, C.; Fisher, M. The Role of Pericytes in Blood-Brain Barrier Function and Stroke. Curr. Pharm. Des. 2012, 18, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castro, B.; Robel, S.; Mishra, A. Astrocyte Endfeet in Brain Function and Pathology: Open Questions. Annu. Rev. Neurosci. 2023, 46, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Chen, L.; Kostich, W.A.; Hamman, B.; Allen, J.; Easton, A.; Bourin, C.; Gulianello, M.; Lippy, J.; Nara, S.; et al. Discovery and Optimization of Biaryl Alkyl Ethers as a Novel Class of Highly Selective, CNS-Penetrable, and Orally Active Adaptor Protein-2-Associated Kinase 1 (AAK1) Inhibitors for the Potential Treatment of Neuropathic Pain. J. Med. Chem. 2022, 65, 4534–4564. [Google Scholar] [CrossRef] [PubMed]
- May-Dracka, T.L.; Gao, F.; Hopkins, B.T.; Hronowski, X.; Chen, T.; Chodaparambil, J.V.; Metrick, C.M.; Cullivan, M.; Enyedy, I.; Kaliszczak, M.; et al. Discovery of Phospholipase D Inhibitors with Improved Drug-like Properties and Central Nervous System Penetrance. ACS Med. Chem. Lett. 2022, 13, 665–673. [Google Scholar] [CrossRef]
- Tanaka, Y.; Seto, M.; Kakegawa, K.; Takami, K.; Kikuchi, F.; Yamamoto, T.; Nakamura, M.; Daini, M.; Murakami, M.; Ohashi, T.; et al. Discovery of Brain-Penetrant Glucosylceramide Synthase Inhibitors with a Novel Pharmacophore. J. Med. Chem. 2022, 65, 4270–4290. [Google Scholar] [CrossRef] [PubMed]
- Hartz, R.A.; Ahuja, V.T.; Nara, S.J.; Kumar, C.M.V.; Manepalli, R.K.V.L.P.; Sarvasiddhi, S.K.; Honkhambe, S.; Patankar, V.; Dasgupta, B.; Rajamani, R.; et al. Bicyclic Heterocyclic Replacement of an Aryl Amide Leading to Potent and Kinase-Selective Adaptor Protein 2-Associated Kinase 1 Inhibitors. J. Med. Chem. 2022, 65, 4121–4155. [Google Scholar] [CrossRef]
- He, Y.; Schild, M.; Grether, U.; Benz, J.; Leibrock, L.; Heer, D.; Topp, A.; Collin, L.; Kuhn, B.; Wittwer, M.; et al. Development of High Brain-Penetrant and Reversible Monoacylglycerol Lipase PET Tracers for Neuroimaging. J. Med. Chem. 2022, 65, 2191–2207. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Gulati, A.; Kattar, S.D.; Johnson, R.E.; Chau, R.W.; Margrey, K.A.; Ardolino, M.J.; Zarate, C.; Poremba, K.E.; Simov, V.; et al. Structure-Guided Discovery of Aminoquinazolines as Brain-Penetrant and Selective LRRK2 Inhibitors. J. Med. Chem. 2022, 65, 838–856. [Google Scholar] [CrossRef]
- Peschiulli, A.; Oehlrich, D.; Van Gool, M.; Austin, N.; Van Brandt, S.; Surkyn, M.; De Cleyn, M.; Vos, A.; Tresadern, G.; Rombouts, F.J.R.; et al. A Brain-Penetrant and Bioavailable Pyrazolopiperazine BACE1 Inhibitor Elicits Sustained Reduction of Amyloid β In Vivo. ACS Med. Chem. Lett. 2022, 13, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.H.; Xin, Z.; Himmelbauer, M.; Dechantsreiter, M.; Enyedy, I.; Hedde, J.; Fang, T.; Coomaraswamy, J.; King, K.W.; Murugan, P.; et al. Discovery of Potent, Selective, and Brain-Penetrant Apoptosis Signal-Regulating Kinase 1 (ASK1) Inhibitors that Modulate Brain Inflammation In Vivo. J. Med. Chem. 2021, 64, 15402–15419. [Google Scholar] [CrossRef]
- Machauer, R.; Lueoend, R.; Hurth, K.; Veenstra, S.J.; Rueeger, H.; Voegtle, M.; Tintelnot-Blomley, M.; Rondeau, J.M.; Jacobson, L.H.; Laue, G.; et al. Discovery of Umibecestat (CNP520): A Potent, Selective, and Efficacious β-Secretase (BACE1) Inhibitor for the Prevention of Alzheimer’s Disease. J. Med. Chem. 2021, 64, 15262–15279. [Google Scholar] [CrossRef]
- Feng, Y.; Park, H.; Ryu, J.C.; Yoon, S.O. N-Aromatic-Substituted Indazole Derivatives as Brain-Penetrant and Orally Bioavailable JNK3 Inhibitors. ACS Med. Chem. Lett. 2021, 12, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.C.; Short, J.L.; Nicolazzo, J.A. Memantine transport across the mouse blood-brain barrier is mediated by a cationic influx H+ antiporter. Mol. Pharm. 2013, 10, 4491–4498. [Google Scholar] [CrossRef] [PubMed]
- Sachkova, A.; Doetsch, D.A.; Jensen, O.; Brockmöller, J.; Ansari, S. How do psychostimulants enter the human brain? Analysis of the role of the proton-organic cation antiporter. Biochem. Pharmacol. 2021, 192, 114751. [Google Scholar] [CrossRef]
- André, P.; Debray, M.; Scherrmann, J.-M.; Cisternino, S.J. Clonidine transport at the mouse blood–brain barrier by a new H+ antiporter that interacts with addictive drugs. Cereb. Blood Flow Metab. 2009, 29, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Okura, T.; Kato, S.; Couraud, P.-O.; Schermann, J.-M.; Terasaki, T.; Deguchi, Y. Functional expression of a proton-coupled organic cation (H+ /OC) antiporter in human brain capillary endothelial cell line hCMEC/D3, a human blood–brain barrier model. Fluids Barriers CNS 2013, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Kitamura, A.; Okura, T.; Deguchi, Y. Memantine transport by a proton-coupled organic cation antiporter in hCMEC/D3 cells, an in vitro human blood-brain barrier model. Drug Metab. Pharmacokinet. 2015, 30, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Ruan, Y.; Lyu, Q.; Qin, X.; Qi, X.; Liu, W.; Kang, L.; Zhang, J.; Wu, C. A proton-coupled organic cation antiporter is involved in the blood-brain barrier transport of Aconitum alkaloids. J. Ethnopharmacol. 2020, 252, 112581. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Gutmann, H.; Krähenbühl, S.; Weksler, B.; Romero, I.; Couraud, P.O.; Tuffin, G.; Drewe, J.; Huwyler, J. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J. Neurochem. 2008, 107, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, B.; Su, H.; Li, J.; Sun, X.; He, Q.; Fu, Y.; Zhang, Z. Pyrilamine-sensitive proton-coupled organic cation (H+/OC) antiporter for brain-specific drug delivery. J. Control. Release 2017, 254, 34–43. [Google Scholar] [CrossRef] [PubMed]
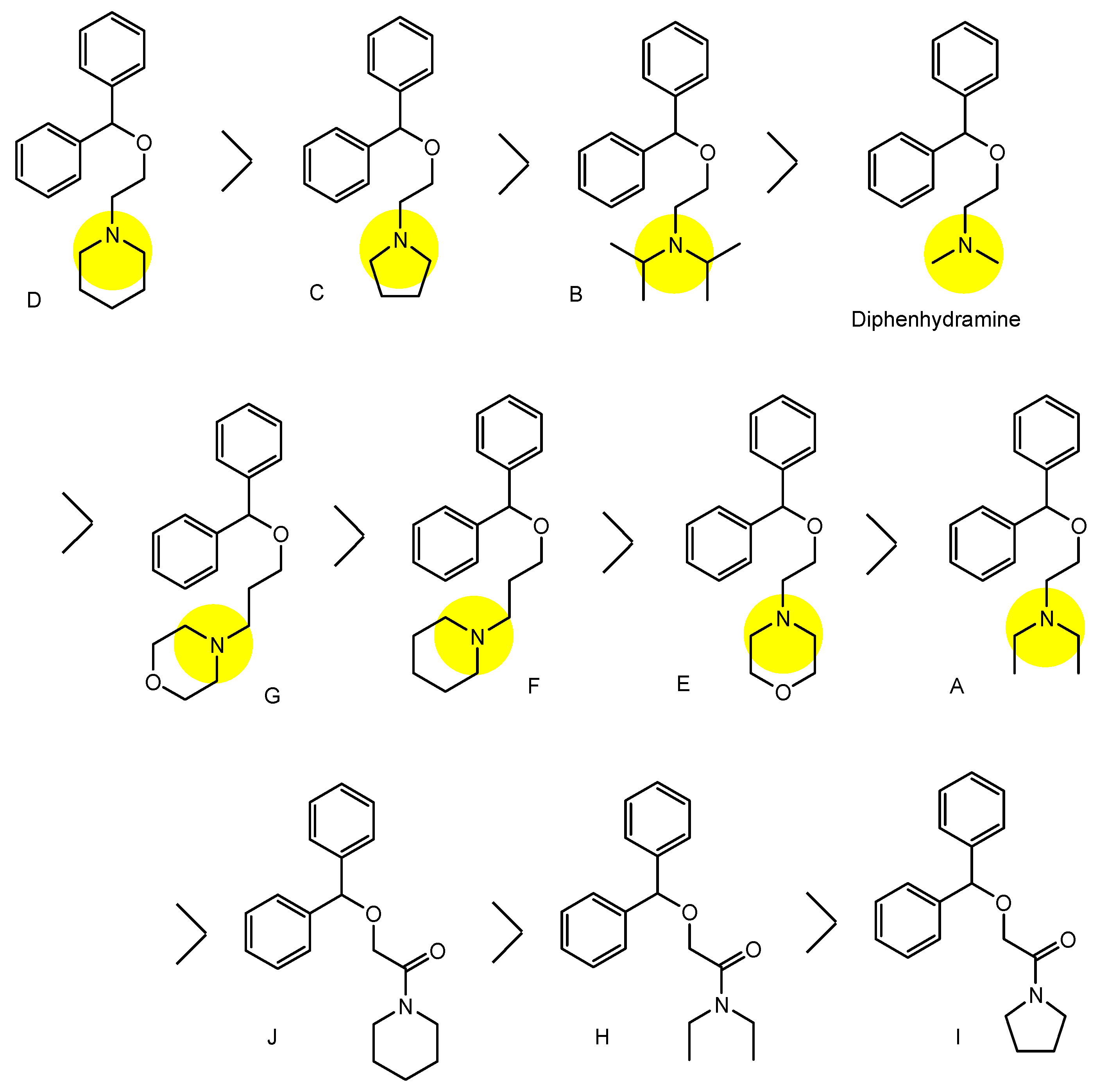
- Tega, Y.; Tabata, H.; Kurosawa, T.; Kitamura, A.; Itagaki, F.; Oshitari, T.; Deguchi, Y. Structural Requirements for Uptake of Diphenhydramine Analogs into hCMEC/D3 Cells Via the Proton-Coupled Organic Cation Antiporter. J. Pharm. Sci. 2021, 110, 397–403. [Google Scholar] [CrossRef]
- Kato, Y.; Sugiura, M.; Sugiura, T.; Wakayama, T.; Kubo, Y.; Kobayashi, D.; Sai, Y.; Tamai, I.; Iseki, S.; Tsuji, A. Organic Cation/Carnitine Transporter OCTN2 (Slc22a5) Is Responsible for Carnitine Transport across Apical Membranes of Small Intestinal Epithelial Cells in Mouse. Mol. Pharmacol. 2006, 70, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017, 139, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.A.; Lawrenson, J.G.; Dolman, D.E.; Arden, G.B.; Abbott, N.J. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res. 2006, 83, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, A.; Kang, Y.S. Blood-to-Retina Transport of Imperatorin Involves the Carrier-Mediated Transporter System at the Inner Blood-Retinal Barrier. J. Pharm. Sci. 2019, 108, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Sharma, P.K.; Malviya, R. Role of Blood Retinal Barrier in Drug Absorption. Pharm. Anal. Acta 2018, 9, 5. [Google Scholar] [CrossRef]
- Kubo, Y.; Tsuchiyama, A.; Shimizu, Y.; Akanuma, S.; Hosoya, K. Involvement of Carrier-Mediated Transport in the Retinal Uptake of Clonidine at the Inner Blood–Retinal Barrier. Mol. Pharm. 2014, 11, 3747–3753. [Google Scholar] [CrossRef]
- Debaisieux, S.; Rayne, F.; Yezid, H.; Beaumelle, B. The Ins and Outs of HIV-1 Tat. Traffic 2012, 13, 355–363. [Google Scholar] [CrossRef]
- Hiranaka, S.; Tega, Y.; Higuchi, K.; Kurosawa, T.; Deguchi, Y.; Arata, M.; Ito, A.; Yoshida, M.; Nagaoka, Y.; Sumiyoshi, T. Design, Synthesis, and Blood-Brain Barrier Transport Study of Pyrilamine Derivatives as Histone Deacetylase Inhibitors. ACS Med. Chem. Lett. 2018, 9, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Xu, C.; Li, Y.; Gong, T.; Sun, X.; Fu, Y.; He, Q.; Zhang, Z. Scopine as a novel brain-targeting moiety enhances the brain uptake of chlorambucil. Bioconjug. Chem. 2014, 25, 2046–2054. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Qi, B.; Gong, T.; Sun, X.; Fu, Y.; Zhang, Z. Brain-Specific Delivery of Dopamine Mediated by N,N-Dimethyl Amino Group for the Treatment of Parkinson’s Disease. Mol. Pharm. 2014, 11, 3174–3185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Jiang, J.; Wang, X.; Fu, Y.; Gong, T.; Sun, X.; Zhang, Z. Mechanism of brain targeting by dexibuprofen prodrugs modified with ethanolamine-related structures. J. Cereb. Blood Flow Metab. 2015, 35, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, J.; Adla, S.K.; Markowicz-Piasecka, M.; Huttunen, K.M. Increased/Targeted Brain (Pro)Drug Delivery via Utilization of Solute Carriers (SLCs). Pharmaceutics 2022, 14, 1234. [Google Scholar] [CrossRef]
- Kawase, A.; Chuma, T.; Irie, K.; Kazaoka, A.; Kakuno, A.; Matsuda, N.; Shimada, H.; Iwaki, M. Increased penetration of diphenhydramine in brain via proton-coupled organic cation antiporter in rats with lipopolysaccharide-induced inflammation. Brain Behav. Immun. Health 2021, 10, 100188. [Google Scholar] [CrossRef] [PubMed]
- Kawase, A.; Kazaoka, A.; Shimada, H.; Iwaki, M. Increased brain penetration of diphenhydramine and memantine in rats with adjuvant-induced arthritis. Brain Res. 2021, 1768, 147581. [Google Scholar] [CrossRef]
- Chapy, H.; Goracci, L.; Vayer, P.; Parmentier, Y.; Carrupt, P.A.; Declèves, X.; Scherrmann, J.M.; Cisternino, S.; Cruciani, G. Pharmacophore-based discovery of inhibitors of a novel drug/proton antiporter in human brain endothelial hCMEC/D3 cell line. Br. J. Pharmacol. 2015, 172, 4888–4904. [Google Scholar] [CrossRef]
- Smirnova, M.; Goracci, L.; Cruciani, G.; Federici, L.; Declèves, X.; Chapy, H.; Cisternino, S. Pharmacophore-Based Discovery of Substrates of a Novel Drug/Proton-Antiporter in the Human Brain Endothelial hCMEC/D3 Cell Line. Pharmaceutics 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Calabrese, M.F.; Bullock, A.N.; Crews, C.M. Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discovery 2019, 18, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Wang, J.; Xing, D. VHL-based PROTACs as potential therapeutic agents: Recent progress and perspectives. Eur. J. Med. Chem. 2022, 227, 113906. [Google Scholar] [CrossRef]
- Barankiewicz, J.; Salomon-Perzyński, A.; Misiewicz-Krzemińska, I.; Lech-Marańda, E. CRL4CRBN E3 Ligase Complex as a Therapeutic Target in Multiple Myeloma. Cancers 2022, 14, 4492. [Google Scholar] [CrossRef]
- Ma, Z.; Ji, Y.; Yu, Y.; Liang, D. Specific non-genetic IAP-based protein erasers (SNIPERs) as a potential therapeutic strategy. Eur. J. Med. Chem. 2021, 216, 113247. [Google Scholar] [CrossRef]
- Chen, M. Permeability Characterization and Potential Transporter(s) Identification for Immunomodulatory Drugs (IMiDs) and Application of Pharmacokinetic Modeling in Resistance in Multiple Myeloma. Doctoral Dissertation, Ohio State University, OhioLINK Electronic Theses and Dissertations Center, Columbus, OH, USA, 2022. Available online: https://rave.ohiolink.edu/etdc/view?acc_num=osu1641214592550088 (accessed on 7 July 2023).
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Rasool, A.; Shaheryar, M.; Sarfraz, A.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare 2023, 11, 32. [Google Scholar] [CrossRef]
- Inuzuka, H.; Liu, J.; Wei, W.; Rezaeian, A.-H. PROTAC technology for the treatment of Alzheimer’s disease: Advances and perspectives. Acta Mater Med. 2022, 1, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Ferguson, F.M.; Cai, Q.; Donovan, K.A.; Nandi, G.; Patnaik, D.; Zhang, T.; Huang, H.T.; Lucente, D.E.; Dickerson, B.C.; et al. Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. eLife 2019, 8, e45457. [Google Scholar] [CrossRef] [PubMed]
- Vagrys, D.; Davidson, J.; Chen, I.; Hubbard, R.E.; Davis, B. Exploring IDP–Ligand Interactions: Tau K18 as a Test Case. Int. J. Mol. Sci. 2020, 21, 5257. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.; Lucas, X.; Ciulli, A. Thioamide substitution to probe the hydroxyproline recognition of VHL ligands. Bioorg. Med. Chem. 2018, 26, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zheng, J.; Wang, X.; Liu, Y.; Deng, M.; Ke, D.; et al. A Novel Small-molecule PROTAC Selectively Promotes Tau Clearance to Improve Cognitive Functions in Alzheimerlike Models. Theranostics 2021, 11, 5279–5295. [Google Scholar] [CrossRef]
- Laughlin, C.D.; D’Aquili, E.G. Biogenetic Structuralism; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Leavy, S.A. Biogenetic Structuralism. Yale J. Biol. Med. 1976, 49, 420–421. [Google Scholar]
- Narum, T. Novel Visible Light Photoactivatable Caged NeurotransmittersBased on a N-Methyl Quinolinium Chromophore. Yakugaku Zasshi 2019, 139, 263–271. [Google Scholar] [CrossRef]
- Subbaraya, I.; Ruiz, C.C.; Helekar, B.S.; Zhao, X.; Gorczyca, W.A.; Pettenati, M.J.; Rao, P.N.; Palczewski, K.; Baehr, W. Molecular characterization of human and mouse photoreceptor guanylate cyclase-activating protein (GCAP) and chromosomal localization of the human gene. J. Biol. Chem. 1994, 269, 31080–31089. [Google Scholar] [CrossRef] [PubMed]
- Young, J.E.; Vogt, T.; Gross, K.W.; Khani, S.C. A short, highly active photoreceptor-specific enhancer/promoter region upstream of the human rhodopsin kinase gene. Investig. Ophthalmol. Vis Sci. 2003, 44, 4076–4085. [Google Scholar] [CrossRef]
- Hammid, A.; Fallon, J.K.; Lassila, T.; Salluce, G.; Smith, P.C.; Tolonen, A.; Sauer, A.; Urtti, A.; Honkakoski, P. Carboxylesterase Activities and Protein Expression in Rabbit and Pig Ocular Tissues. Mol. Pharm. 2021, 18, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.-S. Brain-specific aminopeptidase: From enkephalinase to protector against neurodegeneration. Neurochem. Res. 2007, 32, 2062–2071. [Google Scholar] [CrossRef]
- Frey, A.; Meckelein, B.; Weiler-Güttler, H.; Möckel, B.; Flach, R.; Gassen, H.G. Pericytes of the brain microvasculature express γ-glutamyl transpeptidase. Eur. J. Biochem. 1991, 202, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Dingler, A.; Albrecht, U.; Dehouck, M.-P.; Cecchelli, R. Blood-brain barrier pericytes are the main source of γ-glutamyltranspeptidase activity in brain capillaries. J. Neurochem. 1992, 58, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Bausback, H.H.; Churchill, L.; Ward, P.E. Angiotensin metabolism by cerebral microvascular aminopeptidase a. Biochem. Pharmacol. 1988, 37, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wilk, S.; Healy, D.P. Glutamyl aminopeptidase (aminopeptidase A), the BP-1/6C3 antigen. Adv. Neuroimmunol. 1993, 3, 195–207. [Google Scholar] [CrossRef]
- Song, L.; Wilk, E.; Wilk, S.; Healy, D.P. Localization of immunoreactive glutamyl aminopeptidase in rat brain. I. Association with cerebral microvessels. Brain Res. 1993, 606, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hamblett, K.J.; Jacob, A.P.; Gurgel, J.L.; Tometsko, M.E.; Rock, B.M.; Patel, S.K.; Milburn, R.R.; Siu, S.; Ragan, S.P.; Rock, D.A.; et al. SLC46A3 is required to transport catabolites of noncleavable antibody maytansine conjugates from the lysosome to the cytoplasm. Cancer Res. 2015, 75, 5329–5340. [Google Scholar] [CrossRef] [PubMed]




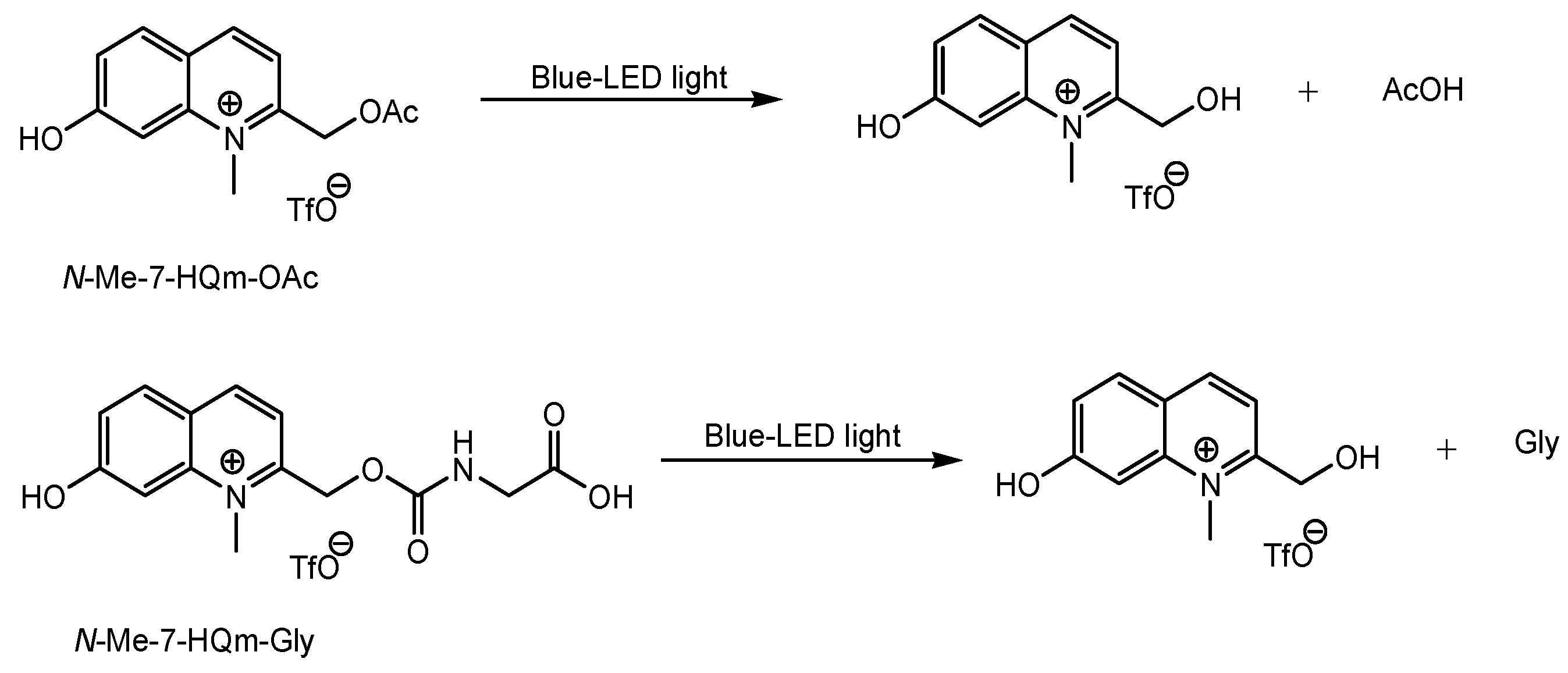
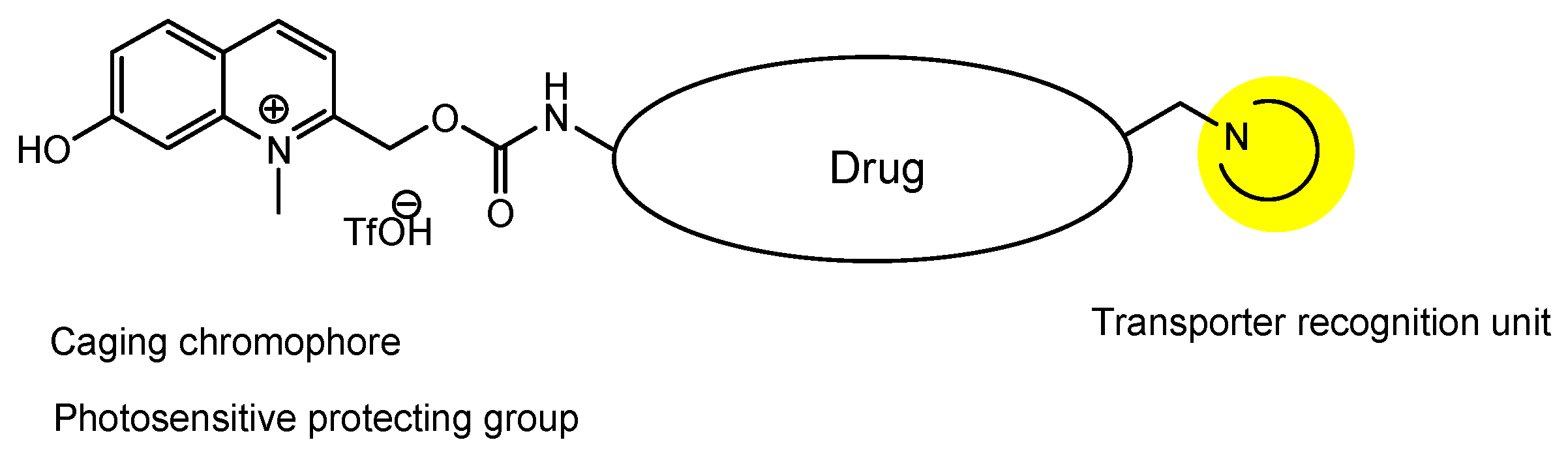
| # | Categories | Transporters/Subtypes | Substrates |
|---|---|---|---|
| (1) | Amine transporters | The proton-coupled organic cation (H+/OC) antiporter, organic cation transporter novel type 1 (OCTN1), OCTN2, OCTN3, multidrug and toxin extrusion protein 1 (MATE1), MATE2, MATE3, plasma membrane monoamine transporter (PMAT) | Cationic amine compounds |
| (2) | Peptide transporters | Peptide transporter 1 (PEPT1), PEPT2 | Peptides |
| (3) | Amino acid transporters | L-type amino acid transporter 1 (LAT1), LAT2, LAT3, LAT4 | Amino acids |
| (4) | Organic cation transporters (OCTs) | OCT1, OCT2, OCT3, OCT4 | Cationic compounds |
| (5) | Organic anion transporters (OATs) | OAT1, OAT2, OAT3, OAT4, OAT5, organic anion transporting peptides (OATP1A2), OATP1B1, OATP1B3, OATP1C1 OATP2A1, OATP2B1, OATP3A1, OATP4A1, OATP4C1, OATP5A1, OATP6A1 | Anionic compounds |
| (6) | Glucose transporters | Glucose transporter1 (GLUT1), GLUT2, GLUT3, GLUT4, GLUT5, GLUT6, GLUT7 | Glucose |
| # | Compounds | The Barrier to Cross | Tissues to Be Absorbed | Status | References |
|---|---|---|---|---|---|
| (1) | 1–10 | BBB | Brain | Basic research | Figure 3, [21,22,23,24,25,26,27,28,29,30] |
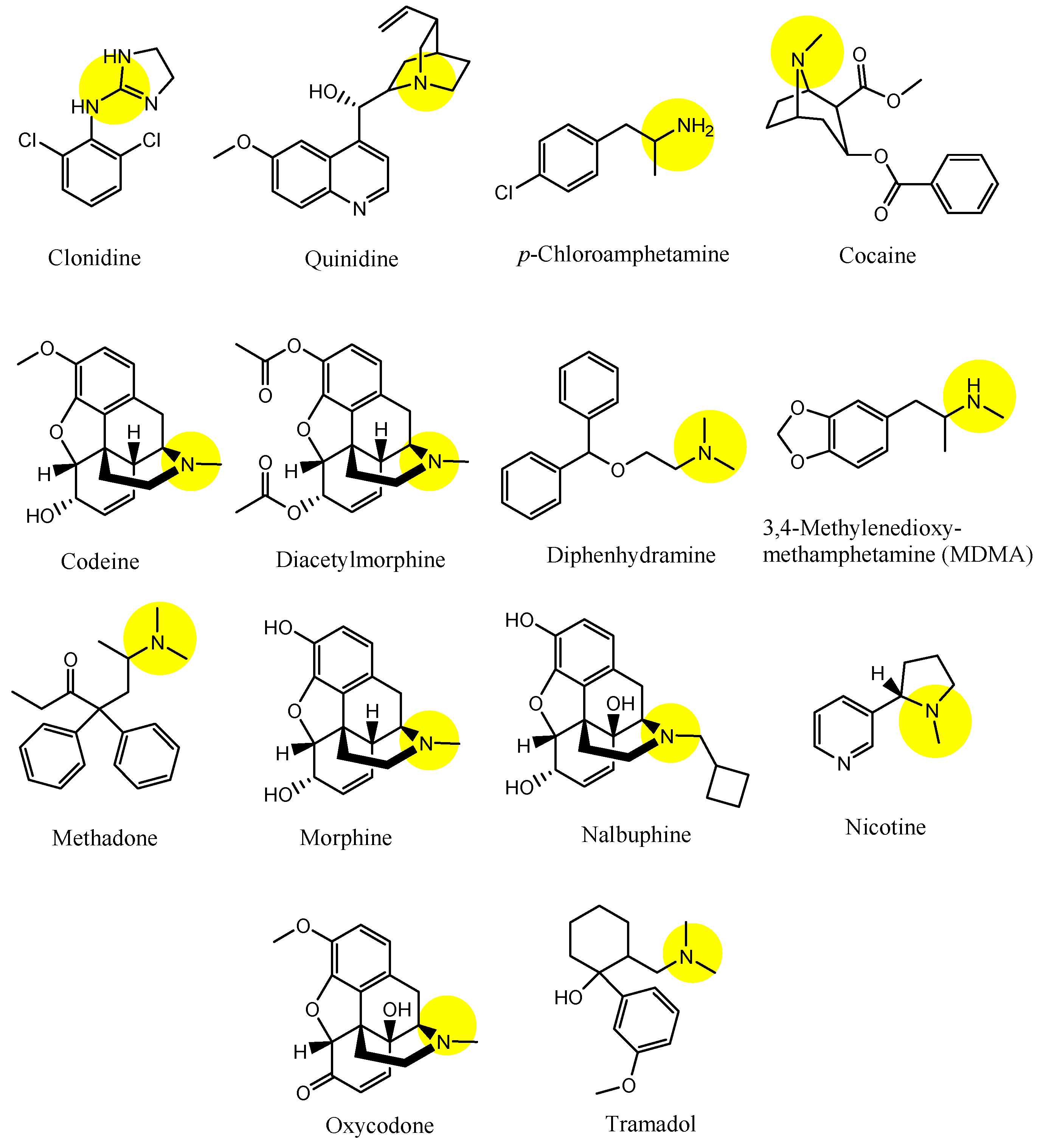
| (2) | Clonidine | BBB, Inner BRB | Brain, eyes | Launched | Figure 4, [33,45] |
| (3) | Quinidine | BBB, Inner BRB | Brain, eyes | Launched | Figure 4 and Figure 5, [33,34,35,36,45] |
| (4) | p-Chloroamphetamine | BBB | Brain | Launched | Figure 4, [33] |
| (5) | Cocaine | BBB | Brain | Launched | Figure 4, [33] |
| (6) | Codeine | BBB | Brain | Launched | Figure 4, [33] |
| (7) | Diacetylmorphine | BBB | Brain | Launched | Figure 4, [33] |
| (8) | Diphenhydramine | BBB | Brain | Launched | Figure 4 and Figure 5, [33,34,35,36] |
| (9) | MDMA (3,4-methylenedioxymethamphetamine) | BBB | Brain | Basic research | Figure 4, [33] |
| (10) | Methadone | BBB | Bain | Launched | Figure 4, [33] |
| (11) | Morphine | BBB | Braun | Launched | Figure 4, [33] |
| (12) | Nalbuphine | BBB | Brain | Launched | Figure 4, [33] |
| (13) | Nicotine | BBB | Brain. | Launched | Figure 4, [33] |
| (14) | Oxycodone | BBB | Brain | Launched | Figure 4, [33] |
| (15) | Tramadol | BBB, Inner BRB | Brain, eyes | Launched | Figure 4, [33,45] |
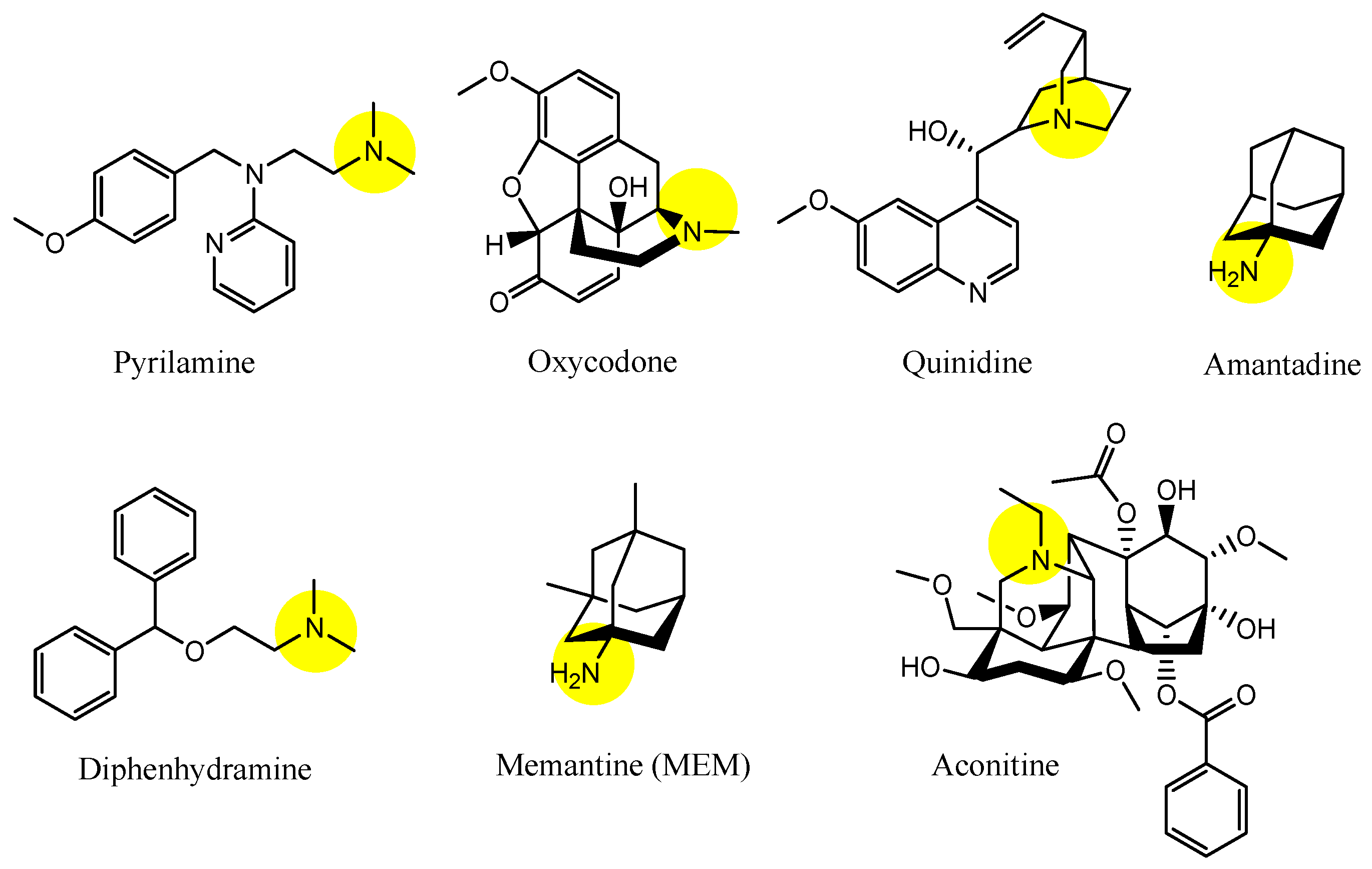
| (16) | Pyrilamine | BBB, Inner BRB | Brain, eyes | Launched | Figure 5, [34,35,36,45] |
| (17) | Oxycodone | BBB | Brain | Launched | Figure 5, [34,35,36] |
| (18) | Amantadine | BBB, Inner BRB | Brain, eyes | Launched | Figure 5, [34,35,36,45] |
| (19) | Memantine (MEM) | BBB | Brain | Launched | Figure 5, [31,34,35,36] |
| (20) | Aconitine | BBB | Brain | Basic research | Figure 5, [34,35,36] |
| (21) | Desipramine | Inner BRB | Eyes | Launched | [45] |
| (22) | Propranolol | Inner BRB | Eyes | Launched | [45] |
| (23) | Verapamil | Inner BRB | Eyes | Launched | [45] |
| (24) | Imipramine | Inner BRB | Eyes | Launched | [45] |
| (25) | Pyrilamine derivative with benzamide | BBB | Brain | Basic research | Figure 8, [47] |
| (26) | Diphenhydramine analogs | BBB | Brain | Basic research | Figure 9, [39] |
| (27) | Chlorambucil-scopine (CHLS) | BBB | Brain | Basic research | Figure 10, [38,48] |
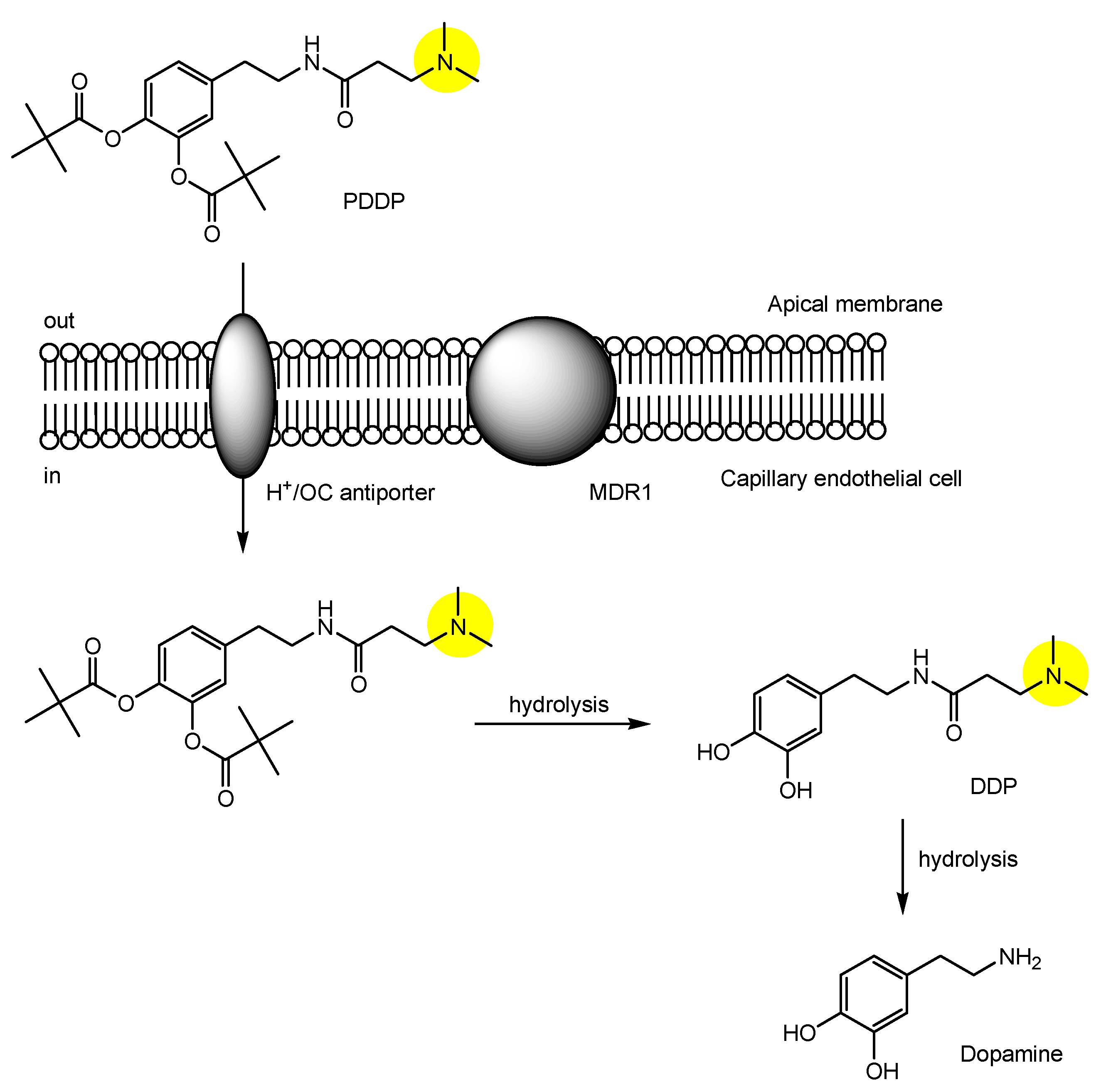
| (28) | N-[3,4-bis(pivaloyloxy)domapine]-3-(dimethylamino)propanamide (PDDP) | BBB | Brain | Basic research | Figure 11, [49] |
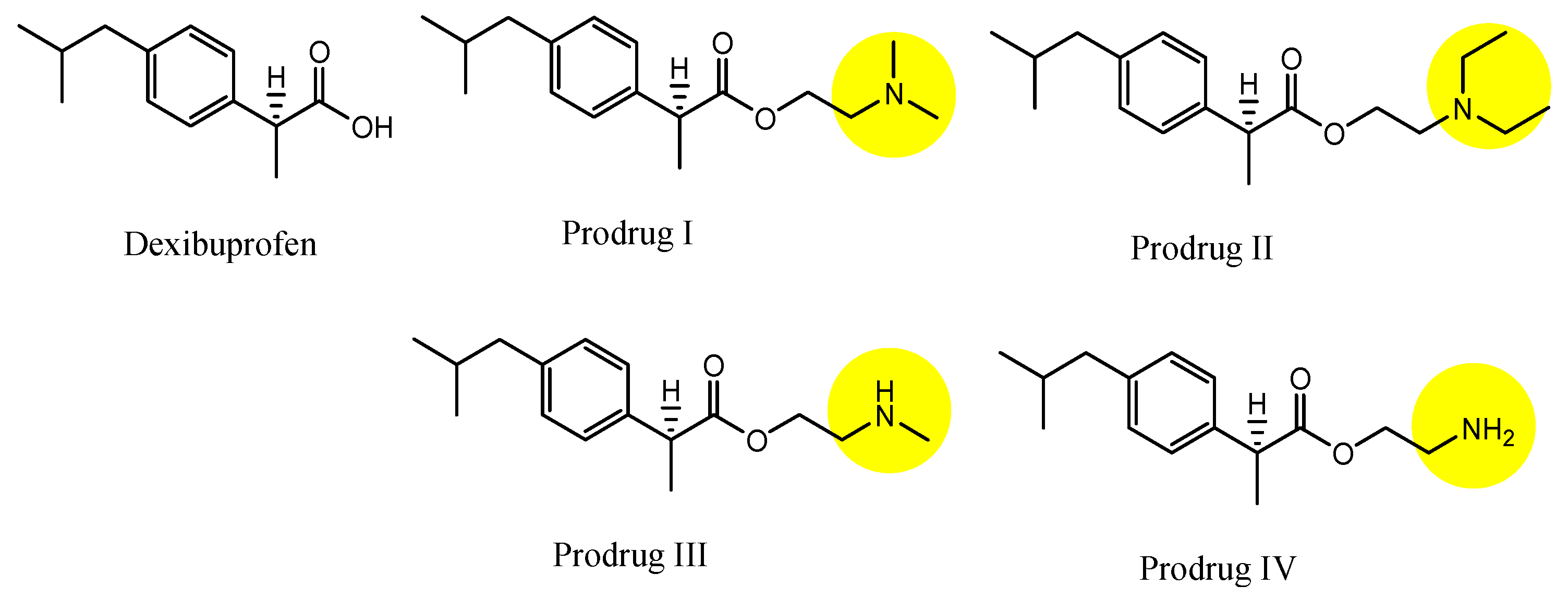
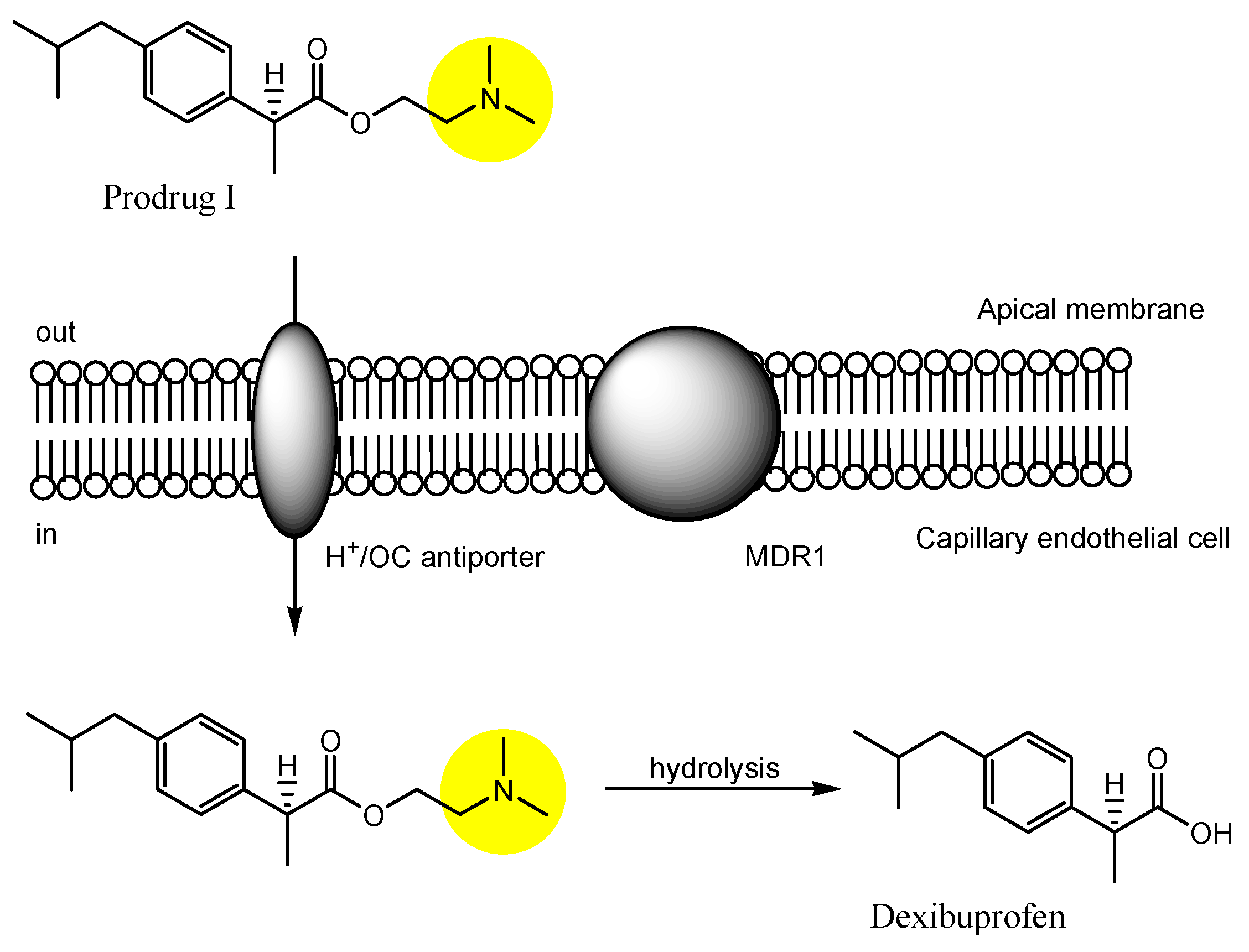
| (29) | Dexibuprofen prodrugs | BBB | Brain | Basic research | Figure 12 and Figure 13, [50] |
| (30) | QC-01-175 | BBB | Brain | Basic research | Figure 16, [66] |
| (31) | C004019 | BBB | Brain | Basic research | Figure 16, [69] |
| (32) | PROTACs with vectors | BBB | Brain | Under analysis in Tashima lab | - |
| (33) | Prodrugs with N-containing group and N-Me-7-HQm | Inner BRB | Eyes | Under analysis in Tashima lab | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashima, T. Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter. Future Pharmacol. 2023, 3, 742-762. https://doi.org/10.3390/futurepharmacol3040046
Tashima T. Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter. Future Pharmacology. 2023; 3(4):742-762. https://doi.org/10.3390/futurepharmacol3040046
Chicago/Turabian StyleTashima, Toshihiko. 2023. "Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter" Future Pharmacology 3, no. 4: 742-762. https://doi.org/10.3390/futurepharmacol3040046
APA StyleTashima, T. (2023). Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter. Future Pharmacology, 3(4), 742-762. https://doi.org/10.3390/futurepharmacol3040046







