Comprehensive Review on the Biomedical Applications of Marine Algal Polysaccharides
Abstract
1. Introduction
2. Biochemical Composition and Structural Characteristics
2.1. Alginic Acid and Its Derivatives
2.2. Agar
2.3. Carrageenan
2.4. Fucoidans
2.5. Ulvan
2.6. Laminarin
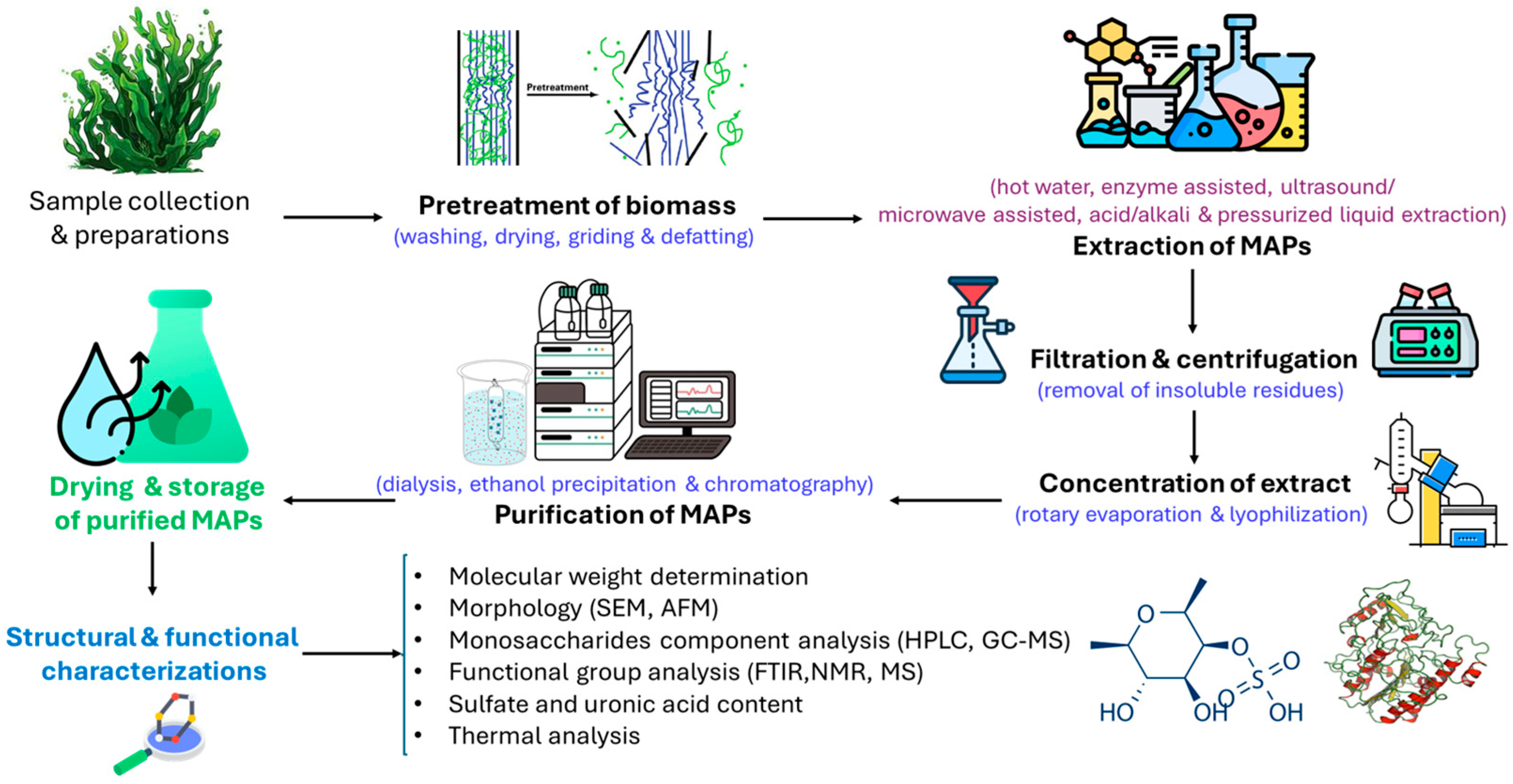
3. Extraction and Purification Methods
4. Advanced Technologies for Polysaccharide Extraction from Marine Brown Algae
- Modern extraction techniques such as pressurized liquid extraction (PLE), ultrasound-assisted extraction, microwave-assisted extraction, and enzyme-assisted extraction are increasingly being used to isolate polysaccharides from algae [98]. However, the extraction method and conditions can significantly influence the characteristics of MAPs, including their viscosity, sulfate content, monosaccharide composition, molecular weight, and overall bioactivity—due to possible degradation during the process. Therefore, optimizing key extraction parameters such as temperature, extraction time, power, and sample-to-solvent ratio is essential to maximize polysaccharide yield while preserving their native structural and functional properties [98].
- Microwave-assisted extraction (MAE) is an efficient technique that overcomes the drawbacks of conventional methods by generating heat directly within a material [98]. This facilitates cell wall rupture and releases intracellular compounds into the extraction solvent. MAE has been successfully utilized for isolating bioactive compounds from seaweeds and polysaccharides from other plants, with notable effects on the chemical structure and bioactivity of the target polysaccharides [100]. Numerous studies have demonstrated the effectiveness of microwave-assisted extraction (MAE) in terms of operational capability and sustainability [101,102]. Microwave irradiation generates heat through dipole rotation when it interacts with the polar compounds in the material, leading to a high yield of extracted compounds [100,101].
- Ultrasound-Assisted Extraction (UAE) has emerged as the most practical industrial technique owing to its simplicity, faster extraction rate, increased yield, reduced cost, and shorter processing time [103]. It can be combined with other technologies, such as enzymatic processing or MAE, utilizing acoustic cavitation to disrupt cell walls, reduce particle size, and enhance contact between the solvent and compounds. UAE induces structural and microstructural modifications in sulfated APS, with efficiency dependent on factors such as ultrasound power, temperature, and solvent ratio, necessitating the optimization of extraction conditions [104,105].
- Pressurized Liquid Extraction (PLE) is an innovative technique that utilizes elevated temperatures and pressures to extract compounds from samples in a short time using less solvent. In addition, pressurized fluid extraction, pressurized solvent extraction, accelerated solvent extraction, and PLE can achieve higher solubility and diffusion rates without boiling the solvent. Various static and dynamic methods have been utilized for polysaccharide extraction from brown algae, with commercial options available since 1995 [106,107].
- Enzyme-Assisted Extraction (EAE) is a valuable technique for improving the extraction efficiency of bioactive compounds from seaweeds, although it is more commonly used with terrestrial plants [108]. EAE offers a higher extraction yield, faster rates, lower energy consumption, and simpler recovery compared to conventional extraction methods. This involves the use of enzymes capable of degrading cell walls or partially breaking down polysaccharides to facilitate extraction. Commercially available carbohydrate hydrolytic enzymes and proteases are commonly used for polysaccharide extraction from seaweeds [109,110].
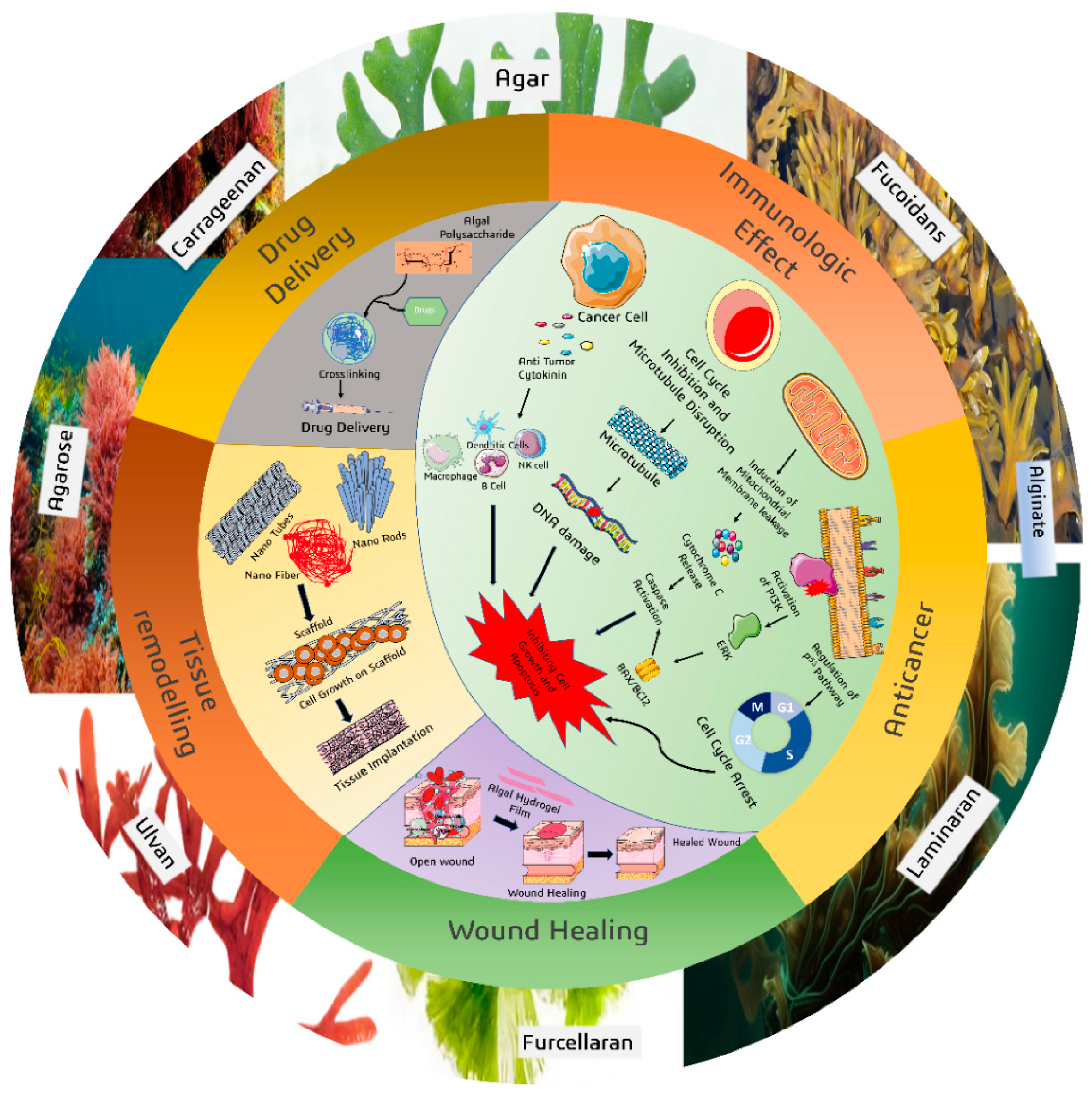
5. Biomedical Applications
5.1. Immunomodulatory Effects
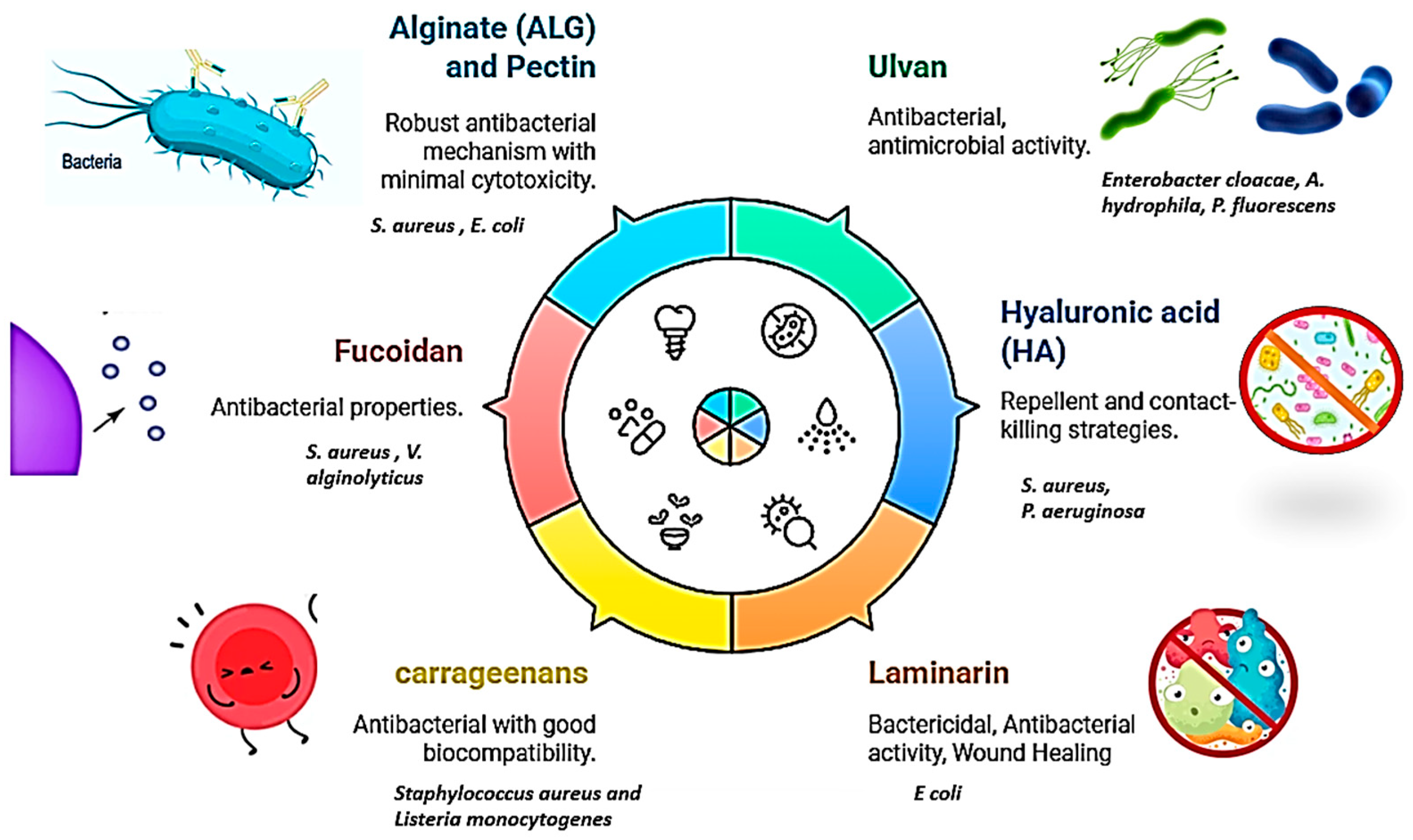
5.2. Antiviral and Antibacterial Activities
5.3. Anticancer Potential
5.4. Wound Healing and Tissue Regeneration
5.5. Antiviral Therapy
5.6. Anti-Diabetic and Anti-Obesity Effects
5.7. Neuroprotective Effects
5.8. Antibacterial Coatings and Medical Implants
5.9. Anticoagulant and Antithrombotic Agent
6. Molecular Mechanisms Underlying the Biomedical Activities of Marine Macroalgae Polysaccharides
7. Interaction with Cellular Receptors, Signal Transduction Pathways, and Immune Cells
8. Challenges and Future Directions
| Polysaccharide | Product/Brand Name | Biomedical Use | Formulation Type | Status | Approved/In Trials | References |
|---|---|---|---|---|---|---|
| Alginate | AlgiMatrix® | 3D cell culture scaffold, Multicellular tumor spheroid (MCTS) assays, organogenesis studies (hepatocytes, cardiomyocytes), co-culture models | Porous sponge matrix | Commercial | Approved (research use) | [202,203] |
| Alginate | Algisite™ M | Wound healing, exudate absorption, high-throughput drug screening, and 3D stem cell differentiation | Calcium alginate dressing | Approved | CE Marked | |
| Carrageenan | Carraguard® | Vaginal microbicide (anti-HIV) | Gel | Clinical trial completed | Phase III (discontinued) | [204] |
| Carrageenan | Iota-Carrageenan nasal spray | Cold & flu treatment relieves nasal symptoms through antiviral action rather than affecting blood vessels or glands. | Nasal spray | Commercial | Approved (EU/OTC) | [205,206] |
| Fucoidan | Maritech® Fucoidan | Effectively reduced osteoarthritis symptoms such as pain and stiffness. Immunity, inflammation, and oncology support | Capsule, powder | Commercial | Approved (nutraceutical) | [128] |
| Fucoidan | Fucoidan wound dressings | Wound healing, angiogenesis | Hydrogel, biofilm | In development | Preclinical | [207] |
| Fucoidan | Fucoidan-cisplatin nanoparticle | Cancer drug delivery | Nanoparticle system | Experimental | In vitro/in vivo | [208] |
| Ulvan | Ulvans in wound healing scaffolds | Antioxidant, antimicrobial, promotes wound healing by maintaining moisture and absorbing exudate. | Ulvan-based hydrogel film | Experimental | In vitro | [133] |
| Ulvan | Ulvan–chitosan hydrogels | Skin regeneration, tissue engineering | Injectable hydrogel | Experimental | Preclinical | [209,210] |
| Laminarin | Laminarin microparticles | Drug delivery, immune modulation | Microparticle system | Experimental | In vitro | [211] |
| Laminarin | Laminarin in vaccine adjuvants | Immune adjuvant | Injectable vaccine adjuvant | Experimental | In vivo (animal models) | [212] |
| Laminarin | Agar-based wound dressings | Wound healing, moist environment | Film/dressing | Experimental | Preclinical | [213] |
| Laminarin | Agar-based hydrogels for drug release | Controlled drug delivery | Hydrogel | Experimental | In vitro/in vivo | [214] |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magnabosco, C.; Santaniello, G.; Romano, G. Microalgae: A Promising Source of Bioactive Polysaccharides for Biotechnological Applications. Molecules 2025, 30, 2055. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Ning, L.; Yao, Z.; Zhu, B. Ulva (Enteromorpha) Polysaccharides and Oligosaccharides: A Potential Functional Food Source from Green-Tide-Forming Macroalgae. Mar. Drugs 2022, 20, 202. [Google Scholar] [CrossRef]
- Anisha, G.S.; Padmakumari, S.; Patel, A.K.; Pandey, A.; Singhania, R.R. Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering 2022, 9, 472. [Google Scholar] [CrossRef]
- Kasanah, N.; Ulfah, M.; Imania, O.; Hanifah, A.N.; Marjan, M.I.D. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules 2022, 27, 7788. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y.I. Cell Wall Polysaccharides of Marine Algae. In Hb25_Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp. 543–590. ISBN 978-3-642-53970-1. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Kang, J.; Jia, X.; Wang, N.; Xiao, M.; Song, S.; Wu, S.; Li, Z.; Wang, S.; Cui, S.W.; Guo, Q. Insights into the Structure-Bioactivity Relationships of Marine Sulfated Polysaccharides: A Review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Mutch, A.L.; Yang, J.; Ferro, V.; A, A.; Grøndahl, L. Sulfated Alginate for Biomedical Applications. Macromol. Biosci. 2024, 24, 2400237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, M.; Liu, Y.; Liu, W.; Peng, C.; Zheng, L. Sulfated Polysaccharides with Anticoagulant Potential: A Review Focusing on Structure-Activity Relationship and Action Mechanism. Chem. Biodivers. 2024, 21, e202400152. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; De Morais, A.; De Morais, R. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-Derived Polysaccharides and Their Potential Health Benefits in Nutraceutical Applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, T.; Małyska, A. Uninformed and Disinformed Society and the GMO Market. Trends Biotechnol. 2015, 33, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wawoczny, A.; Gillner, D. The Most Potent Natural Pharmaceuticals, Cosmetics, and Food Ingredients Isolated from Plants with Deep Eutectic Solvents. J. Agric. Food Chem. 2023, 71, 10877–10900. [Google Scholar] [CrossRef]
- Lesco, K.C.; Williams, S.K.R.; Laurens, L.M.L. Marine Algae Polysaccharides: An Overview of Characterization Techniques for Structural and Molecular Elucidation. Mar. Drugs 2025, 23, 105. [Google Scholar] [CrossRef]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The System of Galactans of the Red Seaweed, Kappaphycus Alvarezii, with Emphasis on Its Minor Constituents. Carbohydr. Res. 2004, 339, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Ustyuzhanina, N.E.; Nifantiev, N.E. Fucoidans of Brown Algae: Comparison of Sulfated Polysaccharides from Fucus Vesiculosus and Ascophyllum Nodosum. Mar. Drugs 2022, 20, 638. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, L.; Li, Y.; Jiang, S.; Sun, Q.; Xie, E.; Chen, H.; Zhao, Z.; Qiao, W.; Xu, J.; et al. Structure of a Laminarin-Type β-(1→3)-Glucan from Brown Algae Sargassum Henslowianum and Its Potential on Regulating Gut Microbiota. Carbohydr. Polym. 2021, 255, 117389. [Google Scholar] [CrossRef]
- Pramanik, S.; Singh, A.; Abualsoud, B.M.; Deepak, A.; Nainwal, P.; Sargsyan, A.S.; Bellucci, S. From Algae to Advancements: Laminarin in Biomedicine. RSC Adv. 2024, 14, 3209–3231. [Google Scholar] [CrossRef] [PubMed]
- Pari, R.F.; Uju, U.; Hardiningtyas, S.D.; Ramadhan, W.; Wakabayashi, R.; Goto, M.; Kamiya, N. Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Mar. Drugs 2025, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.N.; Mikkelsen, M.D.; Truong, H.B.; Vo, H.N.M.; Pham, T.D.; Cao, H.T.T.; Nguyen, T.T.; Meyer, A.S.; Thanh, T.T.T.; Van, T.T.T. Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva Papenfussii. Mar. Drugs 2023, 21, 556. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Mar. Drugs 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Nakazoe, J. Production and Use of Marine AIgae in Japan. JARQ 2001, 35, 281–290. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, J.M. Inclusion of Seaweeds as Healthy Approach to Formulate New Low-Salt Meat Products. Curr. Opin. Food Sci. 2021, 40, 20–25. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A Decade of Change in the Seaweed Hydrocolloids Industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Akter, A.; Sobuj, M.K.A.; Islam, M.S.; Chakroborty, K.; Tasnim, N.; Ayon, M.H.; Hossain, M.F.; Rafiquzzaman, S.M. Seaweed Polysaccharides: Sources, Structure and Biomedical Applications with Special Emphasis on Antiviral Potentials. Future Foods 2024, 10, 100440. [Google Scholar] [CrossRef]
- Yue, Q.; Liu, Y.; Li, F.; Hong, T.; Guo, S.; Cai, M.; Zhao, L.; Su, L.; Zhang, S.; Zhao, C.; et al. Antioxidant and Anticancer Properties of Fucoidan Isolated from Saccharina Japonica Brown Algae. Sci. Rep. 2025, 15, 8962. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Gopal, R.K.; Damodharan, R.; Arumugam, A.; Sampath Kumar, M.; Senthilkumar, N.; Anbalagan, M. In Vitro Anticancer Potential of Laminarin and Fucoidan from Brown Seaweeds. Sci. Rep. 2023, 13, 14452. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.-F., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0864-1. [Google Scholar]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview. In Alginates—Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-641-5. [Google Scholar]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Lu, S.; Na, K.; Wei, J.; Zhang, L.; Guo, X. Alginate Oligosaccharides: The Structure-Function Relationships and the Directional Preparation for Application. Carbohydr. Polym. 2022, 284, 119225. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Yarkent, C.; Aslanbay Guler, B.; Gurlek, C.; Sahin, Y.; Kose, A.; Oncel, S.S.; Imamoglu, E. Algal Alginate in Biotechnology: Biosynthesis and Applications. In Properties and Applications of Alginates; Deniz, I., Imamoglu, E., Keskin-Gundogdu, T., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-499-8. [Google Scholar]
- Zhong, H.; Gao, X.; Cheng, C.; Liu, C.; Wang, Q.; Han, X. The Structural Characteristics of Seaweed Polysaccharides and Their Application in Gel Drug Delivery Systems. Mar. Drugs 2020, 18, 658. [Google Scholar] [CrossRef]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral Iron Supplementation with Feralgine® in Inflammatory Bowel Disease: A Retrospective Observational Study. Minerva Gastroenterol. Dietol. 2019, 65, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Ale, M.; Meyer, A. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Duan, D.; Xu, J.; Gao, X. Preparation and Characterization of Agar, Agarose, and Agaropectin from the Red Alga Ahnfeltia Plicata. J. Ocean. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Vuai, S.A.H. Characterization of Agar Extracted from Gracilaria Species Collected along Tanzanian Coast. Heliyon 2022, 8, e09002. [Google Scholar] [CrossRef]
- Yermak, I.; Mischchenko, N.; Davydova, V.; Glazunov, V.; Tarbeeva, D.; Kravchenko, A.; Pimenova, E.; Sorokina, I. Carrageenans-Sulfated Polysaccharides from Red Seaweeds as Matrices for the Inclusion of Echinochrome. Mar. Drugs 2017, 15, 337. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I. A Modified System of Nomenclature for Red Algal Galactans. Bot. Mar. 1994, 37. [Google Scholar] [CrossRef]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan From Kappaphycus Alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological Properties and Potential of Compounds Extracted from Red Seaweeds. Phytochem. Rev. 2023, 22, 1509–1540. [Google Scholar] [CrossRef]
- Orbita, M.L. Growth Rate and Carrageenan Yield of Kappaphycus Alvarezii (Rhodophyta, Gracilariales Cultivated in Kolambugan, Lanao Del Norte, Mindanao, Philippines. Adv. Agric. Bot. Int. J. Bioflux Soc. 2013, 5, 128–139. [Google Scholar]
- Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A. Synthesis, Characterization and Potential Application of Hydrophobically Modified Carrageenan Derivatives as Pharmaceutical Excipients. Carbohydr. Polym. 2021, 251, 116997. [Google Scholar] [CrossRef] [PubMed]
- Barahona, T.; Prado, H.J.; Bonelli, P.R.; Cukierman, A.L.; Fissore, E.L.; Gerschenson, L.N.; Matulewicz, M.C. Cationization of Kappa- and Iota-Carrageenan—Characterization and Properties of Amphoteric Polysaccharides. Carbohydr. Polym. 2015, 126, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, M.; Chen, S. Synthesis and Characterization of Thermo- and pH-Sensitive Kappa-Carrageenan-g-Poly(Methacrylic Acid)/Poly(N,N-Diethylacrylamide) Semi-IPN Hydrogel. Mater. Chem. Phys. 2009, 115, 339–346. [Google Scholar] [CrossRef]
- Jiang, Y.-P.; Guo, X.-K.; Tian, X.-F. Synthesis and NMR Structural Analysis of O-Succinyl Derivative of Low-Molecular-Weight κ-Carrageenan. Carbohydr. Polym. 2005, 61, 399–406. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan From Undaria Pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharmacol. 2020, 11, 831. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Usoltseva, R.V.; Shevchenko, N.M.; Surits, V.V.; Imbs, T.I.; Malyarenko, O.S.; Besednova, N.N.; Ivanushko, L.A.; Ermakova, S.P. Structural Diversity of Fucoidans and Their Radioprotective Effect. Carbohydr. Polym. 2021, 273, 118551. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Usoltseva, R.V.; Silchenko, A.S.; Zueva, A.O.; Ermakova, S.P. The Combined Metabolically Oriented Effect of Fucoidan from the Brown Alga Saccharina Cichorioides and Its Carboxymethylated Derivative with 2-Deoxy-D-Glucose on Human Melanoma Cells. IJMS 2023, 24, 12050. [Google Scholar] [CrossRef]
- Claus-Desbonnet, H.; Nikly, E.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S.; Pierre, G.; Benbassat, N.; Katsarov, P.; Michaud, P.; Lukova, P.; et al. Polysaccharides and Their Derivatives as Potential Antiviral Molecules. Viruses 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine Fucoidans: Structural, Extraction, Biological Activities and Their Applications in the Food Industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Taran, I.V.; Usoltseva, R.V.; Zvyagintsev, N.V.; Zueva, A.O.; Rubtsov, N.K.; Lembikova, D.E.; Nedashkovskaya, O.I.; Kusaykin, M.I.; Isaeva, M.P.; et al. The Discovery of the Fucoidan-Active Endo-1→4-α-L-Fucanase of the GH168 Family, Which Produces Fucoidan Derivatives with Regular Sulfation and Anticoagulant Activity. IJMS 2023, 25, 218. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Sivagnanam, S.P.; Rai, D.K.; O’Donnell, C.; Conway, G.E.; Heffernan, S.M.; Fitzpatrick, S.; Lyons, H.; Curtin, J.; Tiwari, B.K. Conventional Extraction of Fucoidan from Irish Brown Seaweed Fucus Vesiculosus Followed by Ultrasound-Assisted Depolymerization. Sci. Rep. 2024, 14, 6214. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Yun, H.; Zhang, H.; Zhang, Q. Effect and Mechanism of Fucoidan Derivatives from Laminaria Japonica in Experimental Adenine-Induced Chronic Kidney Disease. J. Ethnopharmacol. 2012, 139, 807–813. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Eco-Friendly Nutrient from Ocean: Exploring Ulva Seaweed Potential as a Sustainable Food Source. J. Agric. Food Res. 2024, 17, 101239. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; De Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Pizzoferrato, L.; Liquori, A.M. A Physico-Chemical Study on the Polysaccharide Ulvan from Hot Water Extraction of the Macroalga Ulva. Int. J. Biol. Macromol. 1999, 25, 309–315. [Google Scholar] [CrossRef]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Ulvan and Ulva Oligosaccharides: A Systematic Review of Structure, Preparation, Biological Activities and Applications. Bioresour. Bioprocess. 2023, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, H.; Wang, P.; Du, C.; Ye, H.; Zuo, S.; Guan, H.; Wang, P. Structural Characterization of Ulvan Extracted from Ulva Clathrata Assisted by an Ulvan Lyase. Carbohydr. Polym. 2020, 229, 115497. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of Extraction Conditions on the Yield and Purity of Ulvan Extracted from Ulva Lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Pengzhan, Y. Antihyperlipidemic Effects of Different Molecular Weight Sulfated Polysaccharides from Ulva Pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Podolean, I.; Coman, S.M.; Bucur, C.; Teodorescu, C.; Kikionis, S.; Ioannou, E.; Roussis, V.; Primo, A.; Garcia, H.; Parvulescu, V.I. Catalytic Transformation of the Marine Polysaccharide Ulvan into Rare Sugars, Tartaric and Succinic Acids. Catal. Today 2022, 383, 345–357. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates From Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–274. ISBN 978-0-12-802772-1. [Google Scholar]
- Tagliapietra, B.L.; Clerici, M.T.P.S. Brown Algae and Their Multiple Applications as Functional Ingredient in Food Production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira Barbata Seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Dhahri, M.; Alghrably, M.; Mohammed, H.A.; Badshah, S.L.; Noreen, N.; Mouffouk, F.; Rayyan, S.; Qureshi, K.A.; Mahmood, D.; Lachowicz, J.I.; et al. Natural Polysaccharides as Preventive and Therapeutic Horizon for Neurodegenerative Diseases. Pharmaceutics 2021, 14, 1. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Role of Algal Derived Compounds in Pharmaceuticals and Cosmetics. In Recent Advances in Micro and Macroalgal Processing; Rajauria, G., Yuan, Y.V., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 537–603. ISBN 978-1-119-54258-2. [Google Scholar]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of Biomolecules from Seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 243–269. ISBN 978-0-12-418697-2. [Google Scholar]
- Choi, J.; Kim, H.-J.; Lee, J.-W. Structural Feature and Antioxidant Activity of Low Molecular Weight Laminarin Degraded by Gamma Irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, S.; Li, L.; Pan, T.; Shi, C.; Liu, H.; Cao, M.; Su, W.; Liu, G. In Vitro and in Vivo Immunomodulatory Activity of Sulfated Polysaccharide from Porphyra Haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef]
- Cognigni, V.; Ranallo, N.; Tronconi, F.; Morgese, F.; Berardi, R. Potential Benefit of β-Glucans as Adjuvant Therapy in Immuno-Oncology: A Review. Explor. Target. Anti-Tumor Ther. 2021, 2, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, Isolation and Purification Methods of Polysaccharides from Natural Products: A Review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of Alginate Extraction Processes: Impact on Alginate Molecular Structure and Techno-Functional Properties. Trends Food Sci. Technol. 2023, 140, 104142. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO fisheries technical paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-104958-7. [Google Scholar]
- Pandya, Y.; Bakshi, M.; Sharma, A. Agar-Agar Extraction, Structural Properties and Applications: A Review. Pharma Innov. J. 2022, 11, 1151–1157. [Google Scholar]
- UNE EN ISO 9000:2015; Quality Management Systems—Fundamentals and Vocabulary (ISO 9000:2015). European Standards: Brussels, Belgium, 2015. Available online: https://www.en-standard.eu/une-en-iso-9000-2015-quality-management-systems-fundamentals-and-vocabulary-iso-9000-2015/ (accessed on 10 November 2025).
- Vo, T.P.; Pham, T.V.; Tran, T.N.H.; Vo, L.T.V.; Vu, T.T.; Pham, N.D.; Nguyen, D.Q. Ultrasonic-Assisted and Microwave-Assisted Extraction of Phenolics and Terpenoids from Abelmoschus sagittifolius (Kurz) Merr Roots Using Natural Deep Eutectic Solvents. ACS Omega 2023, 8, 29704–29716. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar Extraction from Integrated Multitrophic Aquacultured Gracilaria Vermiculophylla: Evaluation of a Microwave-Assisted Process Using Response Surface Methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel Procedures for the Extraction of Fucoidan from Brown Algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Ascophyllum Nodosum and Its Antioxidant Activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-Assisted Hydrothermal Extraction of Sulfated Polysaccharides from Ulva Spp. and Monostroma Latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Brown Seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, Y.; Yang, Y.; Zhang, H.; Ding, C.; Hu, C.; Zhou, L.; Zhang, Z.; Yuan, S.; Chen, Y.; et al. Microwave-Assisted Extraction, Physicochemical Characterization and Bioactivity of Polysaccharides from Camptotheca Acuminata Fruits. Int. J. Biol. Macromol. 2019, 133, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of Microwave-Assisted Extraction Processes of Food Components by Integrating Technologies and Applying Emerging Solvents: A Review of Latest Developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of Grape Pomace Phenolic Compounds through Optimized Extraction and Adsorption Processes. Chem. Eng. Process.—Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochemistry 2023, 101, 106646. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Bioactivities of Nizamuddinia Zanardinii Sulfated Polysaccharides Extracted by Enzyme, Ultrasound and Enzyme-Ultrasound Methods. J. Food Sci. Technol. 2019, 56, 1212–1220. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, Y.-Y.; Ma, H.-L.; Wang, Z.-B. Ultrasonic Effects on the Degradation Kinetics, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Phellinus Linteus Mycelia. Ultrason. Sonochemistry 2016, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.-D.; Woo, H.-C.; Chun, B.-S. Subcritical Water Extraction of Fucoidan from Saccharina Japonica: Optimization, Characterization and Biological Studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, J. Pressurized Liquids as an Alternative Green Process to Extract Antiviral Agents from the Edible Seaweed Himanthalia Elongata. J. Appl. Phycol. 2011, 23, 909–917. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.; Gomes-Dias, J.S.; Cunha, S.A.; Pintado, M.E.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Enzymatic Approach for the Extraction of Bioactive Fractions from Red, Green and Brown Seaweeds. Food Bioprod. Process. 2023, 138, 25–39. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Franco, C.M.M.; Zhang, W. Enzyme-Assisted Extraction of Carbohydrates from the Brown Alga Ecklonia Radiata: Effect of Enzyme Type, pH and Buffer on Sugar Yield and Molecular Weight Profiles. Process Biochem. 2016, 51, 1503–1510. [Google Scholar] [CrossRef]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine Algae Sulfated Polysaccharides for Tissue Engineering and Drug Delivery Approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally Occurring Immune Modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Tuvikene, R. Potential Antiviral Properties of Industrially Important Marine Algal Polysaccharides and Their Significance in Fighting a Future Viral Pandemic. Viruses 2021, 13, 1817. [Google Scholar] [CrossRef]
- Abu-Rabeah, K.; Marks, R.S. Impedance Study of the Hybrid Molecule Alginate–Pyrrole: Demonstration as Host Matrix for the Construction of a Highly Sensitive Amperometric Glucose Biosensor. Sens. Actuators B Chem. 2009, 136, 516–522. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Sodium and Potassium in the Pathogenesis of Hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.; Kim, S. Seaweed Polysaccharides and Their Potential Biomedical Applications. Starch 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Vitko, M.; Valerio, D.M.; Rye, P.D.; Onsøyen, E.; Myrset, A.H.; Dessen, A.; Drumm, M.L.; Hodges, C.A. A Novel Guluronate Oligomer Improves Intestinal Transit and Survival in Cystic Fibrosis Mice. J. Cyst. Fibros. 2016, 15, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.M.; Lee, H.-G.; Nagahawatta, D.P.; Jayawardhana, H.H.A.C.K.; Song, K.-M.; Choi, Y.-S.; Jeon, Y.-J.; Kang, M.-C. Fucoidan from Sargassum Autumnale Inhibits Potential Inflammatory Responses via NF-κB and MAPK Pathway Suppression in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Mar. Drugs 2023, 21, 374. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’Elia, L.; Silva, M.; Barreira, L.; Monti, D.M. Shedding Light on the Hidden Benefit of Porphyridium Cruentum Culture. Antioxidants 2023, 12, 337. [Google Scholar] [CrossRef]
- Goyal, M.; Baranwal, M.; Pandey, S.K.; Reddy, M.S. Hetero-Polysaccharides Secreted from Dunaliella Salina Exhibit Immunomodulatory Activity Against Peripheral Blood Mononuclear Cells and RAW 264.7 Macrophages. Indian. J. Microbiol. 2019, 59, 428–435. [Google Scholar] [CrossRef]
- Park, S.-J.; Sharma, A.; Lee, H.-J. An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus. Mar. Drugs 2024, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Nozid, N.A.C.; Ibrahim, N.H.; Yoshida, A.; Huang, Y.; Hirose, T.; Hirasaka, K. Valorization of Agar Extracted from Gelidium Elegans via Ultrasound-Assisted Extraction as Potential Film Packaging by Optimizing the Incorporation of Chitosan and Curcumin. Int. J. Biol. Macromol. 2025, 327, 147323. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Lloret, V.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Exploring Alternative Red Seaweed Species for the Production of Agar-Based Hydrogels for Food Applications. Food Hydrocoll. 2024, 146, 109177. [Google Scholar] [CrossRef]
- Cholaraj, R.; Venkatachalam, R. Investigation of Antioxidant and Anticancer Potential of Fucoidan (in-Vitro & in-Silico) from Brown Seaweed Padina Boergesenii. Algal Res. 2024, 79, 103442. [Google Scholar] [CrossRef]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a Polysaccharide from Macroalga Ulva Sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Bioactivity and Applications of Polysaccharides from Marine Microalgae. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–38. ISBN 978-3-319-03751-6. [Google Scholar]
- Yang, J. Topical Application of Fucoidan Improves Atopic Dermatitis Symptoms in NC/Nga Mice. Phytother. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef]
- Myers, S.; O’Connor, J.; Fitton, H.; Brooks, L.; Rolfe, M.; Connellan; Wohlmuth, H.; Cheras, P.A.; Morris, C. A Combined Phase I and II Open-Label Study on the Immunomodulatory Effects of Seaweed Extract Nutrient Complex. BTT 2011, 45, 45–60. [Google Scholar] [CrossRef]
- Thomson, A.W.; Fowler, E.F. Carrageenan: A Review of Its Effects on the Immune System. Agents Actions 1981, 11, 265–273. [Google Scholar] [CrossRef]
- Lukova, P.; Kokova, V.; Baldzhieva, A.; Murdjeva, M.; Katsarov, P.; Delattre, C.; Apostolova, E. Alginate from Ericaria Crinita Possesses Antioxidant Activity and Attenuates Systemic Inflammation via Downregulation of Pro-Inflammatory Cytokines. Mar. Drugs 2024, 22, 482. [Google Scholar] [CrossRef]
- Park, J.; Babensee, J.E. Differential Functional Effects of Biomaterials on Dendritic Cell Maturation. Acta Biomater. 2012, 8, 3606–3617. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Rodríguez-Coello, A.; Latire, T.; Bourgougnon, N.; Torres, M.D.; Buján, M.; Muíños, A.; Muiños, A.; Meijide-Faílde, R.; Blanco, F.J.; et al. Anti-Inflammatory Potential of Ulvan. Int. J. Biol. Macromol. 2023, 253, 126936. [Google Scholar] [CrossRef]
- Millotti, G.; Lagast, J.; Jurman, J.; Paliaga, P.; Laffleur, F. Ulvan as an Underrated Material for Wound Dressings: A Review. Int. J. Biol. Macromol. 2025, 319, 145113. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.; Ki, J.-S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- Kraithong, S.; Bunyameen, N.; Theppawong, A.; Ke, X.; Lee, S.; Zhang, X.; Huang, R. Potentials of Ulva Spp.-Derived Sulfated Polysaccharides as Gelling Agents with Promising Therapeutic Effects. Int. J. Biol. Macromol. 2024, 273, 132882. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Marine Sulfated Polysaccharides as Potential Antiviral Drug Candidates to Treat Corona Virus Disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Talarico, L.B.; Noseda, M.D.; Ducatti, D.R.B.; Duarte, M.E.R.; Damonte, E.B. Differential Inhibition of Dengue Virus Infection in Mammalian and Mosquito Cells by Iota-Carrageenan. J. Gen. Virol. 2011, 92, 1332–1342. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in Dengue Virus Adsorption and Uncoating by Carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Geng, M.; Guan, H.; Li, Z. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitr. Zhongguo Hai Yang Yao Wu=Chin. J. Mar. Drugs 2000, 19, 15–18. [Google Scholar]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Jucá, M.B.; Aoyama, H.; Leite, E.L. Inhibition of Reverse Transcriptase Activity of HIV by Polysaccharides of Brown Algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and Anti-Dengue Virus Activity of Sulfated Polysaccharide from a Marine Alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, Chemical Investigation and Antiviral Activity of Polysaccharides from Gracilaria Corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory Activities of Marine Sulfated Polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Schulze, I.T. Reversible Inhibition of Type 2 Dengue Virus by Agar Polysaccharide. Virology 1964, 22, 79–90. [Google Scholar] [CrossRef]
- Zacharopoulos, V.R.; Phillips, D.M. Vaginal Formulations of Carrageenan Protect Mice from Herpes Simplex Virus Infection. Clin. Diagn. Lab. Immunol. 1997, 4, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Panggabean, J.A.; Adiguna, S.P.; Rahmawati, S.I.; Ahmadi, P.; Zainuddin, E.N.; Bayu, A.; Putra, M.Y. Antiviral Activities of Algal-Based Sulfated Polysaccharides. Molecules 2022, 27, 1178. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive Effects of Cladosiphon Fucoidan Against Helicobacter pylori Infection in Mongolian Gerbils. Helicobacter 2003, 8, 59–65. [Google Scholar] [CrossRef]
- Fedorov, S.; Ermakova, S.; Zvyagintseva, T.; Stonik, V. Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum Sp. and Fucus Vesiculosus Reduces Cell Viability of Lung Carcinoma and Melanoma Cells in Vitro and Activates Natural Killer Cells in Mice in Vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Hu, B.; Cao, Q.; Xing, M.; Song, S.; Ji, A. Fucoidan Induces Apoptosis of HT-29 Cells via the Activation of DR4 and Mitochondrial Pathway. Mar. Drugs 2020, 18, 220. [Google Scholar] [CrossRef]
- Choo, G.-S.; Lee, H.-N.; Shin, S.-A.; Kim, H.-J.; Jung, J.-Y. Anticancer Effect of Fucoidan on DU-145 Prostate Cancer Cells through Inhibition of PI3K/Akt and MAPK Pathway Expression. Mar. Drugs 2016, 14, 126. [Google Scholar] [CrossRef]
- Li, Y.; McGowan, E.; Chen, S.; Santos, J.; Yin, H.; Lin, Y. Immunopotentiating Activity of Fucoidans and Relevance to Cancer Immunotherapy. Mar. Drugs 2023, 21, 128. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Usoltseva, R.V.; Zvyagintseva, T.N.; Ermakova, S.P. Laminaran from Brown Alga Dictyota Dichotoma and Its Sulfated Derivative as Radioprotectors and Radiosensitizers in Melanoma Therapy. Carbohydr. Polym. 2019, 206, 539–547. [Google Scholar] [CrossRef]
- Tian, L.; Li, C.-M.; Li, Y.-F.; Huang, T.-M.; Chao, N.-X.; Luo, G.-R.; Mo, F.-R. Laminarin from Seaweed (Laminaria Japonica) Inhibits Hepatocellular Carcinoma Through Upregulating Senescence Marker Protein-30. Cancer Biother. Radiopharm. 2020, 35, 277–283. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-Derived Polysaccharides and Their Therapeutic Potential in Wound Healing Application—A Review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical Potential of Alginate Wound Dressings—From Preclinical Studies to Clinical Applications: A Review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef]
- Sudarsan, S.; Franklin, D.S.; Guhanathan, S. Imbibed Salts and pH-Responsive Behaviours of Sodium Alginate Based Eco-Friendly Biopolymeric Hydrogels—A Solventless Approach. MMAIJ 2015, 11, 24–29. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Lee, Y.W.; Kang, H.W. Multifunctional Heteropolysaccharide Hydrogel under Photobiomodulation for Accelerated Wound Regeneration. Ceram. Int. 2020, 46, 7268–7278. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A. Marine Algal Antioxidants as Potential Vectors for Controlling Viral Diseases. Antioxidants 2020, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Somova, L.M.; Kuznetsova, T.A. Review: Prospects for the Use of Extracts and Polysaccharides from Marine Algae to Prevent and Treat the Diseases Caused by Helicobacter pylori. Helicobacter 2015, 20, 89–97. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral Effects of Sulfated Exopolysaccharide from the Marine Microalga Gyrodinium Impudicum Strain KG03. Mar. Biotechnol. 2004, 6, 17–25. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.-B.; Hayashi, T. Anti-Herpes Simplex Virus Target of an Acidic Polysaccharide, Nostoflan, from the Edible Blue-Green Alga Nostoc Flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef]
- Din, N.A.S.; Aiemcharoen, P.; Sermwittayawong, D.; Lim, S.J.; Ishak, A.A.; Sofian-Seng, N.-S.; Razali, N.S.M.; Rahman, H.A.; Mustapha, W.A.W. Bioactive Properties of Fucoidan from Malaysian Brown Seaweed (Sargassum Binderi) with an Assessment of Its Anti-Diabetic Potential in 3T3-L1 Adipocytes. J. Food Sci. Technol. 2025. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins Isolated from the Edible Brown Alga Ecklonia Stolonifera Exert Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Chellian, J.; Rahmah, N.S.N.; Gan, W.J.; Banerjee, P.; Sanyal, S.; Banerjee, P.; Ghosh, N.; Guith, T.; Das, A.; et al. Hypoglycaemic Molecules for the Management of Diabetes Mellitus from Marine Sources. DMSO 2023, 16, 2187–2223. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A.; Sedki, M.; Ali, S.M.; El-Sherbiny, I.M. Nanoformulated Ellagic Acid Ameliorates Pentylenetetrazol-Induced Experimental Epileptic Seizures by Modulating Oxidative Stress, Inflammatory Cytokines and Apoptosis in the Brains of Male Mice. Metab. Brain Dis. 2020, 35, 385–399. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Shahrukh, S.; Jain, N.; Vambhurkar, G.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Multifaceted Applications of Ulvan Polysaccharides: Insights on Biopharmaceutical Avenues. Int. J. Biol. Macromol. 2023, 234, 123669. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. IJMS 2019, 20, 3061. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Khan, F.; Kim, D.-K.; Cho, K.-J.; Tabassum, N.; Choudhury, A.; Hassan, M.I.; Jung, W.-K.; Kim, H.-W.; Kim, Y.-M. Marine Polysaccharides for Antibiofilm Application: A Focus on Biomedical Fields. Int. J. Biol. Macromol. 2024, 283, 137786. [Google Scholar] [CrossRef]
- Ruan, H.; Aulova, A.; Ghai, V.; Pandit, S.; Lovmar, M.; Mijakovic, I.; Kádár, R. Polysaccharide-Based Antibacterial Coating Technologies. Acta Biomater. 2023, 168, 42–77. [Google Scholar] [CrossRef] [PubMed]
- Nqoro, X.; Taziwa, R. Polymer-Based Functional Materials Loaded with Metal-Based Nanoparticles as Potential Scaffolds for the Management of Infected Wounds. Pharmaceutics 2024, 16, 155. [Google Scholar] [CrossRef]
- Habibi, M.; Golmakani, M.-T.; Eskandari, M.H.; Hosseini, S.M.H. Potential Prebiotic and Antibacterial Activities of Fucoidan from Laminaria Japonica. Int. J. Biol. Macromol. 2024, 268, 131776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, Characterization and Antibacterial Activity of Oxidized κ-Carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Byankina, A.O.; Kalitnik, A.A.; Kim, Y.H.; Bogdanovich, L.N.; Solov’eva, T.F.; Yermak, I.M. Influence of Red Algal Sulfated Polysaccharides on Blood Coagulation and Platelets Activation in Vitro. J. Biomed. Mater. Res. 2014, 102, 1431–1438. [Google Scholar] [CrossRef]
- Nigam, S.; Singh, R.; Bhardwaj, S.K.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the Therapeutic Applications of Algal Polysaccharides. J. Polym. Env. 2022, 30, 785–809. [Google Scholar] [CrossRef]
- Sharma, R.; Mondal, A.S.; Trivedi, N. Anticancer Potential of Algae-Derived Metabolites: Recent Updates and Breakthroughs. Futur. J. Pharm. Sci. 2023, 9, 44. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in Immunoregulation and Therapeutics. Sig Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. JMSE 2020, 8, 481. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; Araújo, R.G.; Rodríguez-Aguayo, A.A.; Guzmán-Román, M.; García-Venegas, J.C.; Nájera-Martínez, E.F.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldivar, R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants 2023, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in Dietary Polysaccharides as Anticancer Agents: Structure-Activity Relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Jeong, B.-E.; Ko, E.-J.; Joo, H.-G. Cytoprotective Effects of Fucoidan, an Algae-Derived Polysaccharide on 5-Fluorouracil-Treated Dendritic Cells. Food Chem. Toxicol. 2012, 50, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Kiselevskiy, M.V.; Anisimova, N.Y.; Ustyuzhanina, N.E.; Vinnitskiy, D.Z.; Tokatly, A.I.; Reshetnikova, V.V.; Chikileva, I.O.; Shubina, I.Z.; Kirgizov, K.I.; Nifantiev, N.E. Perspectives for the Use of Fucoidans in Clinical Oncology. IJMS 2022, 23, 11821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis Pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- Sabu Mathew, S.; Jaiswal, A.K.; Jaiswal, S. Carrageenan-Based Sustainable Biomaterials for Intelligent Food Packaging: A Review. Carbohydr. Polym. 2024, 342, 122267. [Google Scholar] [CrossRef]
- Lopes, A.H.; Silva, R.L.; Fonseca, M.D.; Gomes, F.I.; Maganin, A.G.; Ribeiro, L.S.; Marques, L.M.M.; Cunha, F.Q.; Alves-Filho, J.C.; Zamboni, D.S.; et al. Molecular Basis of Carrageenan-Induced Cytokines Production in Macrophages. Cell Commun. Signal 2020, 18, 141. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Z.-J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and Potential Antitumor Activity of Polysaccharide from Gracilariopsis Lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Sun, X.; Zang, X.; Zhang, X.; Fang, T.; Xu, N. Comparative Transcriptome Analysis Reveals Chitooligosaccharides-Induced Stress Tolerance of Gracilariopsis Lemaneiformis under High Temperature Stress. Aquaculture 2020, 519, 734876. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Herpes Simplex Virus Type 1 Variants Arising after Selection with an Antiviral Carrageenan: Lack of Correlation between Drug Susceptibility and Syn Phenotype. J. Med. Virol. 2002, 68, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Siddhnath; Surasani, V.K.R.; Singh, A.; Singh, S.M.; Hauzoukim; Murthy, L.N.; Baraiya, K.G. Bioactive Compounds from Micro-Algae and Its Application in Foods: A Review. Discov. Food 2024, 4, 27. [Google Scholar] [CrossRef]
- Beetul, K.; Gopeechund, A.; Kaullysing, D.; Mattan-Moorgawa, S.; Puchooa, D.; Bhagooli, R. Challenges and Opportunities in the Present Era of Marine Algal Applications. In Algae—Organisms for Imminent Biotechnology; Thajuddin, N., Dhanasekaran, D., Eds.; InTech: London, UK, 2016; ISBN 978-953-51-2431-3. [Google Scholar]
- AlgiMatrix 3D Cell Culture System—IN. Available online: https://www.thermofisher.com/in/en/home/life-science/cell-culture/organoids-spheroids-3d-cell-culture/extracellular-matrices-ecm/algimatrix-3d-cell-culture-system.html (accessed on 30 October 2025).
- Alginate Dressing AlgiSite M 6 × 8 Inch Rectangle—McKesson. Available online: https://mms.mckesson.com/product/373245/Smith-Nephew-59480300 (accessed on 30 October 2025).
- Friedland, B.A.; Stoner, M.; Chau, M.M.; Plagianos, M.G.; Govender, S.; Morar, N.; Altini, L.; Skoler-Karpoff, S.; Ahmed, K.; Ramjee, G.; et al. Baseline Predictors of High Adherence to a Coitally Dependent Microbicide Gel Based on an Objective Marker of Use: Findings from the Carraguard Phase 3 Trial. AIDS Behav. 2016, 20, 2565–2577. [Google Scholar] [CrossRef]
- Eccles, R.; Meier, C.; Jawad, M.; Weinmüllner, R.; Grassauer, A.; Prieschl-Grassauer, E. Efficacy and Safety of an Antiviral Iota-Carrageenan Nasal Spray: A Randomized, Double-Blind, Placebo-Controlled Exploratory Study in Volunteers with Early Symptoms of the Common Cold. Respir. Res. 2010, 11, 108. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Carrageenan Nasal Spray May Double the Rate of Recovery from Coronavirus and Influenza Virus Infections: Re-analysis of Randomized Trial Data. Pharmacol. Res. Perspec 2021, 9, e00810. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, X.; Hu, C.; Li, P.; Zhao, M.; Lu, J.; Xia, G. A Fucoidan-Gelatin Wound Dressing Accelerates Wound Healing by Enhancing Antibacterial and Anti-Inflammatory Activities. Int. J. Biol. Macromol. 2022, 223, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Lin, X.-Z.; Kuo, K.-L.; Hsu, F.-Y. Fabrication and Cytotoxicity of Fucoidan-Cisplatin Nanoparticles for Macrophage and Tumor Cells. Materials 2017, 10, 291. [Google Scholar] [CrossRef]
- Salamat, Q.; Moradi, R.; Nadizadeh, Z.; Kavehpour, P.; Soylak, M.; Asimov, A.; Zillur Rahman, M.; Kovářík, T.; Babuška, V.; Deshmukh, K. Chitosan Based Smart Injectable Hydrogels for Biomedical Applications: A Comprehensive Review. Bioact. Mater. 2026, 55, 703–753. [Google Scholar] [CrossRef]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel Chitosan-Ulvan Hydrogel Reinforcement by Cellulose Nanocrystals with Epidermal Growth Factor for Enhanced Wound Healing: In Vitro and in Vivo Analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, E.J.; Correia, T.R.; Rodrigues, J.M.M.; Mano, J.F. Novel Biodegradable Laminarin Microparticles for Biomedical Applications. Bull. Chem. Soc. Jpn. 2020, 93, 713–719. [Google Scholar] [CrossRef]
- Sinani, G.; Şenel, S. Advances in Vaccine Adjuvant Development and Future Perspectives. Drug Deliv. 2025, 32, 2517137. [Google Scholar] [CrossRef]
- Athamneh, T.; Hajnal, A.; Al-Najjar, M.A.A.; Alshweiat, A.; Obaidat, R.; Awad, A.A.; Al-Alwany, R.; Keitel, J.; Wu, D.; Kieserling, H.; et al. In Vivo Tests of a Novel Wound Dressing Based on Agar Aerogel. Int. J. Biol. Macromol. 2023, 239, 124238. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
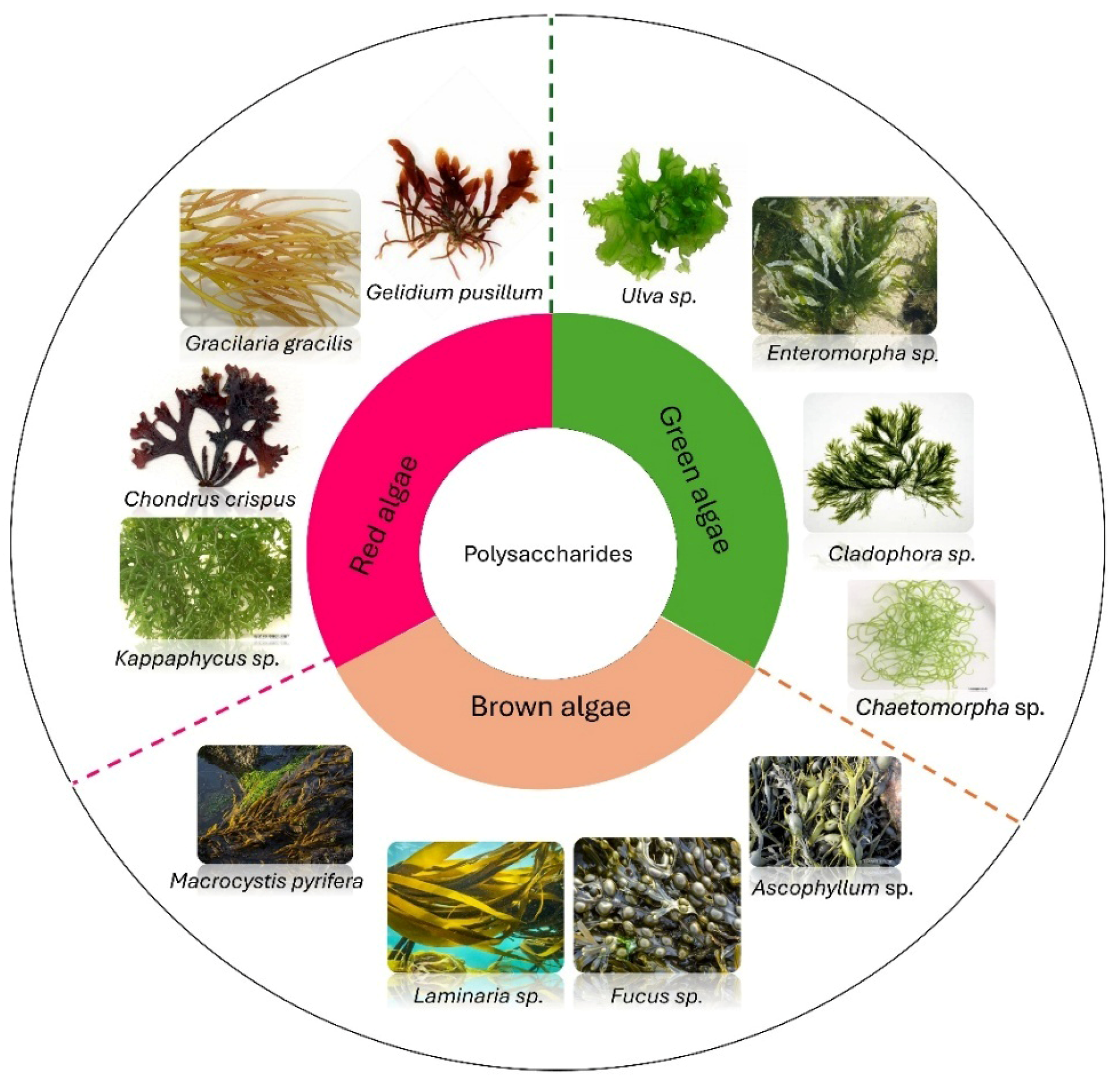
| Polysaccharide | Source Algae | Chemical Composition | Structural Characteristics | Classification | References |
|---|---|---|---|---|---|
| Alginate | Brown algae (Laminaria, Ascophyllum) | β-D-mannuronic acid (M) and α-L-guluronic acid (G) | Linear copolymer of (1→4)-linked M and G residues arranged in blocks (MM, GG, MG) | Structural polysaccharide | [17] |
| Carrageenan | Red algae (Kappaphycus, Chondrus) | Sulfated galactose units | Linear galactans with alternating (1→3)-β-D-galactose and (1→4)-α-D-galactose with sulfate groups | Sulfated galactan | [18,19] |
| Agar | Red algae (Gelidium, Gracilaria) | Galactose and 3,6-anhydro-L-galactose | Repeating disaccharide units of agarose; low sulfate content | Structural galactan | [19] |
| Fucoidan | Brown algae (Fucus, Undaria) | Sulfated L-fucose, with possible galactose, xylose | Highly branched, heterogeneous sulfated polysaccharide with varying structure | Sulfated fucan | [20] |
| Laminarin | Brown algae (Laminaria, Eisenia) | Glucose | Storage β-glucan, mostly linear with some branching | Storage polysaccharide | [21,22] |
| Ulvan | Green algae Ulva | Sulfated rhamnose, glucuronic acid, iduronic acid, xylose | Complex branched polysaccharide with high uronic acid and sulfate content | Sulfated rhamnan | [23,24] |
| Algal Species | Marine Polysaccharide | Biomedical Applications | References |
|---|---|---|---|
| Sargassum swartzii | Fucoidan | Antiviral activity, antibacterial, anticoagulant, anti-inflammatory, antiviral, antithrombosis, anti-tumor, anticancer | [118] |
| Laminaria digitata | Laminaran | anti-inflammatory and anti-oxidative, anti-oxidative and anti-inflammatory | [81] |
| Porphyridium cruentum | Sulfated Polysaccharides (EPS) | Anti-inflammatory, antiviral, wound healing | [119] |
| Dunaliella salina | β-Glucans, Sulfated Polysaccharides | Antioxidant, anti-inflammatory, immune-stimulating | [120] |
| Chondrus crispus (Irish Moss) | Carrageenan (κ-, ι-, and λ-carrageenan) | Thickening agent, anti-inflammatory, potential antitumor | [121] |
| Gelidium amansii | Agar, Agarose | Microbiological media, food industry | [122] |
| Gracilaria spp. | Agar, Agarose | Food gelling agent, microbiology | [123] |
| Laminaria spp. | Alginate | Food thickener, wound dressing | [87] |
| Sargassum spp. | Fucoidan, Alginate | Anti-inflammatory, anticoagulant, potential anticancer | [124] |
| Ulva spp. (Sea Lettuce) | Ulvan | Antioxidant, anti-inflammatory, antiviral | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waoo, A.A.; Singh, S.; Chandra, N.; Mishra, S.; Singh, M.P.; Mal, J.; Jha, A.K.; Joo, J.C.; Pandey, A. Comprehensive Review on the Biomedical Applications of Marine Algal Polysaccharides. Phycology 2025, 5, 80. https://doi.org/10.3390/phycology5040080
Waoo AA, Singh S, Chandra N, Mishra S, Singh MP, Mal J, Jha AK, Joo JC, Pandey A. Comprehensive Review on the Biomedical Applications of Marine Algal Polysaccharides. Phycology. 2025; 5(4):80. https://doi.org/10.3390/phycology5040080
Chicago/Turabian StyleWaoo, Ashwini A., Sukhendra Singh, Neha Chandra, Shaily Mishra, Manish Pratap Singh, Joyabrata Mal, Abhimanyu Kumar Jha, Jeong Chan Joo, and Ashutosh Pandey. 2025. "Comprehensive Review on the Biomedical Applications of Marine Algal Polysaccharides" Phycology 5, no. 4: 80. https://doi.org/10.3390/phycology5040080
APA StyleWaoo, A. A., Singh, S., Chandra, N., Mishra, S., Singh, M. P., Mal, J., Jha, A. K., Joo, J. C., & Pandey, A. (2025). Comprehensive Review on the Biomedical Applications of Marine Algal Polysaccharides. Phycology, 5(4), 80. https://doi.org/10.3390/phycology5040080








