Ocular Drug Delivery into the Eyes Using Drug-Releasing Soft Contact Lens
Abstract
1. Introduction
2. Discussion
2.1. Potential Drug Pathways from DR-SCLs Based on Anatomical Eye Features
2.2. Drug-Releasing Soft Contact Lenses (DR-SCLs)
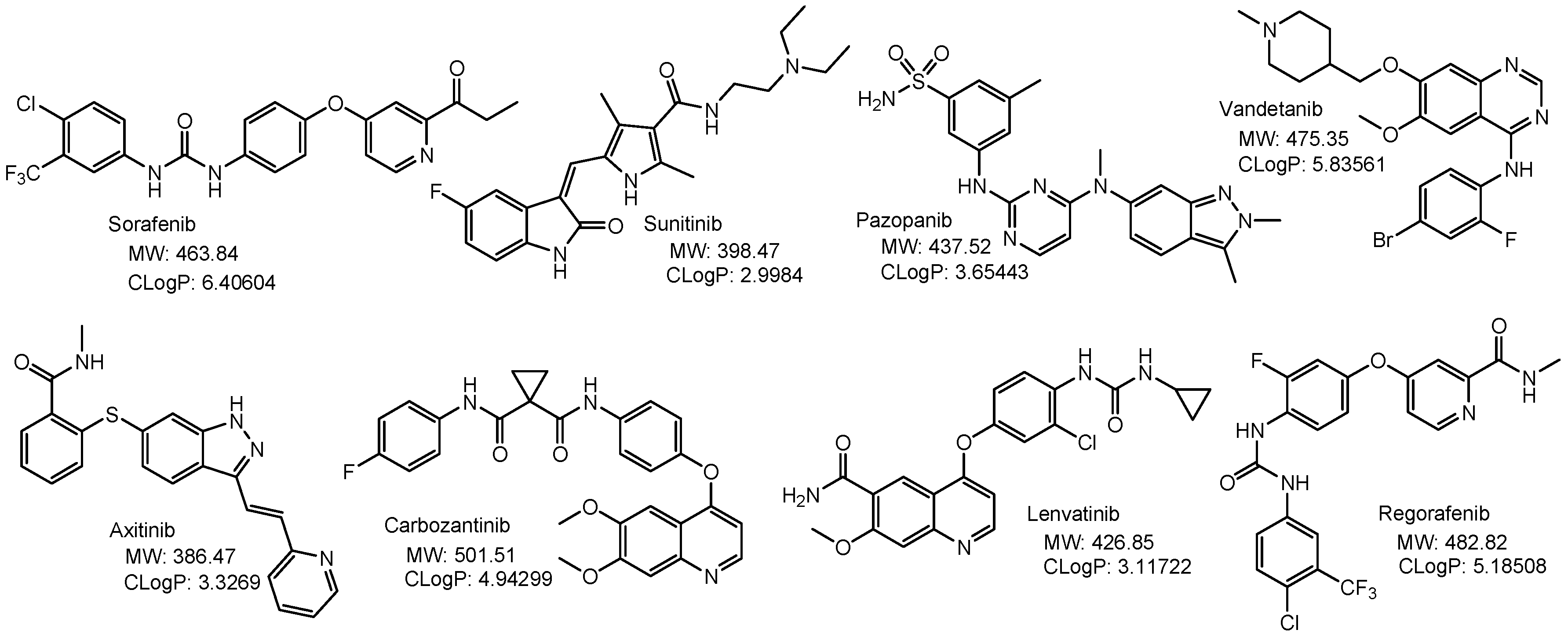
2.3. Ocular Diseases and DR-SCLs
2.3.1. Age-Related Macular Degeneration (AMD)
2.3.2. Diabetic Retinopathy (DR)
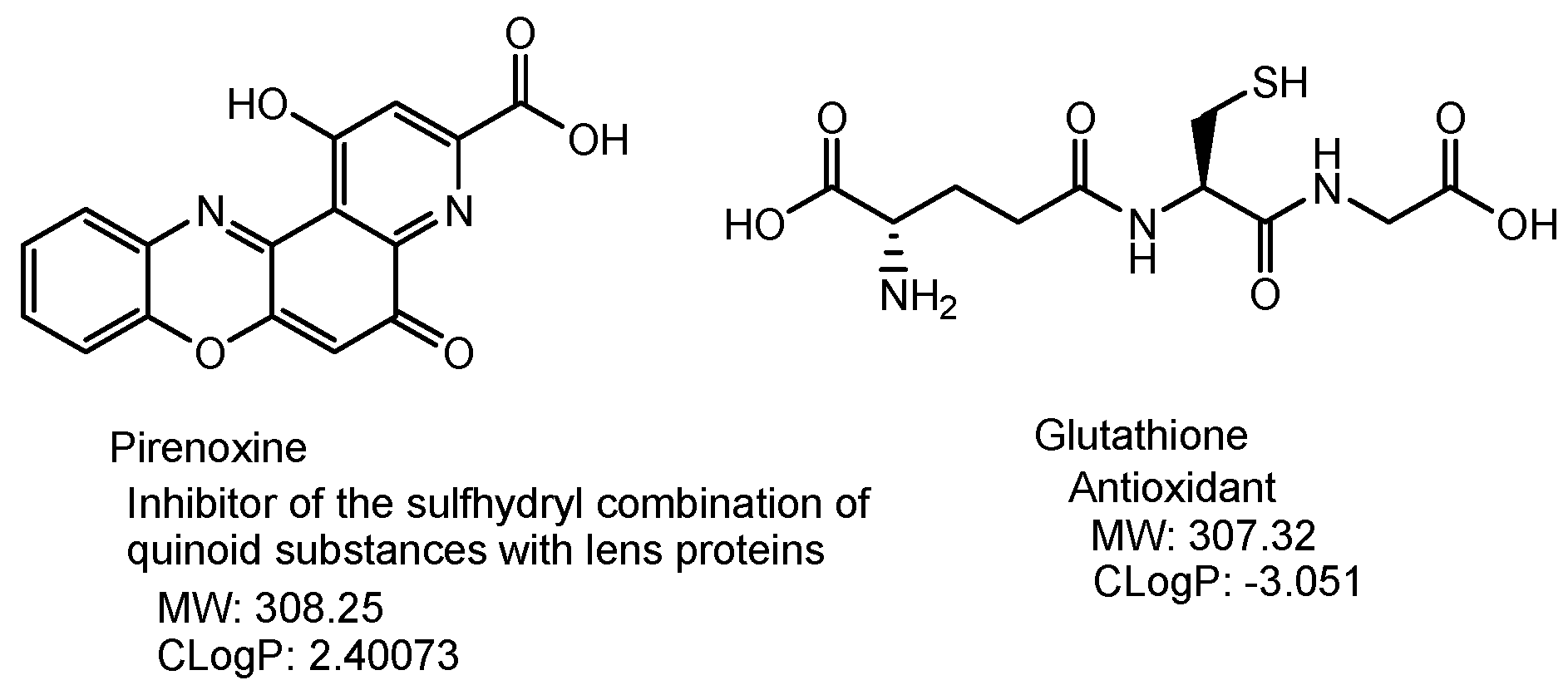
2.3.3. Cataract
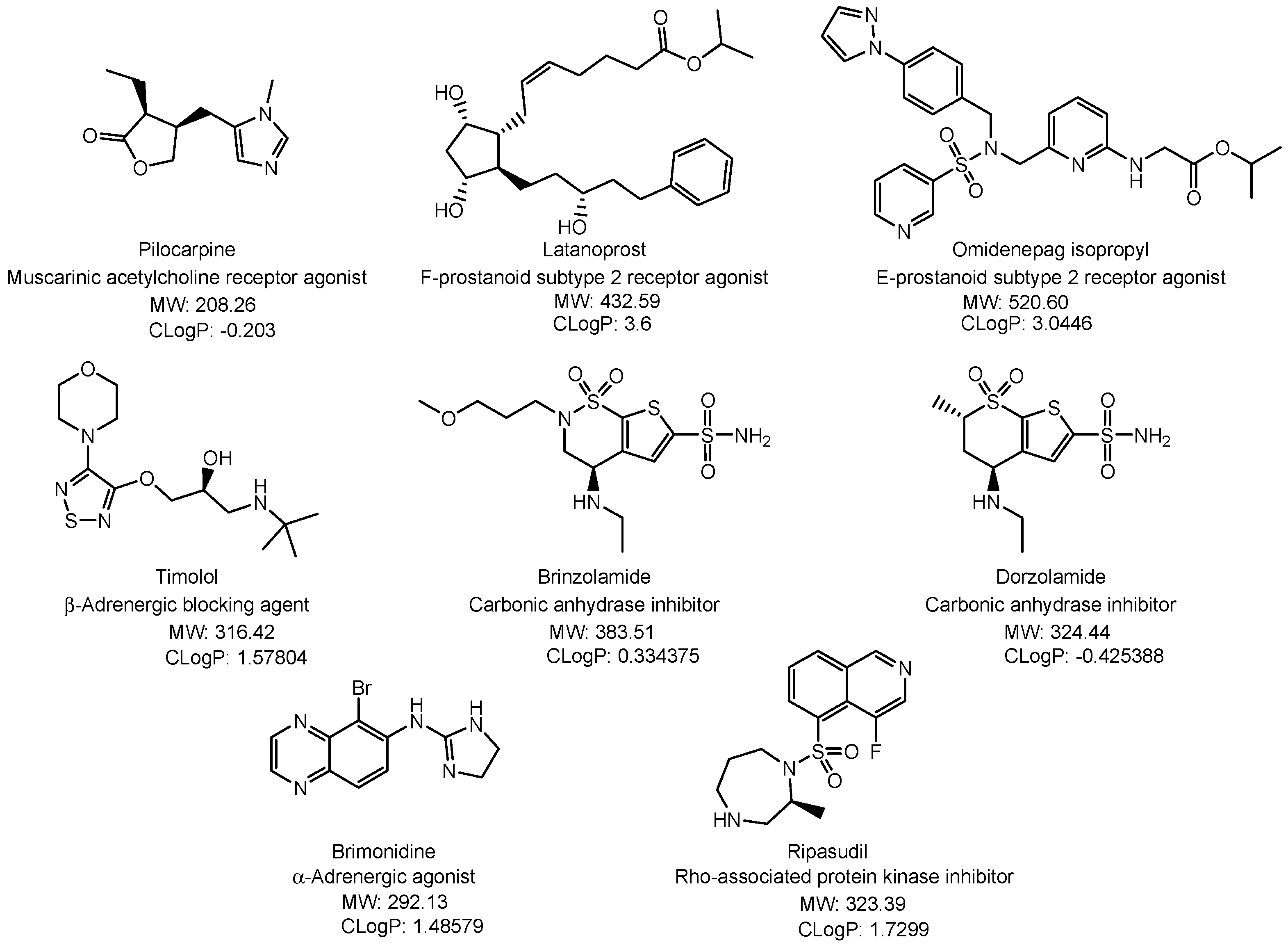
2.3.4. Glaucoma
2.3.5. Dry Eye Disease
2.3.6. Neurotrophic Keratitis
2.3.7. Neuropathic Corneal Pain
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Leary, F.; Campbell, M. The blood–retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Coca-Prados, M. The Blood-Aqueous Barrier in Health and Disease. J. Glaucoma 2014, 23 (Suppl. 1), S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, C.D.; D’Aquili, E.G. Biogenetic Structuralism; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Leavy, S.A. Biogenetic Structuralism. Yale J. Biol. Med. 1976, 49, 420–421. [Google Scholar]
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Carrier-Mediated Delivery of Low-Molecular-Weight N-Containing Drugs across the Blood–Brain Barrier or the Blood–Retinal Barrier Using the Proton-Coupled Organic Cation Antiporter. Future Pharmacol. 2023, 3, 742–762. [Google Scholar] [CrossRef]
- Tashima, T. Substance Delivery across the Blood-Brain Barrier or the Blood-Retinal Barrier using Organic Cation Transporter Novel Type 2 (OCTN2). Future Pharmacol. 2024. under review. [Google Scholar]
- Majumdar, S.; Hingorani, T.; Srirangam, R. Evaluation of Active and Passive Transport Processes in Corneas Extracted from Preserved Rabbit Eyes. J. Pharm. Sci. 2010, 99, 1921–1930. [Google Scholar] [CrossRef]
- Chemuturi, N.; Yanez, J.A. The Role of Xenobiotic Transporters in Ophthalmic Drug Delivery. J. Pharm. Pharm. Sci. 2013, 16, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Morita, S.; Matsuo, M.; Ueda, K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007, 98, 1303–1310. [Google Scholar]
- Arunachalam, D.; Namperumalsamy, V.P.; Prajna, L.; Kuppamuthu, D. Human Corneal Epithelial Cells Internalize Aspergillus flavus Spores by Actin-Mediated Endocytosis. Infect Immun. 2021, 89, e00794-20. [Google Scholar] [CrossRef]
- Bioadhesive glycosylated nanoformulations for extended trans-corneal drug delivery to suppress corneal neovascularization. J. Mater. Chem. B 2021, 9, 4190–4200. [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Danysh, B.P.; Duncan, M.K. The Lens Capsule. Exp. Eye Res. 2009, 88, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Koliakos, G.G.; Kouzi-Koliakos, K.; Furcht, L.T.; Reger, L.A.; Tsilibary, E.C. The Binding of Heparin to Type IV Collagen: Domain Specificity with Identification of Peptide Sequences from the al(1V) and a2(IV) Which Preferentially Bind Heparin. J. Biol. Chem. 1989, 264, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Song, J.; Du, Y.; Ren, C.; Guo, B.; Bi, H. Therapeutic applications of contact lens-based drug delivery systems in ophthalmic diseases. Drug Deliv. 2023, 30, 2219419. [Google Scholar] [CrossRef] [PubMed]
- Toffoletto, N.; Saramago, B.; Serro, A.P.; Chauhan, A. A Physiology-Based Mathematical Model to Understand Drug Delivery from Contact Lenses to the Back of the Eye. Pharm. Res. 2023, 40, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pelis, R.M. Drug Transport by the Blood-Aqueous Humor Barrier of the Eye. Drug Metab. Dispos. 2016, 44, 1675–1681. [Google Scholar] [CrossRef]
- Civan, M.M. Transport by the Ciliary Epithelium of the Eye. Physiology 1997, 12, 158–162. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, M.; Xing, D.; Zhang, J.; Fang, T.; Zhang, F.; Nie, Z.; Liu, Y.; Yang, L.; Li, J.; et al. Contact lens as an emerging platform for ophthalmic drug delivery: A systematic review. Asian J. Pharm. Sci. 2023, 18, 100847. [Google Scholar] [CrossRef]
- Hosoya, K.; Lee, V.H.; Kim, K.J. Roles of the conjunctiva in ocular drug delivery: A review of conjunctival transport mechanisms and their regulation. Eur. J. Pharm. Biopharm. 2005, 60, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Gade, S.; Glover, K.; Sheshala, R.; Singh, T.R.R. Vitreous Humor: Composition, Characteristics and Implication on Intravitreal Drug Delivery. Curr. Eye Res. 2023, 48, 208–218. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Qi, P. Therapeutic contact lenses for ophthalmic drug delivery: Major challenges. J. Biomater. Sci. Polym Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Holgado, M.A.; Anguiano-Domínguez, A.; Martín-Banderas, L. Contact lenses as drug-delivery systems: A promising therapeutic tool. Arch. Soc. Esp. Oftalmol (Engl Ed). 2020, 95, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Armaly, M.F.; Rao, K.R. The effect of pilocarpine Ocusert with different release rates on ocular pressure. Investig. Ophthalmol. 1973, 1, 491–496. [Google Scholar]
- Sakuma, S. Controlled release-driven clinical benefits and next orientation. Drug Deliv. System 2016, 31, 183. [Google Scholar] [CrossRef][Green Version]
- Kobayakawa, S.; Matsunaga, T. The Preventive Effect by the Drug Released Soft Contact Lens against Bacterial Endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2923. [Google Scholar]
- Kobayakawa, S.; Matsunaga, T.; Kakisu, K.; Yamazaki, Y.; Sato, T.; Tochikubo, T. Development of a Drug released Soft Contact Lens that Releases Antibiotics in a Sustained Manner. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6102. [Google Scholar]
- Dixon, P.; Ghosh, T.; Mondal, K.; Konar, A.; Chauhan, A.; Hazra, S. Controlled delivery of pirfenidone through vitamin E-loaded contact lens ameliorates corneal inflammation. Drug Deliv. Transl. Res. 2018, 8, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.-P.-D.; Yang, M.-C. Synthesis and Characterization of Silicone Contact Lenses Based on TRIS-DMA-NVP-HEMA Hydrogels. Polymers 2019, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, S.; Ogawa, S.; Fukuda, Y.; Niida, S.; Shimakawa, S. Slow-Drug-Release Ophthalmic Lens and Manufacturing Method Therefor. WO2014038004A1, 13 March 2014. [Google Scholar]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Moret, E.; Ambresin, A.; Gianniou, C.; Bijon, J.; Besse-Hayat, C.; Bogiatzi, S.; Hohl, D.; Spertini, F.; Mantel, I. Non-immediate drug hypersensitivity reactions secondary to intravitreal anti-vascular endothelial growth factors. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Del Re, M.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye 2018, 32, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Wang, Y.; Lin, C.; Zhang, D.; Chen, J.; Ouyang, L.; Wu, F.; Zhang, J.; Chen, L. Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy. J. Hematol.Oncol. 2022, 15, 89. [Google Scholar] [CrossRef]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration:therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zarling, J.A.; Brunt, V.E.; Vallerga, A.K.; Li, W.; Tao, A.; Zarling, D.A.; Minson, C.T. Nitroxide pharmaceutical development for age-related degeneration and disease. Front. Genet. 2015, 6, 325. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current Understanding of the Molecular and Cellular Pathology of Diabetic Retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Bahr, T.A.; Bakri, S.J. Update on the Management of Diabetic Retinopathy: Anti-VEGF Agents for the Prevention of Complications and Progression of Nonproliferative and Proliferative Retinopathy. Life 2023, 13, 1098. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, R.; Albou-Ganem, C.; Gatinel, D.; Sandali, O. Editorial: Innovations in cataract surgery. Front. Med. 2024, 11, 1367577. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fu, Q.; Chen, X.; Yao, K. Advances in pharmacotherapy of cataracts. Ann. Transl. Med. 2020, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Jayanetti, V.; Sandhu, S.; Lusthaus, J.A. The Latest Drugs in Development That Reduce Intraocular Pressure in Ocular Hypertension and Glaucoma. J. Exp. Pharmacol. 2020, 12, 539–548. [Google Scholar] [CrossRef]
- Shalaby, W.S.; Shankar, V.; Razeghinejad, R.; Katz, L.J. Current and new pharmacotherapeutic approaches for glaucoma. Expert. Opin. Pharmacother. 2020, 21, 2027–2040. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Matsuoka, Y.; Tanito, M. Efficacy and Patient Tolerability of Omidenepag Isopropyl in the Treatment of Glaucoma and Ocular Hypertension. Clin. Ophthalmol. 2022, 16, 1261–1279. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, F.; Fan, Y.; Zhang, J.; Fang, T.; Xing, D.; Zhen, Y.; Nie, Z.; Liu, Y.; Wang, D.; et al. Co-delivery of Brinzolamide and Timolol from Micelles-laden Contact Lenses: In vitro and In Vivo Evaluation. Pharm. Res. 2024, 41, 531–546. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Winkler, N.S.; Fautsch, M.P. Effects of Prostaglandin Analogues on Aqueous Humor Outflow Pathways. J. Ocul Pharmacol. Ther. 2014, 30, 102–109. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.W.; Peart, D.; Purves, R.D.; Carré, D.A.; Peterson-Yantorno, K.; Mitchell, C.H.; Macknight, A.D.; Civan, M.M. Timolol may inhibit aqueous humor secretion by cAMP-independent action on ciliary epithelial cells. Am. J. Physiol. Cell Physiol. 2001, 281, C865–C875. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.S.; Jadhav, G.; Ogidigben, M.; Kompella, U.B. Ocular Pharmacokinetics of Dorzolamide and Brinzolamide After Single and Multiple Topical Dosing: Implications for Effects on Ocular Blood Flow. Drug Metab. Dispos. 2011, 39, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.I. Aqueous Humor Flow in Normal Human Eyes Treated with Brimonidine and Timolol, Alone and in Combination. Arch. Ophthalmol. 2001, 119, 492–495. [Google Scholar] [CrossRef]
- Kusuhara, S.; Nakamura, M. Ripasudil Hydrochloride Hydrate in the Treatment of Glaucoma: Safety, Efficacy, and Patient Selection. Clin. Ophthalmol. 2020, 14, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.C.; Liu, Z.; Baudouin, C.; Kim, H.M.; Messmer, E.M.; Kruse, F.; Liang, L.; Carreno-Galeano, J.T.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Karpecki, P.M.; Meske, M.E.; Reissman, D. Evolving knowledge of the unmet needs in dry eye disease. Am. J. Manag. Care 2021, 27 (Suppl. 2), S23–S32. [Google Scholar] [CrossRef]
- Chaudhary, S.; Ghimire, D.; Basu, S.; Agrawal, V.; Jacobs, D.S.; Shanbhag, S.S. Contact lenses in dry eye disease and associated ocular surface disorders. Indian J. Ophthalmol. 2023, 71, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv Rev. 2022, 191, 114582. [Google Scholar] [CrossRef]
- Nakamura, M.; Imanaka, T.; Sakamoto, A. Diquafosol Ophthalmic Solution for Dry Eye Treatment. Adv. Ther. 2012, 29, 579–589. [Google Scholar] [CrossRef]
- Saad, S.; Abdelmassih, Y.; Saad, R.; Guindolet, D.; Khoury, S.E.; Doan, S.; Cochereau, I.; Gabison, E.E. Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. Ocul. Surf. 2020, 18, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D.; Lamb, Y.N. Cenegermin: A Review in Neurotrophic Keratitis. Drugs 2020, 80, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Sheha, H.; Tighe, S.; Hashem, O.; Hayashida, Y. Update On Cenegermin Eye Drops In The Treatment Of Neurotrophic Keratitis. Clin. Ophthalmol. 2019, 13, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Koay, S.Y.; Larkin, D.F.P. New Pharmacological Approaches for the Treatment of Neurotrophic Keratitis. Front. Pharmacol. 2022, 13, 796854. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Hamrah, P. Understanding Neuropathic Corneal Pain-Gaps and Current Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Moreno, A.; Baudouin, C.; Melik Parsadaniantz, S.; Réaux-Le Goazigo, A. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef]
- Ozmen, M.C.; Dieckmann, G.; Cox, S.M.; Rashad, R.; Paracha, R.; Sanayei, N.; Morkin, M.I.; Hamrah, P. Efficacy and tolerability of nortriptyline in the management of neuropathic corneal pain. Ocul. Surf. 2020, 18, 814–820. [Google Scholar] [CrossRef]






| Eye Drop | Eye Ointment | Local Injection | Oral Administration | Intravenous Administration | Drug-Releasing Soft Contact Lenses (DR-SCLs) | |
|---|---|---|---|---|---|---|
| Long-term effectiveness | × | ◯ | ◯ | × | × | ◯ |
| Patient friendliness | ◯ | △ | × | ◯ | × | ◯ |
| Patient adherence to the drug regimen | × | × | ◯ | × | ◯ | ◯ |
| Low-molecular-weight drugs | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ |
| High-molecular-weight drugs | × | × | ◯ | × | ◯ | ◯ |
| Nanoparticles | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ |
| Dilution with tear fluid | ◯ | ◯ | × | × | × | × |
| Excretion through the nasolacrimal duct | ◯ | ◯ | × | × | × | × |
| The blood–retinal barrier (BRB) | × | × | × | ◯ | ◯ | × |
| The ocular blood–aqueous barrier (BAB) | × | × | × | ◯ | ◯ | × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashima, T. Ocular Drug Delivery into the Eyes Using Drug-Releasing Soft Contact Lens. Future Pharmacol. 2024, 4, 336-351. https://doi.org/10.3390/futurepharmacol4020019
Tashima T. Ocular Drug Delivery into the Eyes Using Drug-Releasing Soft Contact Lens. Future Pharmacology. 2024; 4(2):336-351. https://doi.org/10.3390/futurepharmacol4020019
Chicago/Turabian StyleTashima, Toshihiko. 2024. "Ocular Drug Delivery into the Eyes Using Drug-Releasing Soft Contact Lens" Future Pharmacology 4, no. 2: 336-351. https://doi.org/10.3390/futurepharmacol4020019
APA StyleTashima, T. (2024). Ocular Drug Delivery into the Eyes Using Drug-Releasing Soft Contact Lens. Future Pharmacology, 4(2), 336-351. https://doi.org/10.3390/futurepharmacol4020019





