Waste-to-Resource Strategies: The Potential of Agro-Industrial Residues for Microalgal Bioproducts in Indonesia
Abstract
1. Introduction
2. Agro-Industrial Waste in Indonesia
2.1. Palm Oil Mill Effluent (POME)
2.2. Cassava Wastes
2.3. Sugarcane Wastes
2.4. Soybean Waste
3. Microalgae Cultivation on Agro-Industrial Wastewaters
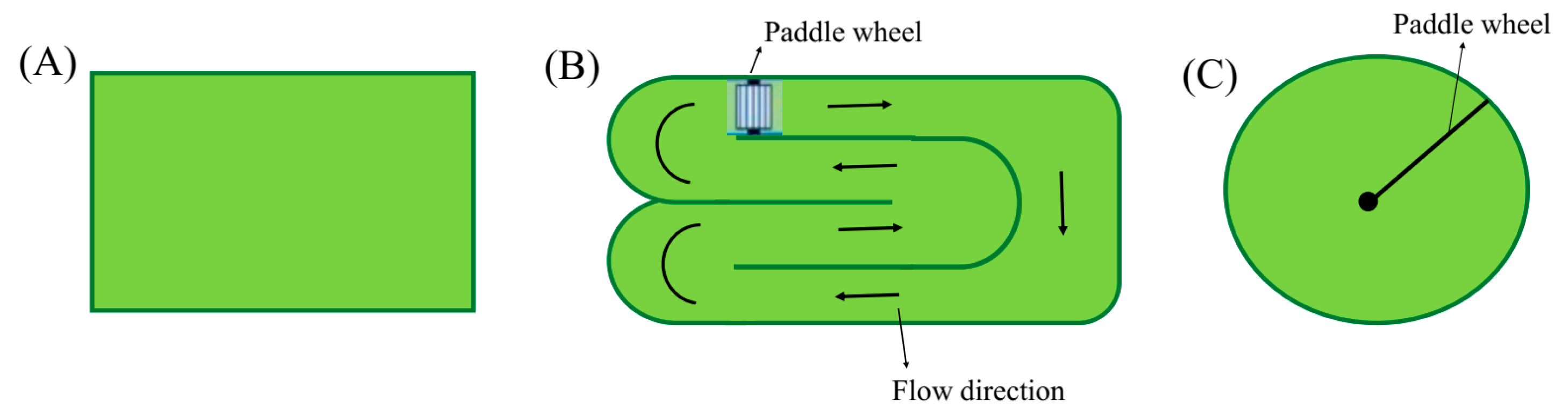
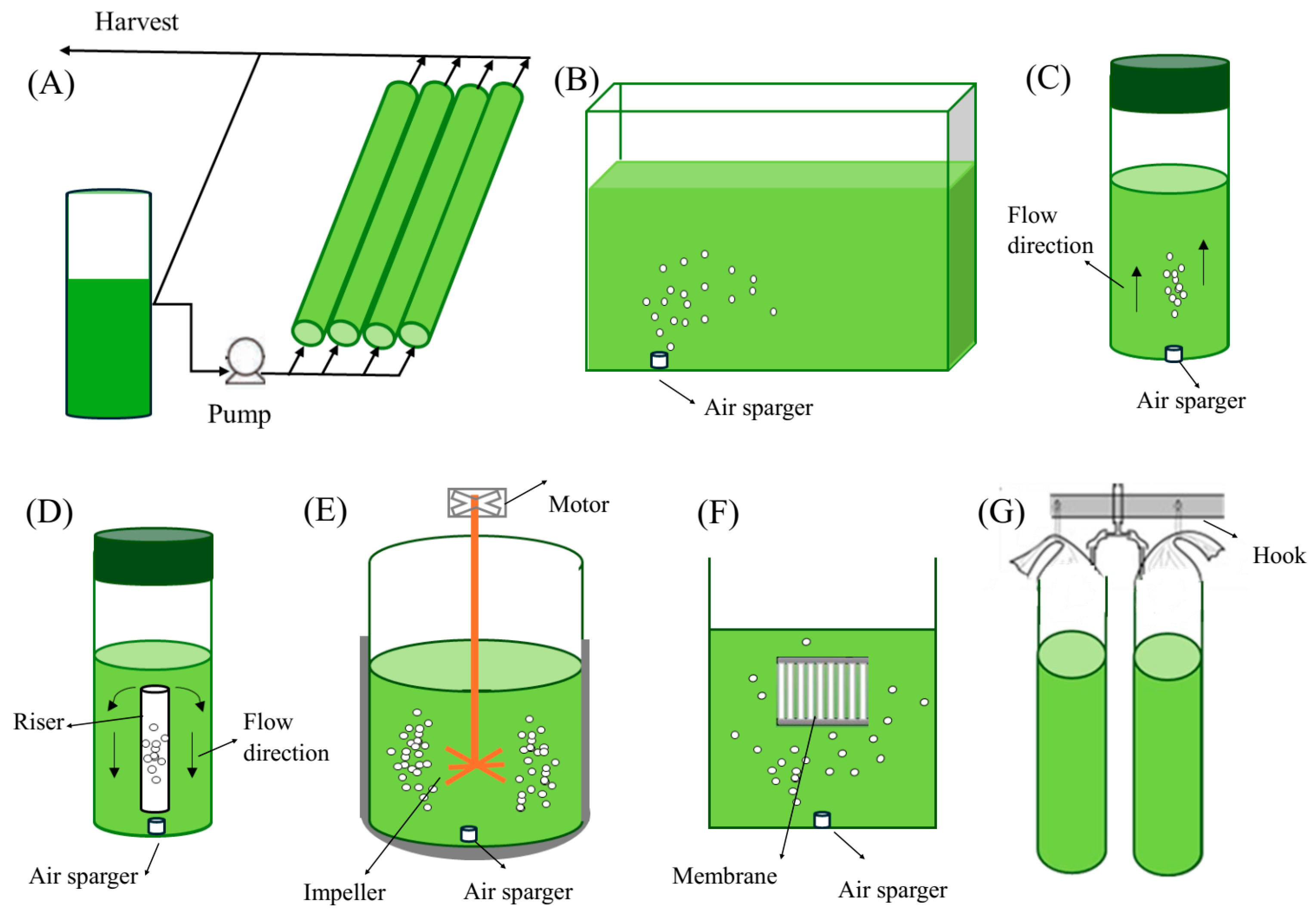
3.1. Growth Modes
3.2. Cultivation Systems
3.3. Cultivation of Microalgae on Different Agro-Industrial Wastewaters
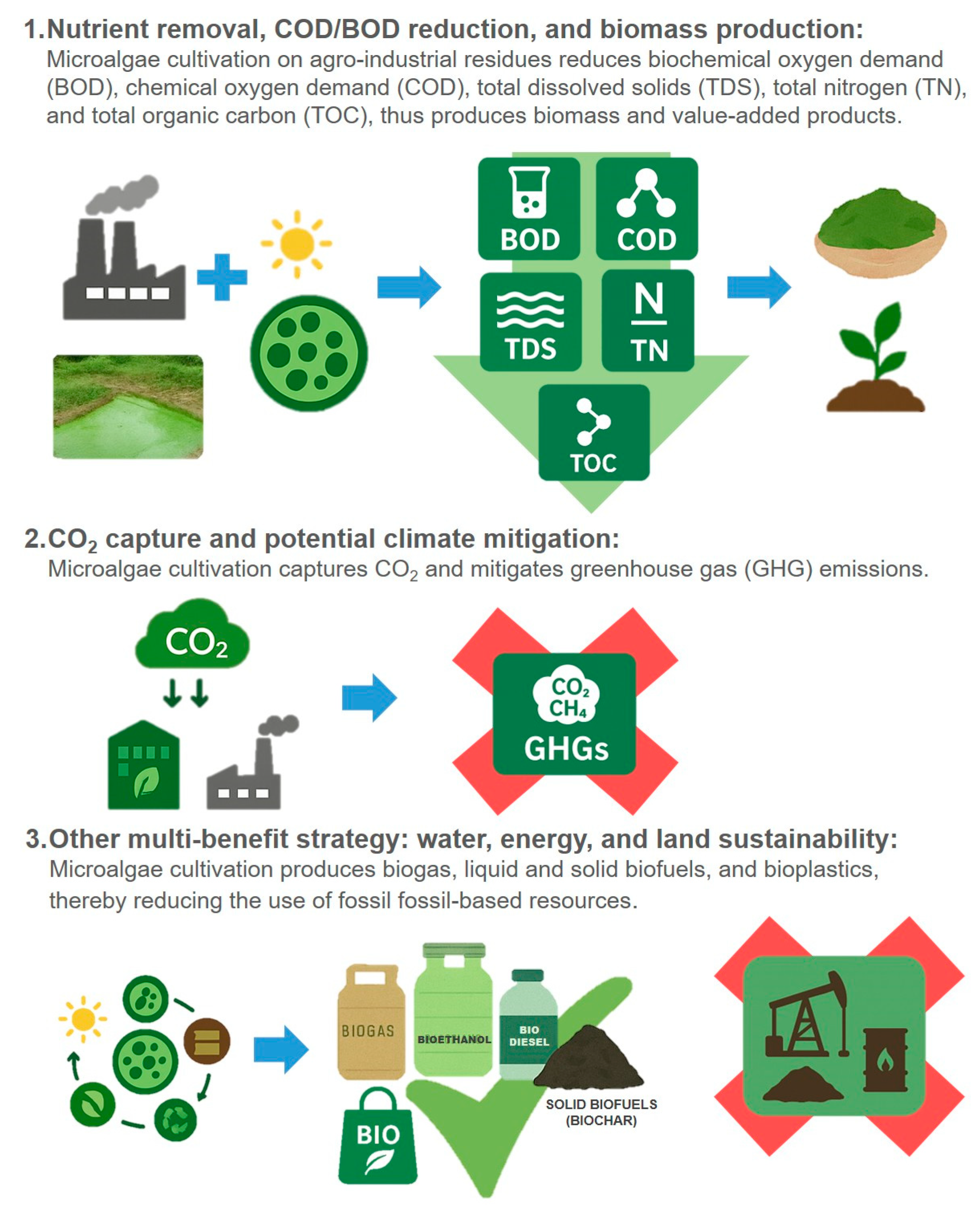
4. Environmental Benefits of Bioproducts from Agro-Industrial Residues
4.1. Nutrient Removal, COD/BOD Reduction, and Biomass Production
4.2. CO2 Capture and Potential Climate Mitigation
4.3. Other Multi-Benefit Strategies for Water, Energy, and Land Sustainability
5. Value-Added Bioproducts from Microalgal Biomass
5.1. Bioenergy
5.2. Pigments and Nutraceuticals
5.3. Biofertilizers, Biostimulants, Biocontrol Agents
5.4. Animal and Aquaculture Feed
6. Environmental Conditions and Opportunities for Microalgae Cultivation in Indonesia
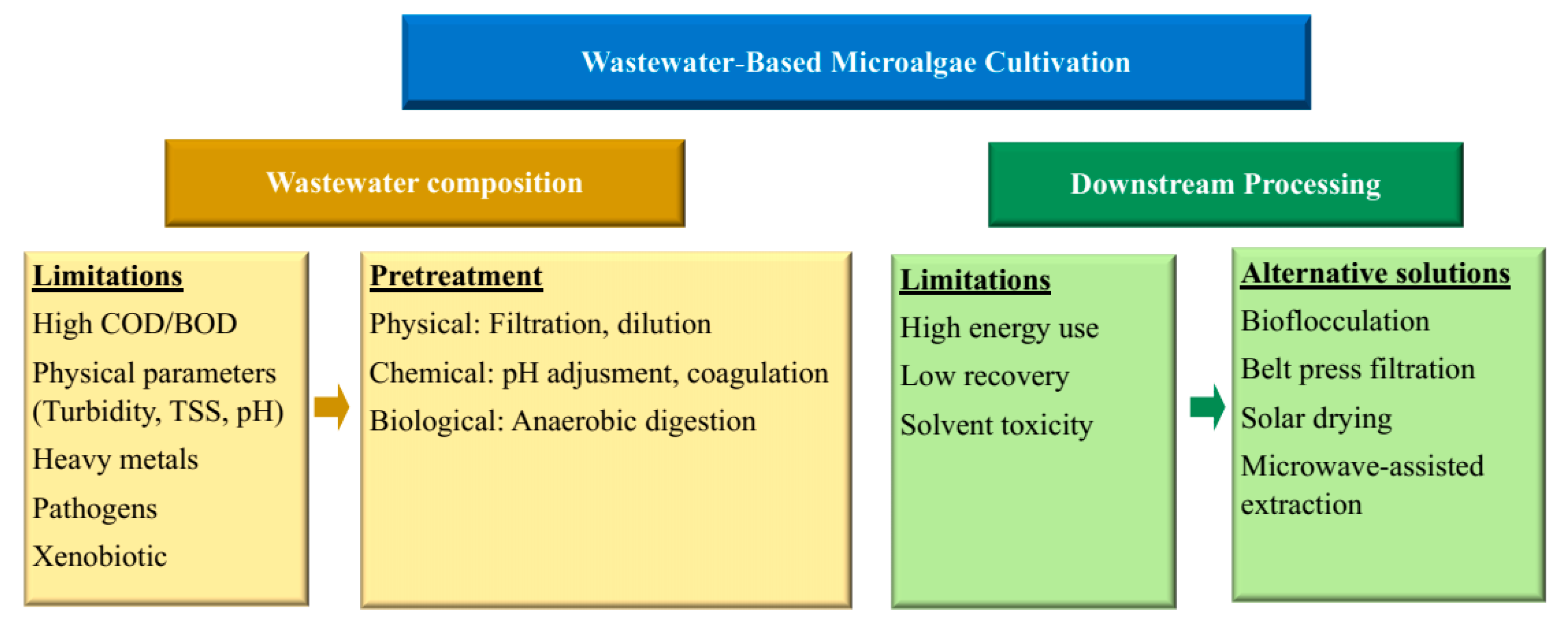
7. Challenges, Limitations, and Strategies for Microalgae Cultivation Using Agro-Industrial Wastewaters in Indonesia
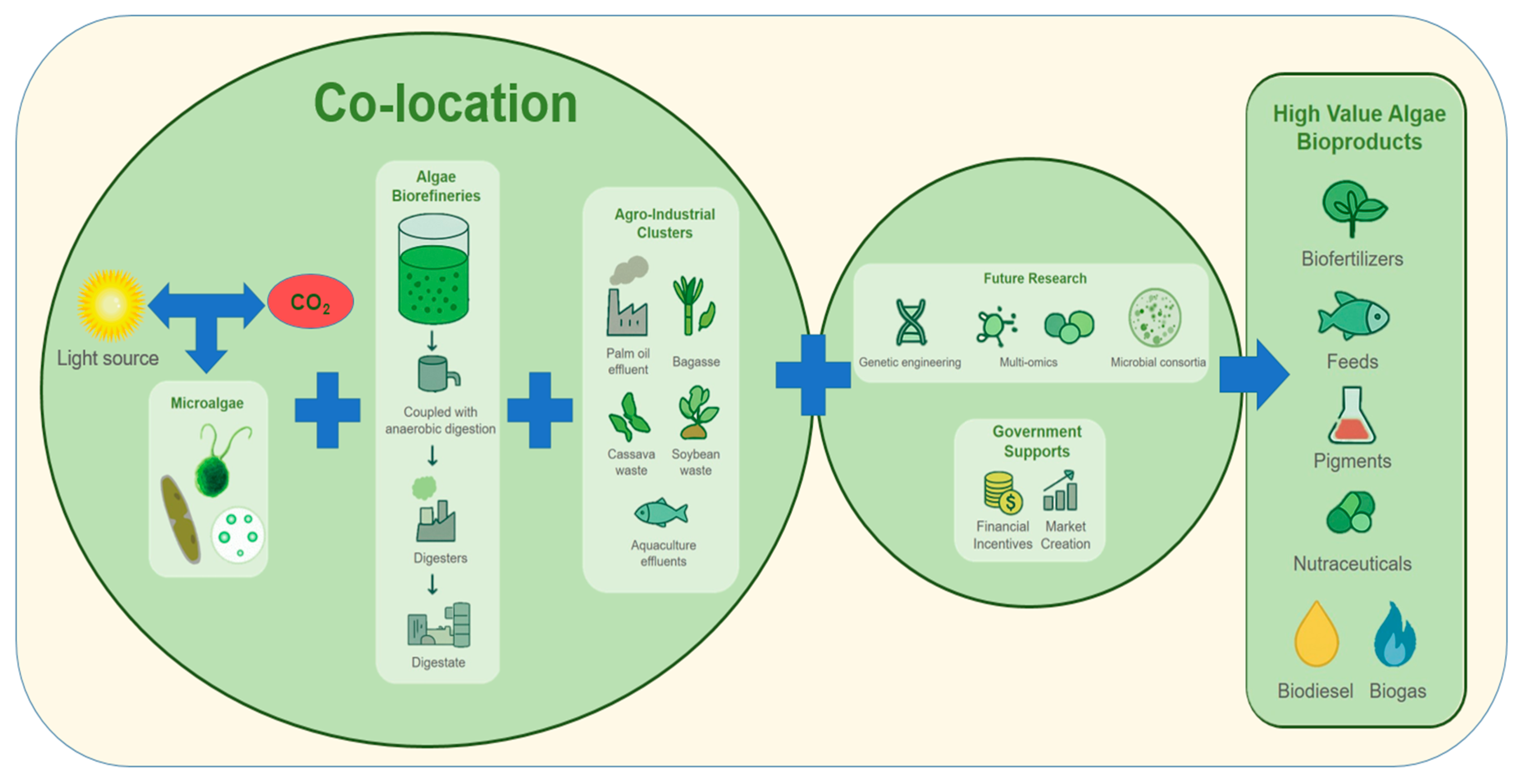
8. Future Perspectives: Advancing Waste-to-Resource Strategies for Microalgal Bioproducts in Indonesia
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| BOD | Biochemical Oxygen Demand |
| CBEW | Cassava Biogas Effluent Wastewater |
| COD | Chemical Oxygen Demand |
| CPW | Cassava Processing Wastewater |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic Acid |
| FAME | Fatty Acid Methyl Esters |
| GDP | Gross Domestic Product |
| GHG | Greenhouse gas |
| HC | Hydrocarbon |
| HRAP | High-Rate Algal Pond |
| MBBR | Moving-Bed Biofilm Reactor |
| MBS | Microalgal Biostimulants |
| PBR | Photobioreactors |
| POME | Palm Oil Mill Effluent |
| PUFA | Polyunsaturated Fatty Acid |
| TDS | Total Dissolved Solids |
| TN | Total Nitrogen |
| TP | Total Phosphorus |
| TOC | Total Organic Carbon |
| TWW | Tofu Whey Wastewater |
References
- Yuniarto, B.; Nurani, R.; Apriyono, A.; Rahmawati, R.; Nurfadilah, N. The Role of Geopolitics on Economic Growth in Indonesia. Opsearch Am. J. Open Res. 2024, 3, 251–257. [Google Scholar] [CrossRef]
- Nugraha, H.; Nurmalina, R.; Achsani, N.A.; Suroso, A.I.; Suprehatin, S. Indonesia’s Position and Participation in The Global Value Chain of The Agriculture Sector. J. Manaj. Dan Agribisnis 2025, 22, 105. [Google Scholar] [CrossRef]
- Sarma, M.; Septiani, S.; Nanere, M. The Role of Entrepreneurial Marketing in the Indonesian Agro-Based Industry Cluster to Face the ASEAN Economic Community. Sustainability 2022, 14, 6163. [Google Scholar] [CrossRef]
- Mileno, M.F. Industi Agro Indonesia Jadi Magnet Investasi, Serap Hingga 9 Juta Tenaga Kerja. Available online: https://www.goodnewsfromindonesia.id/2025/04/02/industi-agro-indonesia-jadi-magnet-investasi-serap-hingga-9-juta-tenaga-kerja (accessed on 18 September 2025).
- Kurniawan, R.D. Industri Agro Melemah di Q1 2025, Kemenperin Ungkap Biang Masalahnya. Available online: https://wartaekonomi.co.id/read570500/industri-agro-melemah-di-q1-2025-kemenperin-ungkap-biang-masalahnya (accessed on 18 September 2025).
- Hidayat, N.; Suhartini, S.; Arinda, T.; Elviliana, E.; Melville, L. Literature Review on Ability of Agricultural Crop Residues and Agro-Industrial Waste for Treatment of Wastewater. J. Recycl. Org. Waste Agric. 2022, 11, 553–585. [Google Scholar] [CrossRef]
- Widayat; Philia, J.; Wibisono, J. Cultivation of Microalgae Chlorella sp. on Fresh Water and Waste Water of Tofu Industry. In Proceedings of the E3S Web of Conferences, Semarang, Indonesia, 14–15 August 2018; Volume 31, p. 04009. [Google Scholar] [CrossRef]
- Hadiyanto, H. Ozone Application for Tofu Waste Water Treatment and Its Utilisation for Growth Medium of Microalgae Spirulina sp. In Proceedings of the E3S Web of Conferences, Semarang, Indonesia, 15–17 August 2017; Volume 31, p. 03002. [Google Scholar] [CrossRef]
- Dwi Januari, A.; Warno Utomo, S.; Agustina, H. Estimation and Potential of Palm Oil Empty Fruit Bunches Based on Crude Palm Oil Forecasting in Indonesia. In Proceedings of the E3S Web of Conferences, Virtual, 28–30 September 2020; Volume 211, p. 05003. [Google Scholar] [CrossRef]
- Dianursanti; Rizkytata, B.T.; Gumelar, M.T.; Abdullah, T.H. Industrial Tofu Wastewater as a Cultivation Medium of Microalgae Chlorella vulgaris. Energy Procedia 2014, 47, 56–61. [Google Scholar] [CrossRef]
- Zahroh, S.F.; Syamsu, K.; Haditjaroko, L.; Kartawiria, I.S. Potential and Prospect of Various Raw Materials for Bioethanol Production in Indonesia: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 749, 012060. [Google Scholar] [CrossRef]
- Marthalia, L.; Tumuyu, S.S.; Asteria, D. Economy Circular Adoption toward Sustainable Business (Study Case: Agro-Industry Company in Indonesia). J. Pengelolaan Sumberd. Alam Dan Lingkung. J. Nat. Resour. Environ. Manag. 2024, 14, 58–65. [Google Scholar] [CrossRef]
- Mesa, J.A.; Sierra-Fontalvo, L.; Ortegon, K.; Gonzalez-Quiroga, A. Advancing Circular Bioeconomy: A Critical Review and Assessment of Indicators. Sustain. Prod. Consum. 2024, 46, 324–342. [Google Scholar] [CrossRef]
- Pagels, F.; Amaro, H.M.; Tavares, T.G.; Amil, B.F.; Guedes, A.C. Potential of Microalgae Extracts for Food and Feed Supplementation-A Promising Source of Antioxidant and Anti-Inflammatory Compounds. Life 2022, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Suhartini, S.; Indah, S.H.; Rahman, F.A.; Rohma, N.A.; Rahmah, N.L.; Nurika, I.; Hidayat, N.; Melville, L. Enhancing Anaerobic Digestion of Wild Seaweed Gracilaria verrucosa by Co-Digestion with Tofu Dregs and Washing Pre-Treatment. Biomass Convers. Biorefinery 2023, 13, 4255–4277. [Google Scholar] [CrossRef]
- Sahaq, A.B. Hadiyanto the Bioelectricity of Tofu Wastewater in Microalgae-Microbial Fuel Cell (MMFC) System. Int. Adv. Res. J. Sci. Eng. Technol. 2019, 6, 37–39. [Google Scholar] [CrossRef]
- Elystia, S.; Saragih, L.R.; Muria, S.R. Algal-Bacterial Synergy for Lipid Production and Nutrient Removal in Tofu Liquid Waste. Int. J. Technol. 2021, 12, 287. [Google Scholar] [CrossRef]
- Mishra, B.; Mohanta, Y.K.; Reddy, C.N.; Reddy, S.D.M.; Mandal, S.K.; Yadavalli, R.; Sarma, H. Valorization of Agro-Industrial Biowaste to Biomaterials: An Innovative Circular Bioeconomy Approach. Circ. Econ. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Rahmanulloh, A. Oilseeds and Products Annual: Indonesia (Report No. ID2025-0015); U.S. Department of Agriculture, Foreign Agricultural Service: Washington, DC, USA, 2025. [Google Scholar]
- Sodri, A.; Septriana, F.E. Biogas Power Generation from Palm Oil Mill Effluent (POME): Techno-Economic and Environmental Impact Evaluation. Energies 2022, 15, 7265. [Google Scholar] [CrossRef]
- Putra, S.E.; Dewata, I.; Barlian, E.; Syah, N.; Iswandi, U.; Gusman, M.; Amran, A.; Fariani, A. Analysis of Palm Oil Mill Effluent Quality. In Proceedings of the E3S Web of Conferences, Malang, Indonesia, 22–23 August 2023; Volume 481, p. 03001. [Google Scholar] [CrossRef]
- Handayani, T.; Djarot, I.N.; Widyastuti, N.; Arianti, F.D.; Rifai, A.; Sitomurni, A.I.; Nur, M.M.A.; Dewi, R.N.; Nuha, N.; Haryanti, J.; et al. Biogas Quality and Nutrient Remediation in Palm Oil Mill Effluent through Chlorella vulgaris Cultivation Using a Photobioreactor. Glob. J. Environ. Sci. Manag. 2024, 10, 1519–1542. [Google Scholar] [CrossRef]
- Handayani, T.; Santoso, A.D.; Widyastuti, N.; Djarot, I.N.; Sitomurni, A.I.; Rifai, A.; Aziz, A.; Apriyanto, H.; Nadirah, N.; Kusrestuwardhani, K.; et al. Opportunity of Smart Aquaculture and Eco-Farming Integration in POME Bioremediation and Phycoremediation System for Environmental Sustainability. Evergreen 2025, 12, 903–931. [Google Scholar] [CrossRef]
- Sari, F.Y.A.; Suryajaya, I.M.A.; Christwardana, M.; Hadiyanto, H. Cultivation of Microalgae Spirulina platensis in Palm Oil Mill Effluent (POME) Media with Variations of POME Concentration and Nutrient Composition. J. Bioresour. Environ. Sci. 2022, 1, 57–62. [Google Scholar] [CrossRef]
- Firdaus, N.; Prasetyo, B.T.; Sofyan, Y.; Siregar, F. Palm Oil Mill Effluent (POME): Biogas Power Plant. Distrib. Gener. Altern. Energy J. 2017, 32, 6–18. [Google Scholar] [CrossRef]
- Hasanudin, U.; Sugiharto, R.; Haryanto, A.; Setiadi, T.; Fujie, K. Palm Oil Mill Effluent Treatment and Utilization to Ensure the Sustainability of Palm Oil Industries. Water Sci. Technol. 2015, 72, 1089–1095. [Google Scholar] [CrossRef]
- Nuryadi, A.P.; Raksodewanto, A.A.; Susanto, H.; Peryoga, Y. Analysis on the Feasibility of Small-Scale Biogas from Palm Oil Mill Effluent (POME)—Study Case: Palm Oil Mill in Riau-Indonesia. MATEC Web Conf. 2019, 260, 03004. [Google Scholar] [CrossRef]
- Sinaga, A.R.I.; Nur, T.; Surya, I. Aspen Plus Simulation Analysis on Palm Oil Mill Effluent (POME) Recycling System into Bioethanol. J. Adv. Res. Fluid Mech. Therm. Sci. 2023, 109, 41–50. [Google Scholar] [CrossRef]
- Hariyadi, T.P.; Jelita, M. Analisis Potensi Dan Evaluasi Emisi Biodiesel Dari Palm Oil Mill Effluent. J. Al-AZHAR Indones. SERI SAINS DAN Teknol. 2024, 9, 115. [Google Scholar] [CrossRef]
- Ratnasari, I.F.D.; Devi, D.; Yanuar Setyawan, I.A. Aplikasi Limbah Palm Oil Mill Effluent (POME) Terhadap Sifat Kimia Tanah Pada Perkebunan Kelapa Sawit. J. Media Pertan. 2024, 9, 113. [Google Scholar] [CrossRef]
- Minister of Environment of the Republic of Indonesia. Regulation of the Minister of Environment of the Republic of Indonesia Number 5 of 2014 concerning Wastewater Quality Standards; Ministry of Environment: Jakarta, Indonesia, 2014. [Google Scholar]
- Bisnis Sawit. Regulasi Baru Limbah Sawit Segera Terbit, KLHK Dorong Industri Hijau. Available online: https://bisnissawit.com/regulasi-baru-limbah-sawit-segera-terbit-klhk-dorong-industri-hijau/ (accessed on 29 September 2025).
- Pujono, H.R.; Kukuh, S.; Evizal, R.; Afandi; Rahmat, A. The Effect of POME Application on Production and Yield Components of Oil Palm in Lampung, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 16–18 September 2020; Volume 648, p. 012058. [Google Scholar] [CrossRef]
- Siahaan, M.; Sakiah; Sutanto, A.S.; Lawary, A.; Mahmuda, R. The Study of Soil Biological and Chemical Properties on Palm Oil Plant Rhizosphere with and without Palm Oil Mill Effluent Applications. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kuala Lumpur, Malaysia, 11 November 2020; Volume 819, p. 012003. [Google Scholar] [CrossRef]
- Marsa Chairani, A.; Lestari, P.; Yuni Susanti, D.; Ngadisih; Clara Dione, N.; Labiba Azzahra, R.; Shiba Dhiyaul Rohma, A. Tropical Agricultural Waste Management: Exploring the Utilization of Cassava Husk for Extracting Cellulose and Starch as Sustainable Biomass Resources. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Virtual, 19–20 September 2024; Volume 1438, p. 012072. [Google Scholar] [CrossRef]
- Edama, N.A.; Sulaiman, A.; Abd Rahim, S.N. Enzymatic Saccharification of Tapioca Processing Wastes into Biosugars through Immobilization Technology. Biofuel Res. J. 2014, 1, 2–6. [Google Scholar] [CrossRef]
- Amalia, A.V.; Fibriana, F.; Widiatningrum, T.; Hardianti, R.D. Bioconversion and Valorization of Cassava-Based Industrial Wastes to Bioethanol Gel and Its Potential Application as a Clean Cooking Fuel. Biocatal. Agric. Biotechnol. 2021, 35, 102093. [Google Scholar] [CrossRef]
- Setyawati, R.; Katayama-Hirayama, K.; Kaneko, H.; Hirayaman, K. Current Tapioca Starch Wastewater (TSW) Management in Indonesia. World Appl. Sci. J. 2011, 14, 658–665. [Google Scholar]
- Kurniadie, D.; Wijaya, D.; Widayat, D.; Umiyati, U.; Iskandar. Constructed Wetland to Treat Tapioca Starch Wastewater in Indonesia. Asian J. Water Environ. Pollut. 2018, 15, 107–113. [Google Scholar] [CrossRef]
- Hasanudin, U.; Kustyawati, M.E.; Iryani, D.A.; Haryanto, A.; Triyono, S. Estimation of Energy and Organic Fertilizer Generation from Small Scale Tapioca Industrial Waste. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Malang, Indonesia, 17–19 September 2018; Volume 230, p. 012084. [Google Scholar] [CrossRef]
- Triani, H.; Yuniza, A.; Marlida, Y.; Husmaini, H.; Astuti, W.; Yanti, G. A Novel Bacterial Approach to Cassava Waste Fermentation: Reducing Cyanide Toxicity and Improving Quality to Ensure Livestock Feed Safety. Open Vet. J. 2025, 15, 1358–1369. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Hartanto, D.; Rohman, H.A.; Mitamaytawati; Qudus, N.; Daniyanto. Valorization of Sugarcane-Based Bioethanol Industry Waste (Vinasse) to Organic Fertilizer. In Valorisation of Agro-industrial Residues—Volume II: Non-Biological Approaches; Zakaria, Z.A., Aguilar, C.N., Kusumaningtyas, R.D., Binod, P., Eds.; Applied Environmental Science and Engineering for a Sustainable Future; Springer International Publishing: Cham, Switzerland, 2020; pp. 203–223. ISBN 978-3-030-39207-9. [Google Scholar]
- Adila, H.; Wisnu, W.; Handayani, E.T.; Fathin, H.R.; Rusdianto, A.S. Effectiveness of Sugarcane Bagasse Adsorbent Combined with Aquaponics System as an Innovation for Absorbing Contaminants in Sugar Industry Wastewater. Int. J. Food Agric. Nat. Resour. 2023, 4, 70–76. [Google Scholar] [CrossRef]
- Restiawaty, E.; Gani, K.P.; Dewi, A.; Arina, L.A.; Kurniawati, K.I.; Budhi, Y.W.; Akhmaloka, A. Bioethanol Production from Sugarcane Bagasse Using Neurospora intermedia in an Airlift Bioreactor. Int. J. Renew. Energy Dev. 2020, 9, 247–253. [Google Scholar] [CrossRef]
- Suryaningrum, L.H. Challenges and Strategies for the Utilization of Sugarcane Bagasse (By-Products of Sugar Industry) as Freshwater Fish Feed Ingredient. Perspektif 2022, 21, 26. [Google Scholar] [CrossRef]
- Kustiyah, E.; Novitasari, D.; Wardani, L.A.; Hasaya, H.; Widiantoro, M. Utilization of Sugarcane Bagasses for Making Biodegradable Plastics with the Melt Intercalation Method. J. Teknol. Lingkung. 2023, 24, 300–306. [Google Scholar] [CrossRef]
- Jamir, L.; Kumar, V.; Kaur, J.; Kumar, S.; Singh, H. Composition, Valorization and Therapeutical Potential of Molasses: A Critical Review. Environ. Technol. Rev. 2021, 10, 131–142. [Google Scholar] [CrossRef]
- Bandriyo, M.T.I.A.; Saukat, Y.; Rifin, A. Indonesian Molasses Export Supply in World Trade. J. Perspekt. Pembiayaan Dan Pembang. Drh. 2022, 10, 23–32. [Google Scholar] [CrossRef]
- Ginting, E.; Elisabeth, D.A.A.; Khamidah, A.; Rinaldi, J.; Ambarsari, I.; Antarlina, S.S. The Nutritional and Economic Potential of Tofu Dreg (Okara) and Its Utilization for High Protein Food Products in Indonesia. J. Agric. Food Res. 2024, 16, 101175. [Google Scholar] [CrossRef]
- Pakpahan, M.R.; Ruhiyat, R.; Hendrawan, D. Evaluation of Wastewater Quality of Tempeh Industry (Case Study of Tempeh Semanan Industrial Estate). In Proceedings of the Symposium on Advance of Sustainable Engineering 2021 (SIMASE 2021): Post Covid-19 Pandemic: Chal-lenges and Opportunities in Environment, Science, and Engineering Research, Bandung, Indonesia, 18–19 August 2021; p. 020015. [Google Scholar]
- Wulansarie, R.; Fardhyanti, D.S.; Ardhiansyah, H.; Nuroddin, H.; Salsabila, C.A.; Alifiananda, T. Combination of Adsorption Using Activated Carbon and Advanced Oxidation Processes (AOPs) Using O3/H2O2 in Decreasing BOD of Tofu Liquid Waste. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Virtual, 20–23 September 2023; Volume 1381, p. 012041. [Google Scholar] [CrossRef]
- Bintari, S.H.; Herlina, L.; Wicaksono, D.; Sunyoto; Purnamaningrum, S.P.D.; Laksana, W.D.; Ulfa, B.M. Training on Making Liquid Organic Fertilizer from Tofu Whey Waste to Reduce Environmental Pollution. Abdimas J. Pengabdi. Masy. Univ. Merdeka Malang 2025, 10, 157–166. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, N.; Singh, G.; Mishra, S.; Lodhiyal, N. Microalgae Cultivation and Value-Based Products from Wastewater: Insights and Applications. Blue Biotechnol. 2024, 1, 20. [Google Scholar] [CrossRef]
- Muthukumaran, M.; Rawindran, H.; Noorjahan, A.; Parveen, M.; Barasarathi, J.; Blessie, J.P.J.; Ali, S.S.; Sayyed, R.Z.; Awasthi, M.K.; Hassan, S.; et al. Microalgae-Based Solutions for Palm Oil Mill Effluent Management: Integrating Phycoremediation, Biomass and Biodiesel Production for a Greener Future. Biomass Bioenergy 2024, 191, 107445. [Google Scholar] [CrossRef]
- Santos, B.; Freitas, F.; Sobral, A.J.F.N.; Encarnação, T. Microalgae and Circular Economy: Unlocking Waste to Resource Pathways for Sustainable Development. Int. J. Sustain. Eng. 2025, 18, 2501488. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Buma, A.G.J. Opportunities and Challenges of Microalgal Cultivation on Wastewater, with Special Focus on Palm Oil Mill Effluent and the Production of High Value Compounds. Waste Biomass Valorization 2019, 10, 2079–2097. [Google Scholar] [CrossRef]
- Dias, R.R.; Deprá, M.C.; De Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae Cultivation in Wastewater: How Realistic Is This Approach for Value-Added Product Production? Processes 2025, 13, 2052. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.L.S.; Dutra, D.A.; Schneider, A.T.; Dias, R.R.; Deprá, M.C.; Zepka, L.Q.; Jacob-Lopes, E. Future Prospect of Development of Integrated Wastewater and Algal Biorefineries and Its Impact on Biodiversity and Environment. In Algal Biorefinery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 371–383. ISBN 978-0-443-23967-0. [Google Scholar]
- Velásquez-Orta, S.B.; Yáñez-Noguez, I.; Ramírez, I.M.; Ledesma, M.T.O. Pilot-Scale Microalgae Cultivation and Wastewater Treatment Using High-Rate Ponds: A Meta-Analysis. Environ. Sci. Pollut. Res. 2024, 31, 46994–47021. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Juan, J.C.; Chang, J.-S.; Ling, T.C. Enhancing Biomass and Lipid Productions of Microalgae in Palm Oil Mill Effluent Using Carbon and Nutrient Supplementation. Energy Convers. Manag. 2018, 164, 188–197. [Google Scholar] [CrossRef]
- Alavianghavanini, A.; Shayesteh, H.; Bahri, P.A.; Vadiveloo, A.; Moheimani, N.R. Microalgae Cultivation for Treating Agricultural Effluent and Producing Value-Added Products. Sci. Total Environ. 2024, 912, 169369. [Google Scholar] [CrossRef]
- da Silva, T.L.; Moniz, P.; Silva, C.; Reis, A. The Role of Heterotrophic Microalgae in Waste Conversion to Biofuels and Bioproducts. Processes 2021, 9, 1090. [Google Scholar] [CrossRef]
- Chia, S.R.; Ling, J.; Chia, W.Y.; Nomanbhay, S.; Kurniawan, T.A.; Chew, K.W. Future Bioenergy Source by Microalgae–Bacteria Consortia: A Circular Economy Approach. Green. Chem. 2023, 25, 8935–8949. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent Advancements in Photo-Bioreactors for Microalgae Cultivation: A Brief Overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic Cultivation of Microalgae: Production of Metabolites of Commercial Interest: Heterotrophic Cultivation of Microalgae: Products. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Do, S.; Du, Z.-Y. Exploring the Impact of Environmental Conditions and Bioreactors on Microalgae Growth and Applications. Energies 2024, 17, 5218. [Google Scholar] [CrossRef]
- Braun, J.C.A.; Balbinot, L.; Beuter, M.A.; Rempel, A.; Colla, L.M. Mixotrophic Cultivation of Microalgae Using Agro-Industrial Waste: Tolerance Level, Scale up, Perspectives and Future Use of Biomass. Algal Res. 2024, 80, 103554. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, Y.-S.; Yoon, H.-S. Effect of Different Cultivation Modes (Photoautotrophic, Mixotrophic, and Heterotrophic) on the Growth of Chlorella sp. and Biocompositions. Front. Bioeng. Biotechnol. 2021, 9, 774143. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Kim, B.-H.; Ramanan, R.; Choi, J.-E.; Yang, J.-W.; Oh, H.-M.; Kim, H.-S. A Cost Analysis of Microalgal Biomass and Biodiesel Production in Open Raceways Treating Municipal Wastewater and under Optimum Light Wavelength. J. Microbiol. Biotechnol. 2015, 25, 109–118. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Prashanth, P. Bubble Column Photobioreactor for Chlorella pyrenoidosa Cultivation and Validating Gas Hold up and Volumetric Mass Transfer Coefficient. Energy Sources Part. Recovery Util. Environ. Eff. 2023, 45, 9779–9793. [Google Scholar] [CrossRef]
- Abdur Razzak, S.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae Cultivation in Photobioreactors: Sustainable Solutions for a Greener Future. Green. Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Shekh, A.; Sharma, A.; Schenk, P.M.; Kumar, G.; Mudliar, S. Microalgae Cultivation: Photobioreactors, CO2 Utilization, and Value-added Products of Industrial Importance. J. Chem. Technol. Biotechnol. 2022, 97, 1064–1085. [Google Scholar] [CrossRef]
- Ting, H.; Haifeng, L.; Shanshan, M.; Zhang, Y.; Zhidan, L.; Na, D. Progress in Microalgae Cultivation Photobioreactors and Applications in Wastewater Treatment: A Review. Int. J. Agric. Biol. Eng. 2017, 10, 1–29. [Google Scholar] [CrossRef]
- Shokravi, Z.; Mehravar, N. Optimization of Photobioreactor and Open Pond Systems for Sustainable Microalgal Biomass Production: Challenges, Solutions, and Scalability. In Microalgal Biofuels; Elsevier (Woodhead Publishing): Cambridge, MA, USA, 2025; pp. 45–62. ISBN 978-0-443-24110-9. [Google Scholar] [CrossRef]
- Manu, L.; Mokolensang, J.F.; Ben Gunawan, W.; Setyawardani, A.; Salindeho, N.; Syahputra, R.A.; Iqhrammullah, M.; Nurkolis, F. Photobioreactors Are Beneficial for Mass Cultivation of Microalgae in Terms of Areal Efficiency, Climate Implications, and Metabolites Content. J. Agric. Food Res. 2024, 18, 101282. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for Cultivation and Synthesis: Specifications, Challenges, and Perspectives. Eng. Life Sci. 2022, 22, 712–724. [Google Scholar] [CrossRef]
- Kwon, M.H.; Yeom, S.H. Evaluation of Closed Photobioreactor Types and Operation Variables for Enhancing Lipid Productivity of Nannochloropsis sp. KMMCC 290 for Biodiesel Production. Biotechnol. Bioprocess. Eng. 2017, 22, 604–611. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Wang, J.; Liu, T. Biomass Productivity of Scenedesmus dimorphus (Chlorophyceae) Was Improved by Using an Open Pond–Photobioreactor Hybrid System. Eur. J. Phycol. 2019, 54, 127–134. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Karp, S.G.; Manzoki, M.C.; Medeiros, A.B.P.; Rodrigues, C.; Scapini, T.; Vandenberghe, L.P.D.S.; Vieira, S.; et al. Agro-Industrial Wastewaters for Algal Biomass Production, Bio-Based Products, and Biofuels in a Circular Bioeconomy. Fermentation 2022, 8, 728. [Google Scholar] [CrossRef]
- Low, S.S.; Bong, K.X.; Mubashir, M.; Cheng, C.K.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Munawaroh, H.S.H.; Show, P.L. Microalgae Cultivation in Palm Oil Mill Effluent (POME) Treatment and Biofuel Production. Sustainability 2021, 13, 3247. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Djarot, I.N.; Boelen, P.; Hadiyanto; Heeres, H.J. Co-Cultivation of Microalgae Growing on Palm Oil Mill Effluent under Outdoor Condition for Lipid Production. Environ. Pollut. Bioavailab. 2022, 34, 537–548. [Google Scholar] [CrossRef]
- Fernando, J.S.R.; Premaratne, M.; Dinalankara, D.M.S.D.; Perera, G.L.N.J.; Ariyadasa, T.U. Cultivation of Microalgae in Palm Oil Mill Effluent (POME) for Astaxanthin Production and Simultaneous Phycoremediation. J. Environ. Chem. Eng. 2021, 9, 105375. [Google Scholar] [CrossRef]
- Ding, G.T.; Mohd Yasin, N.H.; Takriff, M.S.; Kamarudin, K.F.; Salihon, J.; Yaakob, Z.; Mohd Hakimi, N.I.N. Phycoremediation of Palm Oil Mill Effluent (POME) and CO2 Fixation by Locally Isolated Microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J. Water Process Eng. 2020, 35, 101202. [Google Scholar] [CrossRef]
- Palanisamy, K.M.; Bhuyar, P.; Ab Rahim, M.H.; Govindan, N.; Maniam, G.P. Cultivation of Microalgae Spirulina Platensis Biomass Using Palm Oil Mill Effluent for Phycocyanin Productivity and Future Biomass Refinery Attributes. Int. J. Energy Res. 2023, 2023, 2257271. [Google Scholar] [CrossRef]
- Sorgatto, V.G.; Soccol, C.R.; Molina-Aulestia, D.T.; De Carvalho, M.A.; De Melo Pereira, G.V.; De Carvalho, J.C. Mixotrophic Cultivation of Microalgae in Cassava Processing Wastewater for Simultaneous Treatment and Production of Lipid-Rich Biomass. Fuels 2021, 2, 521–532. [Google Scholar] [CrossRef]
- Padri, M.; Boontian, N.; Teaumroong, N.; Piromyou, P.; Piasai, C. Application of Two Indigenous Strains of Microalgal Chlorella sorokiniana in Cassava Biogas Effluent Focusing on Growth Rate, Removal Kinetics, and Harvestability. Water 2021, 13, 2314. [Google Scholar] [CrossRef]
- Cerqueira, K.; Coêlho, D.; Rodrigues, J.; Souza, R. Production of Scenedesmus sp. Microalgae in Cassava (Cassava Wastewater) for Extraction of Lipids. Int. J. Dev. Res. 2021, 11, 48393–48398. Available online: https://www.journalijdr.com/production-scenedesmus-sp-microalgae-cassava-cassava-wastewater-extraction-lipids (accessed on 23 September 2025).
- Tamil Selvan, S. Sustainable Bioremediation of Cassava Waste Effluent Using Nannochloropsis salina TSD06: An Eco-Technological Approach. Discov. Appl. Sci. 2024, 6, 642. [Google Scholar] [CrossRef]
- Silva, S.; Melo, L.B.U.; Borrego, B.B.; Gracioso, L.H.; Perpetuo, E.A.; Do Nascimento, C.A.O. Sugarcane Vinasse as Feedstock for Microalgae Cultivation: From Wastewater Treatment to Bioproducts Generation. Braz. J. Chem. Eng. 2024, 41, 911–921. [Google Scholar] [CrossRef]
- Serejo, M.L.; Ruas, G.; Braga, G.B.; Paulo, P.L.; Boncz, M.À. Chlorella vulgaris Growth on Anaerobically Digested Sugarcane Vinasse: Influence of Turbidity. An. Acad. Bras. Ciênc. 2021, 93, e20190084. [Google Scholar] [CrossRef]
- Candido, C.; Cardoso, L.G.; Lombardi, A.T. Bioprospecting and Selection of Tolerant Strains and Productive Analyses of Microalgae Grown in Vinasse. Braz. J. Microbiol. 2022, 53, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef]
- Putri, N.A.; Dewi, R.N.; Lestari, R.; Yuniar, R.A.; Ma’arif, L.M.; Erianto, R. Microalgae as A Bioremediation Agent for Palm Oil Mill Effluent: Production of Biomass and High Added Value Compounds. J. Rekayasa Kim. Lingkung. 2023, 18, 149–161. [Google Scholar] [CrossRef]
- Ajijah, N.; Tjandra, B.C.; Hamidah, U.; Widyarani; Sintawardani, N. Utilization of Tofu Wastewater as a Cultivation Medium for Chlorella vulgaris and Arthrospira platensis. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Tangerang, Indonesia, 23–24 October 2019; Volume 483, p. 012027. [Google Scholar] [CrossRef]
- Elystia, S.; Nasution, F.H.M.; Sasmita, A. Rotary Algae Biofilm Reactor (RABR) Using Microalgae Chlorella Sp. for Tofu Wastewater Treatment. Mater. Today Proc. 2023, 87, 263–271. [Google Scholar] [CrossRef]
- Coutinho Rodrigues, O.H.; Itokazu, A.G.; Rörig, L.; Maraschin, M.; Corrêa, R.G.; Pimentel-Almeida, W.; Moresco, R. Evaluation of Astaxanthin Biosynthesis by Haematococcus pluvialis Grown in Culture Medium Added of Cassava Wastewater. Int. Biodeterior. Biodegrad. 2021, 163, 105269. [Google Scholar] [CrossRef]
- Nunes, N.S.P.; De Almeida, J.M.O.; Fonseca, G.G.; De Carvalho, E.M. Clarification of Sugarcane (Saccharum officinarum) Vinasse for Microalgae Cultivation. Bioresour. Technol. Rep. 2022, 19, 101125. [Google Scholar] [CrossRef]
- Naser, A.S.; Asiandu, A.P.; Sadewo, B.R.; Tsurayya, N.; Putra, A.S.; Kurniawan, K.I.A.; Suyono, E.A. The Effect of Salinity and Tofu Whey Wastewater on the Growth Kinetics, Biomass, and Primary Metabolites in Euglena Sp. J. Appl. Biol. Biotechnol. 2023, 11, 124–132. [Google Scholar] [CrossRef]
- Taufikurahman, T.; Irene, J.; Melani, L.; Marwani, E.; Purba, L.D.A.; Susanti, H. Optimizing Microalgae Cultivation in Tofu Wastewater for Sustainable Resource Recovery: The Impact of Salicylic Acid on Growth and Astaxanthin Production. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Wang, S.-K.; Wang, X.; Miao, J.; Tian, Y.-T. Tofu Whey Wastewater Is a Promising Basal Medium for Microalgae Culture. Bioresour. Technol. 2018, 253, 79–84. [Google Scholar] [CrossRef]
- Oktaviani, Z.; Hendrasarie, N. Efektivitas Konsorsium Mikroalga Chlorella sp. dan Mikroba Indigenous Dalam Menurunkan BOD, COD, dan TN Air Limbah Industri Kecap Menggunakan MBBR. J. Serambi Eng. 2024, 9, 10724–10730. [Google Scholar]
- Faturrahman, A.; Rahmawati, A.; Anggoro, A.D.; Rahmah, A.F.; Haksara, M.F. Optimasi Konsorsium Mikroalga Chlorella Sebagai Upaya Revitalisasi Lingkungan Berbasis Biodegradasi Limbah POME (Palm Oil Mill Effluent). J. Ilmu Lingkung. 2025, 23, 721–729. [Google Scholar] [CrossRef]
- Basra, I.; Silalahi, L.; Pratama, W.D.; Joelyna, F.A. Pretreatment of Palm Oil Mill Effluent (POME) for Spirulina Cultivation. J. Emerg. Sci. Eng. 2023, 1, 57–62. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Mohd Yasin, N.H.; Ba-Abbad, M.M.; Mohd Hakimi, N.I.N. Potential of the Microalgae-Based Integrated Wastewater Treatment and CO2 Fixation System to Treat Palm Oil Mill Effluent (POME) by Indigenous Microalgae; Scenedesmus sp. and Chlorella sp. J. Water Process Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- Sun, Z.; Bo, C.; Cao, S.; Sun, L. Enhancing CO2 Fixation in Microalgal Systems: Mechanistic Insights and Bioreactor Strategies. Mar. Drugs 2025, 23, 113. [Google Scholar] [CrossRef]
- Kumar, A.; Yuan, X.; Sahu, A.K.; Dewulf, J.; Ergas, S.J.; Van Langenhove, H. A Hollow Fiber Membrane Photo-bioreactor for CO2 Sequestration from Combustion Gas Coupled with Wastewater Treatment: A Process Engineering Approach. J. Chem. Technol. Biotechnol. 2010, 85, 387–394. [Google Scholar] [CrossRef]
- Samoraj, M.; Çalış, D.; Trzaska, K.; Mironiuk, M.; Chojnacka, K. Advancements in Algal Biorefineries for Sustainable Agriculture: Biofuels, High-Value Products, and Environmental Solutions. Biocatal. Agric. Biotechnol. 2024, 58, 103224. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, P.K.; Chintala, V.; Khatri, N.; Patel, A. Environment-Friendly Biodiesel/Diesel Blends for Improving the Exhaust Emission and Engine Performance to Reduce the Pollutants Emitted from Transportation Fleets. Int. J. Environ. Res. Public. Health 2020, 17, 3896. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-Fertilizers: Between Current Situation and Future Prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Barata, A.; Batista, A.P.; Gouveia, L. Scenedesmus obliquus in Poultry Wastewater Bioremediation. Environ. Technol. 2019, 40, 3735–3744. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-Based Biodiesel Production and Its Challenges and Future Opportunities: A Review. Green. Technol. Sustain. 2024, 2, 100060. [Google Scholar] [CrossRef]
- Wiley, P.E.; Campbell, J.E.; McKuin, B. Production of Biodiesel and Biogas from Algae: A Review of Process Train Options. Water Environ. Res. 2011, 83, 326–338. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, F.; Wu, Y.-R. Emerging Technologies for Conversion of Sustainable Algal Biomass into Value-Added Products: A State-of-the-Art Review. Sci. Total Environ. 2021, 784, 147024. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A.M. Chemical Composition, Bioactivities, and Applications of Spirulina (Limnospira platensis) in Food, Feed, and Medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Georgiopoulou, I.; Antoniou, N.; Tzima, S.; Kontou, M.; Louli, V.; Fatouros, C.; Magoulas, K.; Kolisis, F.N. Extracts from Chlorella vulgaris Protect Mesenchymal Stromal Cells from Oxidative Stress Induced by Hydrogen Peroxide. Plants 2023, 12, 361. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Innovative Microalgae Pigments as Functional Ingredients in Nutrition. In Handbook of Marine Microalgae; Elsevier (Academic Press): London, UK, 2015; pp. 233–243. ISBN 978-0-12-800776-1. [Google Scholar]
- Prates, J.A.M. Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review. Foods 2025, 14, 1524. [Google Scholar] [CrossRef]
- García-Encinas, J.P.; Ruiz-Cruz, S.; Juárez, J.; Ornelas-Paz, J.D.J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E. Proteins from Microalgae: Nutritional, Functional and Bioactive Properties. Foods 2025, 14, 921. [Google Scholar] [CrossRef]
- Strauch, S.M.; Barjona Do Nascimento Coutinho, P. Bioactive Molecules from Microalgae. In Natural Bioactive Compounds; Elsevier (Academic Press): London, UK, 2021; pp. 453–470. ISBN 978-0-12-820655-3. [Google Scholar]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Conde, T.; Neves, B.; Couto, D.; Melo, T.; Lopes, D.; Pais, R.; Batista, J.; Cardoso, H.; Silva, J.L.; Domingues, P.; et al. Polar Lipids of Marine Microalgae Nannochloropsis Oceanica and Chlorococcum Amblystomatis Mitigate the LPS-Induced Pro-Inflammatory Response in Macrophages. Mar. Drugs 2023, 21, 629. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Encapsulation of Microalgae-Based Products for Food and Feed Applications. In Handbook of Food and Feed from Microalgae; Elsevier (Academic Press): London, UK, 2023; pp. 371–393. ISBN 978-0-323-99196-4. [Google Scholar]
- Martínez-Ruiz, F.; Andrade-Bustamante, G.; Holguín-Peña, R.; Renganathan, P.; Gaysina, L.; Sukhanova, N.; Puente, E. Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass 2025, 5, 25. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public. Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of Microalgal Biomasses as Functional Food Ingredients: Focus on the Composition of Cell Wall Related Polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Eltanahy, E.; Torky, A. CHAPTER 1. Microalgae as Cell Factories: Food and Feed-Grade High-Value Metabolites. In Microalgal Biotechnology; Shekh, A., Schenk, P., Sarada, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–35. ISBN 978-1-83916-003-5. [Google Scholar]
- Dhandwal, A.; Bashir, O.; Malik, T.; Salve, R.V.; Dash, K.K.; Amin, T.; Shams, R.; Wani, A.W.; Shah, Y.A. Sustainable Microalgal Biomass as a Potential Functional Food and Its Applications in Food Industry: A Comprehensive Review. Environ. Sci. Pollut. Res. 2024, 32, 19110–19128. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; Heneash, A.M.M.; Hussein, N.R. Cultivation of Arthrospira (Spirulina) platensis Using Confectionary Wastes for Aquaculture Feeding. J. Genet. Eng. Biotechnol. 2015, 13, 145–155. [Google Scholar] [CrossRef]
- Idenyi, J.N.; Eya, J.C.; Nwankwegu, A.S.; Nwoba, E.G. Aquaculture Sustainability through Alternative Dietary Ingredients: Microalgal Value-Added Products. Eng. Microbiol. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A Techno-Economic Analysis of Industrial Production of Marine Microalgae as a Source of EPA and DHA-Rich Raw Material for Aquafeed: Research Challenges and Possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Hossain, N.; Hasan, M.H.; Mahlia, T.M.I.; Shamsuddin, A.H.; Silitonga, A.S. Feasibility of Microalgae as Feedstock for Alternative Fuel in Malaysia: A Review. Energy Strategy Rev. 2020, 32, 100536. [Google Scholar] [CrossRef]
- Sarker, N.K.; Salam, P.A. Indoor and Outdoor Cultivation of Chlorella vulgaris and Its Application in Wastewater Treatment in a Tropical City—Bangkok, Thailand. SN Appl. Sci. 2019, 1, 1645. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Shahbazian-Yassar, R.; Din, M.F.M.; Khademi, T.; Kumar, A.; Rezania, S. Evaluation of Lipid Content in Microalgae Biomass Using Palm Oil Mill Effluent (POME). JOM 2017, 69, 1361–1367. [Google Scholar] [CrossRef]
- Andrew, A.R.; Yong, W.T.L.; Misson, M.; Anton, A.; Chin, G.J.W.L. Selection of Tropical Microalgae Species for Mass Production Based on Lipid and Fatty Acid Profiles. Front. Energy Res. 2022, 10, 912904. [Google Scholar] [CrossRef]
- Palanisamy, K.M.; Paramasivam, P.; Jayakumar, S.; Maniam, G.P.; Rahim, M.H.A.; Govindan, N. Economical Cultivation System of Microalgae Spirulina platensis for Lipid Production. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Pahang, Malaysia, 19 June 2020; Volume 641, p. 012022. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Borghetti, I.A.; Cartas, L.C.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Biorefinery Integration of Microalgae Production into Cassava Processing Industry: Potential and Perspectives. Bioresour. Technol. 2018, 247, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Kongsil, P.; Ceballos, H.; Siriwan, W.; Vuttipongchaikij, S.; Kittipadakul, P.; Phumichai, C.; Wannarat, W.; Kositratana, W.; Vichukit, V.; Sarobol, E.; et al. Cassava Breeding and Cultivation Challenges in Thailand: Past, Present, and Future Perspectives. Plants 2024, 13, 1899. [Google Scholar] [CrossRef] [PubMed]
- Padri, M.; Boontian, N.; Teaumroong, N.; Piromyou, P.; Piasai, C. Co-Culture of Microalga Chlorella Sorokiniana with Syntrophic Streptomyces thermocarboxydus in Cassava Wastewater for Wastewater Treatment and Biodiesel Production. Bioresour. Technol. 2022, 347, 126732. [Google Scholar] [CrossRef]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using Agro-Industrial Wastes for the Cultivation of Microalgae and Duckweeds: Contamination Risks and Biomass Safety Concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.; Hariyanti, J.; Pinardi, D.; Kusretuwardani, K.; Widyastuti, N.; Djarot, I.; Handayani, T.; Sitomurni, A.; Apriyanto, H. Sustainability Index Analysis of Microalgae Cultivation from Biorefinery Palm Oil Mill Effluent. Glob. J. Environ. Sci. Manag. 2023, 9, 559–576. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Qv, M.; Dai, D.; Liu, D.; Wang, W.; Tang, C.; Zhu, L. Microalgal Cultivation for the Upgraded Biogas by Removing CO2, Coupled with the Treatment of Slurry from Anaerobic Digestion: A Review. Bioresour. Technol. 2022, 364, 128118. [Google Scholar] [CrossRef]
- Cavalcanti Pessôa, L.; Pinheiro Cruz, E.; Mosquera Deamici, K.; Bomfim Andrade, B.; Santana Carvalho, N.; Rocha Vieira, S.; Alves Da Silva, J.B.; Magalhães Pontes, L.A.; Oliveira De Souza, C.; Druzian, J.I.; et al. A Review of Microalgae-Based Biorefineries Approach for Produced Water Treatment: Barriers, Pretreatments, Supplementation, and Perspectives. J. Environ. Chem. Eng. 2022, 10, 108096. [Google Scholar] [CrossRef]
- Djarot, I.N.; Pawignya, H.; Handayani, T.; Widyastuti, N.; Nuha, N.; Arianti, F.D.; Pertiwi, M.D.; Rifai, A.; Isharyadi, F.; Wijayanti, S.P.; et al. Enhancing Sustainability: Microalgae Cultivation for Biogas Enrichment and Phycoremediation of Palm Oil Mill Effluent—A Comprehensive Review. Environ. Pollut. Bioavailab. 2024, 36, 2347314. [Google Scholar] [CrossRef]
- Akter, F.; Songsomboon, K.; Ralph, P.J.; Kuzhiumparambil, U. A Comprehensive Overview of Microalgae- and Cyanobacteria-Derived Polysaccharides: Extraction, Structural Chemistry, Techno-Functional and Bioactive Properties. Bioresour. Technol. Rep. 2025, 31, 102280. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Harun, M.R.; Lau, S.Y.; Sewu, D.D.; Danquah, M.K. Microalgal Biomass Generation via Electroflotation: A Cost-Effective Dewatering Technology. Appl. Sci. 2020, 10, 9053. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.-R.; Pack, S.P. Recent Progress in Flocculation, Dewatering, and Drying Technologies for Microalgae Utilization: Scalable and Low-Cost Harvesting Process Development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef]
- Government of the Republic of Indonesia. Law of the Republic of Indonesia Number 18 of 2012 concerning Food; State Secretariat of the Republic of Indonesia: Jakarta, Indonesia, 2012; Available online: https://peraturan.bpk.go.id/Details/39100 (accessed on 27 October 2025).
- Government of the Republic of Indonesia. Law of the Republic of Indonesia Number 86 of 2019 concerning Food Safety; State Secretariat of the Republic of Indonesia: Jakarta, Indonesia, 2019; Available online: https://peraturan.bpk.go.id/Details/129230/pp-no-86-tahun-2019 (accessed on 27 October 2025).
- Food and Drug Supervisory Agency. Regulation of the National Agency of Drug and Food Control Number 9 of 2022 concerning Requirements for Heavy Metal Contaminants in Processed Food; Food and Drug Supervisory Agency: Jakarta, Indonesia, 2022; Available online: https://peraturan.bpk.go.id/Details/223968/peraturan-bpom-no-9-tahun-2022 (accessed on 27 October 2025).
- National Research and Innovation Agency. Regulation of the National Research and Innovation Agency Number 6 of 2023 concerning the Strategic Plan of the National Research and Innovation Agency for 2022–2024; National Research and Innovation Agency: Jakarta, Indonesia, 2023; Available online: https://peraturan.bpk.go.id/Details/325271 (accessed on 27 October 2025).
- Minister of Agriculture of the Republic of Indonesia. Regulation of the Minister of Agriculture Number 22/Permentan/PK.110/6/2017 of 2017 concerning Registration and Business Licensing for Horticulture; Ministry of Agriculture of the Republic of Indonesia: Jakarta, Indonesia, 2017; Available online: https://peraturan.bpk.go.id/Details/161900/permentan-no-22permentanpk11062017-tahun-2017 (accessed on 27 October 2025).
- Minister of Agriculture of the Republic of Indonesia. Regulation of the Minister of Agriculture Number 01/PERMENTAN/PK.330/1/2019 of 2019 concerning Registration and Business Licensing for Horticulture; Ministry of Agriculture of the Republic of Indonesia: Jakarta, Indonesia, 2019; Available online: https://peraturan.bpk.go.id/Details/161054/permentan-no-01-tahun-2019 (accessed on 27 October 2025).
| Parameters | National Threshold [32] | Study 1 [34] | Study 2 [35] | Study 3 [22] |
|---|---|---|---|---|
| pH | 5–9 | 8 | 7.71 | 7.5–8.9 |
| Total solid | 250 mg L−1 | 96 mg L−1 | 45 mg L−1 | 30–40 mg L−1 |
| Total nitrogen | 50 mg L−1 | 265.25 mg L−1 | 160 mg L−1 * | 1–18 mg L−1 |
| BOD | 100 mg L−1 | 189 mg L−1 | 180 mg L−1 | 20–300 mg L−1 |
| COD | 350 mg L−1 | 402 mg L−1 | 593 mg L−1 | 30–200 mg L−1 |
| Parameters | National Threshold [32] | Study 1 [40] | |
|---|---|---|---|
| Pre | Post | ||
| pH | 6–9 | n.a. | n.a. |
| Total solid | 100 mg L−1 | 739.53 mg L−1 | 51 mg L−1 |
| BOD | 150 mg L−1 | 2472.94 mg L−1 | 53.67 mg L−1 |
| COD | 300 mg L−1 | 4000.86 mg L−1 | 56.81 mg L−1 |
| Cyanide | 0.3 mg L−1 | 0.144 mg L−1 | 0.065 mg L−1 |
| Parameters | Tofu Industry National Threshold [32] | Tofu Wastewater [52] | Tempe Industry National Threshold [32] | Tempeh Wastewater [51] |
|---|---|---|---|---|
| pH | 6–9 | 4–5 | 6–9 | n.a. |
| Total solid | 200 mg L−1 | 6000–8000 mg L−1 | 100 mg L−1 | 1712.78 mg L−1 |
| BOD | 150 mg L−1 | 5000–10,000 mg L−1 | 300 mg L−1 | 6097.49 mg L−1 |
| COD | 300 mg L−1 | 7000–12,000 mg L−1 | 150 mg L−1 | 29,695.13 mg L−1 |
| Agro-Industrial Effluent | Microalgae | Medium Pretreatment | Cultivation System | Biomass Production/ Growth Rate | Product | Removal Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| POME | Co-cultivation Dunaliella sp., Spirulina sp., Nannochloropsis sp., Chaetoceros calciltrans | F, D, A | Outdoor, 200 mL plastic bag, 75% POME added urea 450 mg L−1 | Growth rate 0.35 d−1 | Lipid 40% | - | [83] |
| POME | Haematococcus pluvialis | F, D, A | Indoor, 2 L glass bottle, 7.5% POME | Growth rate 0.21 d−1 | Astaxanthin 22.43 mg L−1 | 50.9% COD, 49.3% TN, 69.4% TP | [84] |
| POME | Chlorella sorokiniana UKM2 | F, D, A | Indoor, 2 L flask, 10% POME, 1% CO2 mixed with air | Growth rate 1.06 d−1 | - | 567 mg L−1 d−1 CO2 uptake rate, 100% ammonium, 65% TN, 56% TP | [85] |
| POME | Spirulina platensis | F, C, D, A | Indoor, 1 L conical flask, 30% POME | Biomass production 1.16 g L −1 | Phycocyanin 175.12 mg, Lipid 28.6% | - | [86] |
| CPW | Haematococcus pluvialis, Neochloris oleoabundans | F, D, A | Indoor, 2 L flask, 25% CPW | Biomass production 3.18 and 1.79 g L−1, respectively | Lipid 0.018 and 0.041 g L−1 d−1, respectively | 60.80% and 69.16% COD, 51.06% and 58.19% TN, 54.68% and 69.84% TP, respectively | [87] |
| CBEW | Chlorella sorokiniana P21 and WB1DG | F | Indoor, 12 L acrylamide flask, 100% CBEW | Biomass production 2.6 and 1.3 g L−1, respectively | - | 73.78% and 63.42% COD, 92.11% and 91.68% TP, 67.33% and 70.66% TN, respectively | [88] |
| CPW | Scenedesmus sp. | F, D, A | Indoor, 500 mL Erlenmeyer flask, synthetic medium supplemented with 5–10% CPW | Biomass 0.7 g L−1 | Lipid 35.5% | - | [89] |
| CPW | Nannochloropsis salina | F | Indoor, PBR 1500 L, 100% CPW | Biomass 7.25 g L−1 | Lipid 210.32 mg g−1, carbohydrates 125.34 mg ml−1, biodiesel 3.75 mL g−1 | 8.26% nitrate, 93.94% phosphate, 97.43% sulfate | [90] |
| Sugarcane vinasse | Coelastrella sp. | C, CL, DC | Indoor, 250 mL Drechsler flask, 20% vinasse with 0.04% CO2 | Biomass 3.16 g L−1 | Carbohydrate 30%, lipid 20% | 53.9% COD | [91] |
| Sugarcane vinasse | Mixed culture is predominantly composed of Chlorella vulgaris | No pretreatment | Indoor, 3 L glass bottle, raw vinasse containing anaerobic sludge from reactor treating vinasse | Biomass 2.7 g L−1 | Lipid 265 mg L−1 | 98% TN | [92] |
| Sugarcane vinasse | Chlorella vulgaris | C, D | Indoor, 250 mL flask, 20% vinasse | Growth rate 1.41 d−1 | Protein 45.98 mg L−1, carbohydrate 6.67 mg L−1 | - | [93] |
| Sugarcane vinasse | Chlorella vulgaris | n.a. | Indoor, tubular 6 L air-lift reactors, fully dark, 1% CO2, 75% vinasse | Biomass 8.7 g L−1, growth rate 0.72 g L−1 d−1 | Protein 45.95%, lipid 1.67% | - | [94] |
| TW | Spirulina sp., Nannochloropsis oculata | D, A | Indoor, 1 L polyethylene flask, 20% TW | Biomass 0.23 and 0.53 g L−1, respectively | Lipid 2.44% and 1.21%, respectively. Protein 1.71% and 1.51%, respectively | - | [95] |
| TW, TW-ADE | Chlorella vulgaris, Arthrospira platensis | D, A | Indoor, 1 L polyethylene flask, 5% TW, 3% TW, 100% TW-ADE | Biomass: C. vulgaris 2.0 g L−1 in 5% TW, A. plantesis 1.4 g L−1 in 5% TW; No growth at TW-ADE | Protein: C. vulgaris 135.8 mg L−1 in 5% TW, A. platensis 42.5 mg L−1 in 3% TW, Protein was not detected in TW-ADE | - | [96] |
| TW | Chlorella sp. | D | Indoor, 18 L rotating algal biofilm reactor, 40% TW | Microalgae cells 3.99 × 106 cells m L−1 | - | 75.88% COD, 80.45% NH3 | [97] |
| No | Microalgal Species | Main Productions | Applications | Health Benefits | References |
|---|---|---|---|---|---|
| 1 | Spirulina (Arthrospira platensis/Limnospira platensis) | Phycocyanin (blue pigment); Proteins; Bioactive peptides | Natural blue food colorant, protein powders, supplements | Antioxidant, neuroprotective, immunomodulatory, antihypertensive | [14,116] |
| 2 | Chlorella vulgaris/C. pyrenoidosa | Chlorophylls; Proteins; Vitamin B12; Folate; Sulphated polysaccharides | Detox/immune supplements, baked goods & beverage enrichment, vegan protein | Detoxification, gut microbiota modulation, antioxidant, ACE-inhibitory, antidiabetic | [117,118,119] |
| 3 | Haematococcus pluvialis | Astaxanthin; Carotenoids; PUFAs | Anti-ageing nutraceuticals, sports nutrition, antioxidant-rich supplements | Potent antioxidant, cardiovascular & skin protection, anti-inflammatory | [120,121,122] |
| 4 | Dunaliella salina | β-carotene; Luteins | Natural orange-red colorant, provitamin A supplements, functional foods | Eye health, antioxidant, and immune support | [121,123] |
| 5 | Nannochloropsis spp. | Eicosapentaenoic acid (EPA); Proteins; Peptides; Chlorophyll; Carotenoids; Phytosterols | Vegan Omega-3 oil, aquafeed, functional beverages | Cardiovascular health, lipid metabolism, cognitive support, anticancer peptides | [124,125] |
| 6 | Isochrysis galbana | Docosahexaenoic acid (DHA); Proteins; Fucoxanthin; Phytosterols | Infant formulas, nutraceuticals | Neurological development, cardiovascular health, neuroprotective, antioxidant | [121,126,127] |
| 7 | Scenedesmus spp. | Lutein; Proteins; Carotenoids | Functional foods, eye health supplements | Antioxidant, ocular health, anti-inflammatory | [121,128] |
| 8 | Porphyridium spp. | Sulphated polysaccharides; Phycoerythrin (red pigment) | Food stabilizers, antiviral nutraceuticals | Antiviral, immune modulation, prebiotic functions | [129,130] |
| 9 | Muriellopsis spp. | Lutein | Eye health supplements, natural yellow colorants | Antioxidant, visual health | [131,132] |
| 10 | Schizochytrium | DHA (long-chain omega-3); EPA | Infant nutrition, vegan omega-3 oils | Brain & eye development, anti-inflammatory; cardiovascular health | [14,124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budinarta, W.; Ajijah, N.; Hermosaningtyas, A.A. Waste-to-Resource Strategies: The Potential of Agro-Industrial Residues for Microalgal Bioproducts in Indonesia. Phycology 2025, 5, 81. https://doi.org/10.3390/phycology5040081
Budinarta W, Ajijah N, Hermosaningtyas AA. Waste-to-Resource Strategies: The Potential of Agro-Industrial Residues for Microalgal Bioproducts in Indonesia. Phycology. 2025; 5(4):81. https://doi.org/10.3390/phycology5040081
Chicago/Turabian StyleBudinarta, Widyah, Nur Ajijah, and Anastasia Aliesa Hermosaningtyas. 2025. "Waste-to-Resource Strategies: The Potential of Agro-Industrial Residues for Microalgal Bioproducts in Indonesia" Phycology 5, no. 4: 81. https://doi.org/10.3390/phycology5040081
APA StyleBudinarta, W., Ajijah, N., & Hermosaningtyas, A. A. (2025). Waste-to-Resource Strategies: The Potential of Agro-Industrial Residues for Microalgal Bioproducts in Indonesia. Phycology, 5(4), 81. https://doi.org/10.3390/phycology5040081





