Influence of Pelagic Sargassum spp. On Soil Amelioration for Seed Germination and Seedling Growth of Corn (Zea mays), Scotch Bonnet Pepper (Capsicum chinense), and Tomato (Solanum lycopersicum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sargassum Collection and Processing
2.2. Plant Experimental Design and Layout
2.2.1. Seed Germination
2.2.2. Seedling Growth
2.3. Collection and Processing of Plant Material
2.4. Analysis of Other Plant Parameters
2.4.1. Germination Experiment
2.4.2. Seedling Growth Experiment
2.5. Elemental Analysis of Plant and Soil Materials
2.6. Statistical Analysis
3. Results
3.1. Seed Germination Parameters
3.2. Seedling Growth Parameters
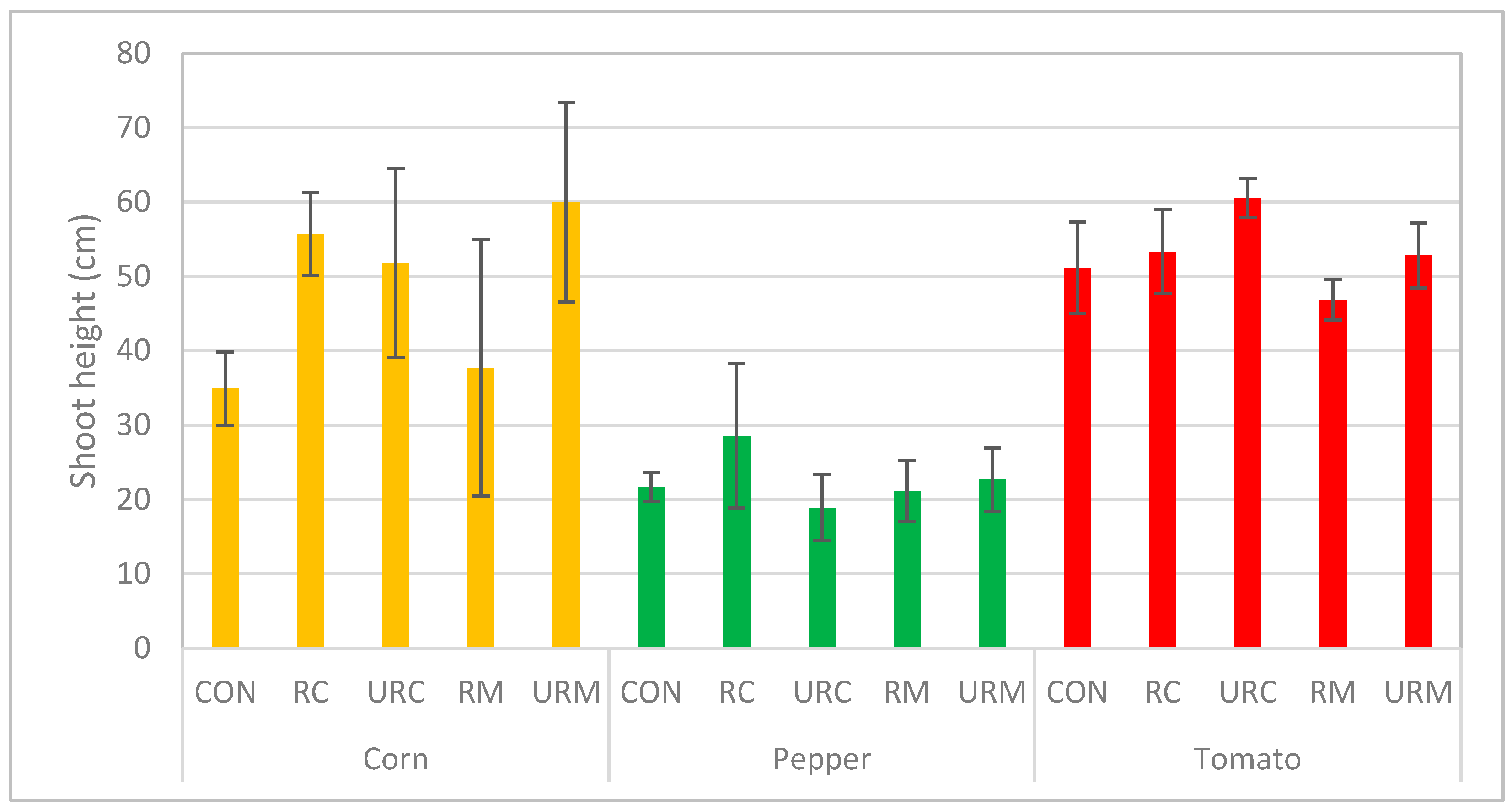
3.2.1. Plant Shoot Height
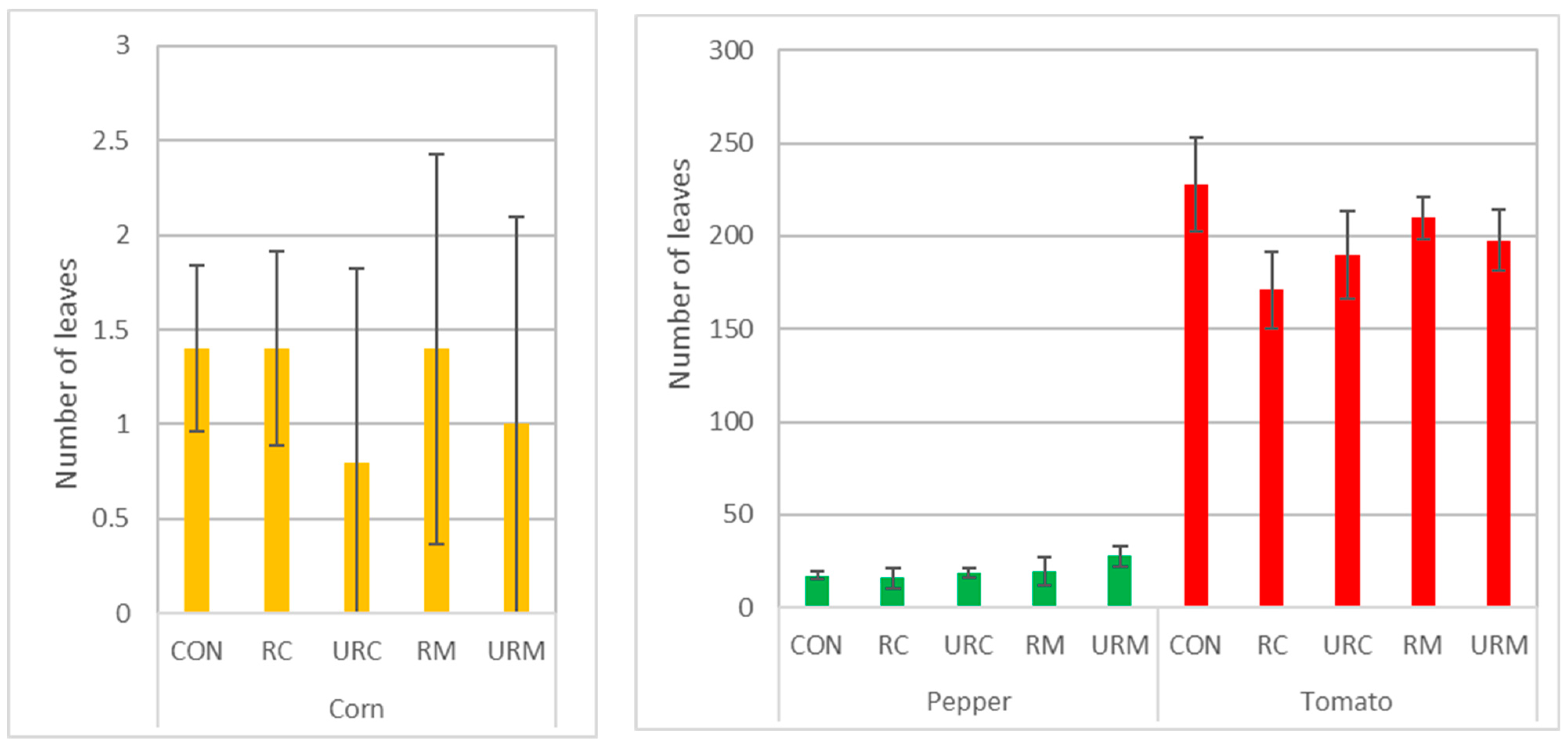
3.2.2. Number of Leaves
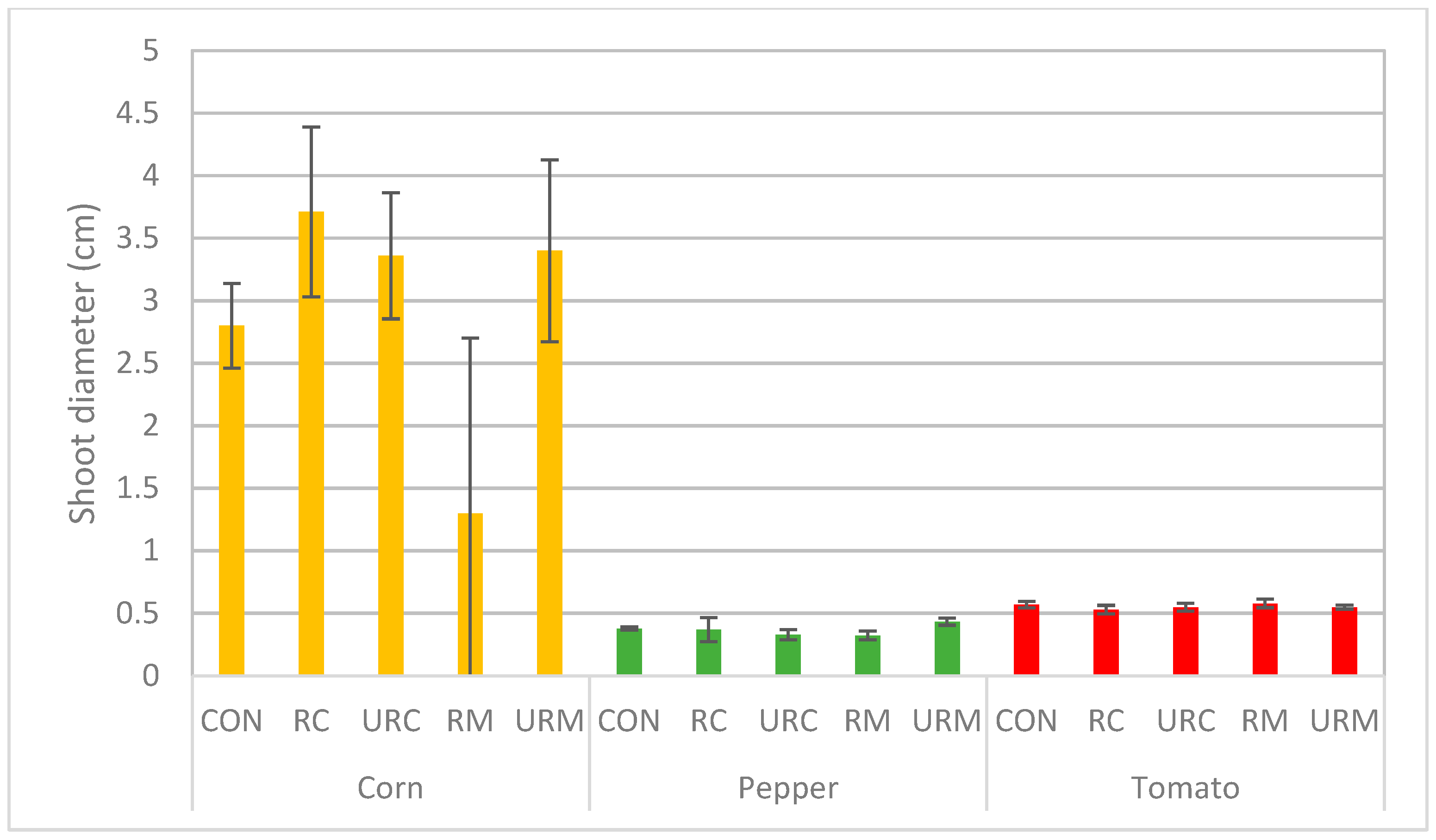
3.2.3. Plant Shoot Diameter
3.3. Description of Plant Health
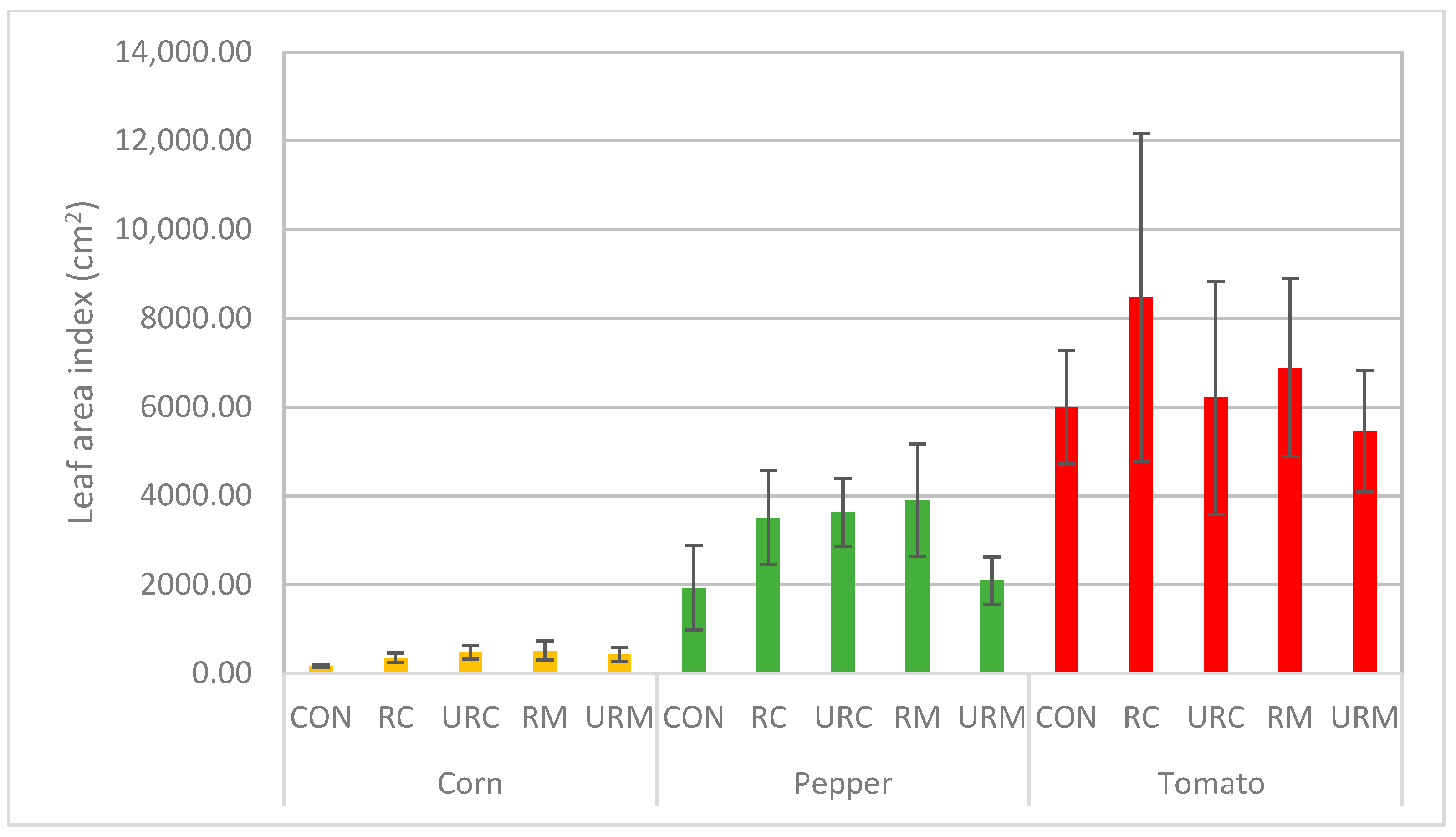
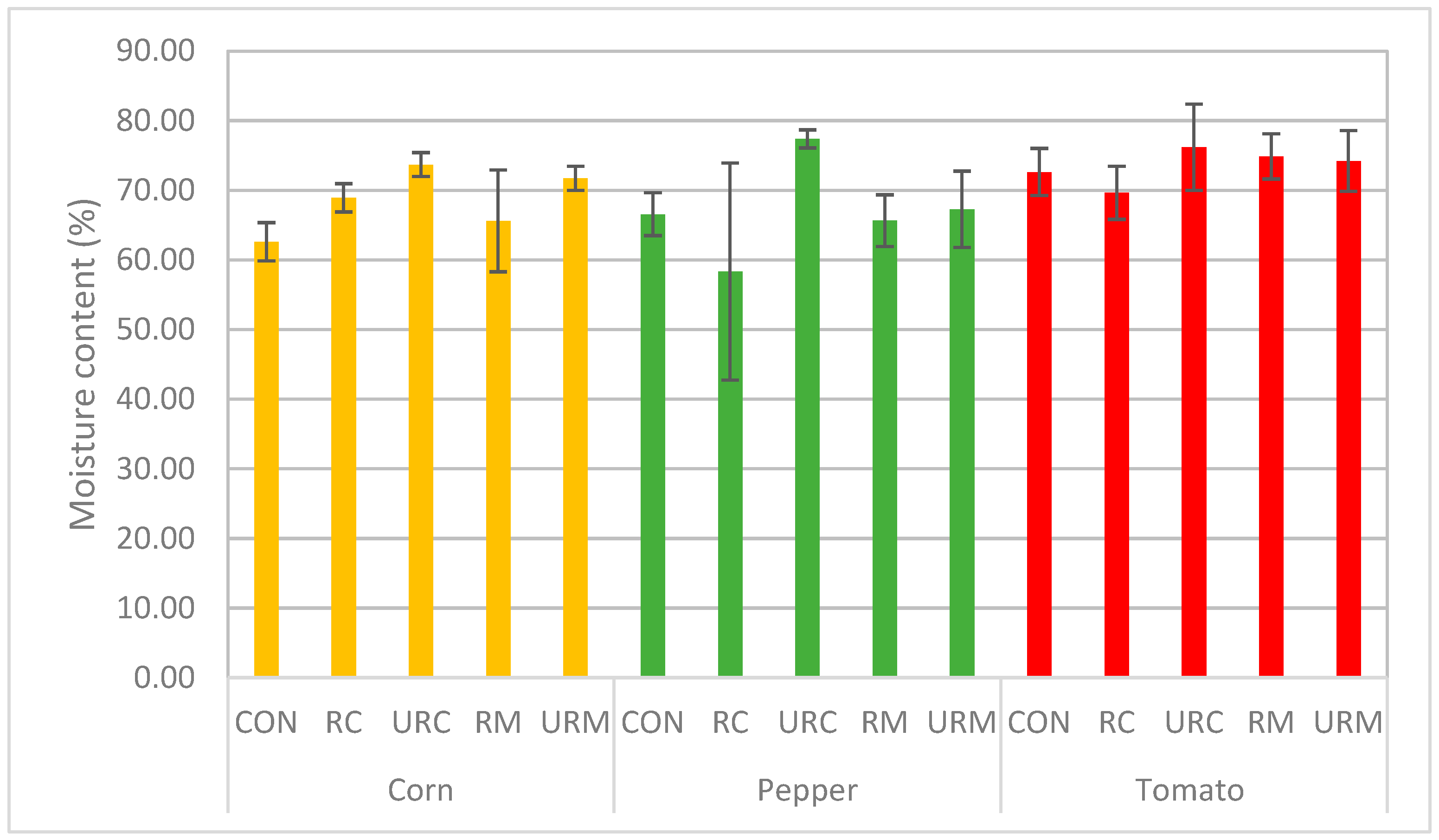
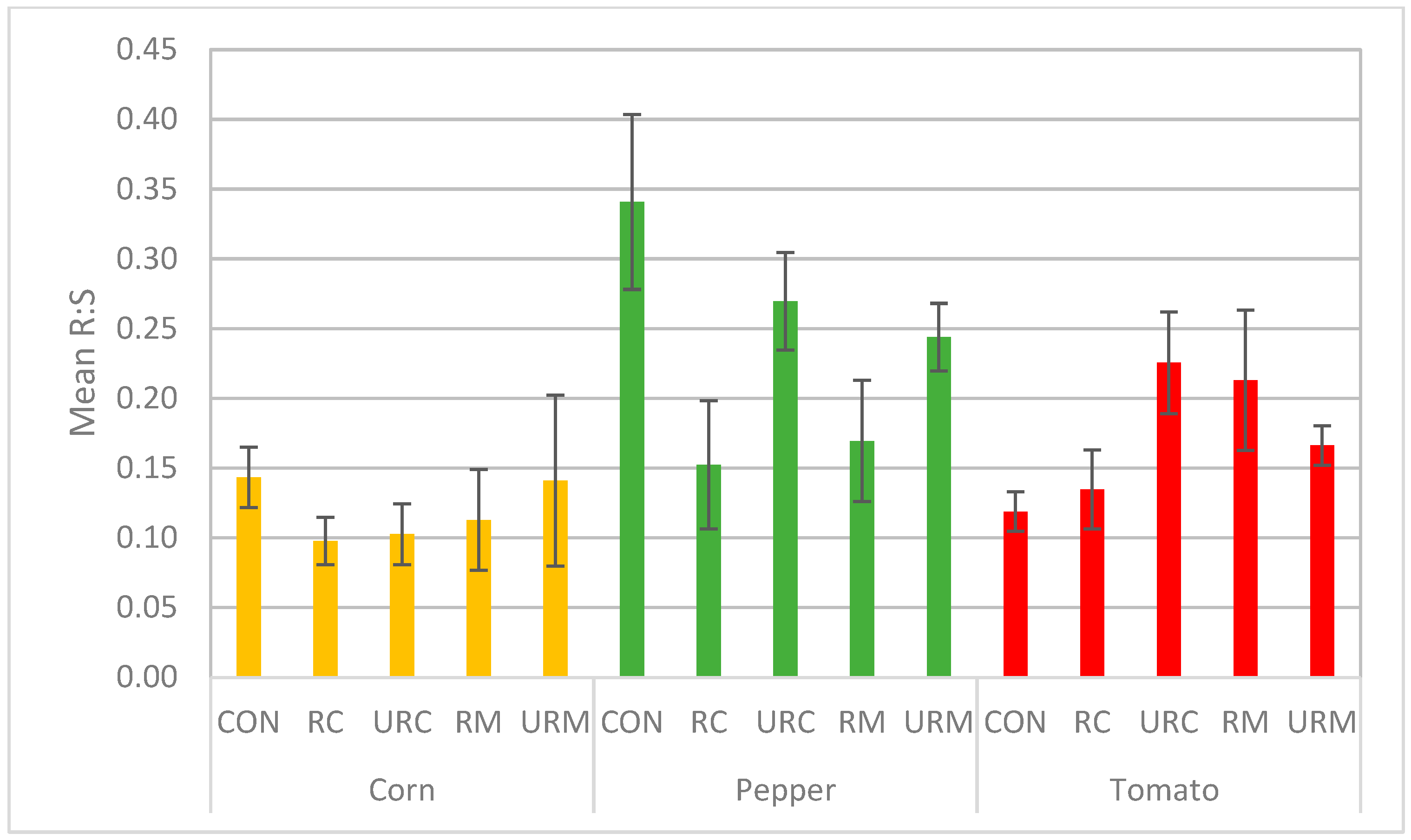
3.4. Analysis of Plant Post-Harvest Parameters
3.5. Elemental Analysis of Mulch, Compost, and Plant (Tomato and Pepper) Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef]
- Jouanno, J.; Almar, R.; Muller-Karger, F.; Morvan, G.; van Tussenbroek, B.; Benshila, R.; Marchesiello, P.; Addo, K.A. Socio-ecological vulnerability assessment to Sargassum arrivals. Sci. Rep. 2025, 22, 9998. [Google Scholar] [CrossRef]
- Mohan, P.; Strobl, E. Tourism and marine crises: The impact of Sargassum invasion on Caribbean small island developing states. Ocean Cost. Manag. 2024, 251, 107091. [Google Scholar] [CrossRef]
- Banydeen, R.; Lacavalerie, M.R.; Florentin, J.; Boullanger, C.; Medhaoui, H.; Resiere, D.; Neviere, R. Central sleep apnea and exposure to ambient hydrogen sulfide emissions from massive strandings of decomposing Sargassum in the Caribbean. Sci. Total Environ. 2024, 912, 168886. [Google Scholar] [CrossRef]
- Qi, L.; Cheng, P.; Wang, M.; Hu, C.; Xie, Y.; Mao, K. Where does floating Sargassum in the East China Sea come from? Harmful Algae 2023, 129, 102523. [Google Scholar] [CrossRef]
- Bennett, J.P.; Robinson, L.F.; Gomez, L. Valorisation strategies for brown seaweed biomass production in a European context. Algal Res. 2023, 75, 103248. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R. Massive influx of pelagic Sargassum spp. on the coasts of the Mexican Caribbean 2014–2020: Challenges and opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar]
- Desrochers, A.; Cox, S.-A.; Oxenford, H.A.; van Tussenbroek, B.I. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers; Report Prepared for the Climate Change Adaptation in the Eastern Caribbean Fisheries Sector (CC4FISH) Project of the Food and Agriculture Organization (FAO) and the Global Environment Facility (GEF); Centre for Resource Management and Environmental Studies (CERMES), University of the West Indies, Cave Hill Campus: Bridgetown, Barbados, 2020. [Google Scholar]
- van der Plank, S.; Cumberbatch, J.; Thomas, B.; Corbett, J.; Tompkins, E.L. Analysis of the Real-Time Phases of Adaptation Through the Lens of an Emergent Risk: Sargassum Adaptation Policy Analysis in the Caribbean. Phycology 2025, 5, 2. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Enhancing soil health and productivity of Lycopersicon esculentum Mill. using Sargassum johnstonii Setchell & Gardner as a soil conditioner and fertilizer. J. Appl. Phycol. 2013, 25, 1225–1235. [Google Scholar]
- Vijayanand, N.; Sivasangari Ramya, S.; Rathinavel, S. Potential of liquid extracts of Sargassum wightii on growth, biochemical and yield parameters of cluster bean plant. Asian Pac. J. Reprod. 2014, 3, 150–155. [Google Scholar] [CrossRef]
- Sasikala, M.; Indumathi, E.; Radhika, S.; Sasireka, R. Effect of seaweed extract (Sargassum tenerrimum) on seed germination and growth of tomato plant. Int. J. ChemTech Res. 2016, 9, 285–293. [Google Scholar]
- Oyesiku, O.O.; Egunyomi, A. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. Afr. J. Biotechnol. 2014, 13, 1188–1193. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef]
- Cipolloni, O.A.; Gigault, J.; Dassié, É.P.; Baudrimont, M.; Gourves, P.Y.; Amaral-Zettler, L.; Pascal, P.Y. Metals and metalloids concentrations in three genotypes of pelagic Sargassum from the Atlantic Ocean Basin-scale. Mar. Pollut. Bull. 2022, 178, 113564. [Google Scholar] [CrossRef]
- Tonon, T.; Machado, C.B.; Webber, M.; Webber, D.; Smith, J.; Pilsbury, A.; Cicéron, F.; Herrera-Rodriguez, L.; Jimenez, E.M.; Suarez, J.V.; et al. Biochemical and elemental composition of pelagic Sargassum biomass harvested across the Caribbean. Phycology 2022, 2, 204–215. [Google Scholar] [CrossRef]
- Alleyne, K.S.T.; Neat, F.; Oxenford, H.A. An analysis of arsenic concentrations associated with sargassum influx events in Barbados. Mar. Pollut. Bull. 2023, 192, 115064. [Google Scholar] [CrossRef]
- Bousso, N.C.; Brehmer, P.; Ndiaye, W.; Stiger-Pouvreau, V.; Kane, C.; Gautier, M.; Faye, M.; Fricke, A.; Diadhiou, H.D.; Aroui Boukbida, H.; et al. Unusual holopelagic Sargassum mass beaching in Northwest Africa: Morphotypes, chemical composition, and potential valorisation. Sci. Total Environ. 2024, 955, 177018. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Enhancing biogas production from Caribbean pelagic Sargassum utilising hydrothermal pretreatment and anaerobic co-digestion with food waste. Chemosphere 2021, 275, 130035. [Google Scholar] [CrossRef] [PubMed]
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. The Biostimulant Effect of Hydroalcoholic Extracts of Sargassum spp. in Tomato Seedlings under Salt Stress. Plants 2022, 11, 3180. [Google Scholar] [CrossRef]
- Smith, J.; Pilsbury, A.; Kumar, V.; Karamerou, E.E.; Chuck, C.J.; Herrera-Rodriguez, L.; Suarez, J.V.; Allen, M.J. Accessing the Efficacy of Sargassum-Based Aqueous Phase Products Derived from Hydrothermal Carbonisation and Hydrothermal Liquefaction on Plant Growth. Phycology 2024, 4, 53–64. [Google Scholar] [CrossRef]
- Paredes-Camacho, R.M.; Robledo-Olivo, A.; González-Morales, S.; Benavides-Mendoza, A.; Rodríguez-Jasso, R.M.; González-Fuentes, J.A.; Charles-Rodríguez, A.V. Evaluation of the Fermented Extract of Sargassum spp., for the Biostimulation in the Germination of Tomato Seeds and Seedlings (Solanum lycopersicum L.). J. Soil Sci. Plant. Nutr. 2024, 24, 4856–4867. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Slocum, A.H.; Ceballos, M.L.; Aponte, P.; Bisonó-León, A.G. Beyond the Bloom: Invasive Seaweed Sargassum spp. as a Catalyst for Sustainable Agriculture and Blue Economy—A Multifaceted Approach to Biodegradable Films, Biostimulants, and Carbon Mitigation. Sustainability 2025, 17, 3498. [Google Scholar] [CrossRef]
- Sembera, J.A.; Meier, E.J.; Waliczek, T.M. Composting as an Alternative Management Strategy for Sargassum Drifts on Coastlines. HortTechnology 2018, 28, 80–84. [Google Scholar] [CrossRef]
- Walsh, K.T.; Waliczek, T.M. Examining the Quality of a Compost Product Derived from Sargassum. HortTechnology 2020, 30, 331–336. [Google Scholar] [CrossRef]
- Abdool-Ghany, A.A.; Blare, T.; Solo-Gabriele, H.M. Assessment of Sargassum spp. Management Strategies in Southeast Florida. Resour. Conserv. Recycl. Adv. 2023, 19, 200175. [Google Scholar] [CrossRef]
- Blare, T.; Abdool-Ghany, A.A.; Solo-Gabriele, H.M. Cost estimates for producing Sargassum spp. compost. Univ. Fla. Inst. Food Agric. Sci. EDIS 2023, 2023, FE1128. [Google Scholar] [CrossRef]
- Ramdwar, M.N.A.; Stoute, V.A.; Abraham, B.S. An evaluation of Sargassum seaweed media compositions on the performance of hot pepper (Capsicum chinense Jacq.) seedling production. Cogent Food Agric. 2016, 2, 1263428. [Google Scholar] [CrossRef]
- Abdool-Ghany, A.A.; Pollier, C.G.L.; Oehlert, A.M.; Swart, P.K.; Blare, T.; Moore, K.; Solo-Gabriele, H.M. Assessing quality and beneficial uses of Sargassum compost. Waste Manag. 2023, 171, 545–556. [Google Scholar] [CrossRef]
- Trench, C.; Thomas, S.; Thorney, D.; Maddix, G.; Francis, P.; Small, H.; Machado, C.B.; Webber, D.M.; Tonon, T.; Webber, M.K. Application of stranded pelagic Sargassum biomass as compost for seedling production in the context of mangrove restoration. Front. Env. Sci. 2022, 10, 932293. [Google Scholar] [CrossRef]
- Caribbean Agricultural Research and Development Institute. Sargassum Seaweed and Its Use in Crop and Livestock Production: Possible Agri-Business Opportunities; CARDI Policy Brief; Caribbean Agricultural Research and Development Institute: St. Augustine, Trinidad and Tobago, 2015. [Google Scholar]
- Eyras, M.C.; Defosse, G.E.; Dellatorre, F. Seaweed compost as an amendment for horticultural soils in Patagonia, Argentina. Compos. Sci. Util. 2008, 16, 119–124. [Google Scholar] [CrossRef]
- Haase, D. Understanding forest seedling quality: Measurements and interpretation. Tree Plant. Notes 2008, 52, 24–30. [Google Scholar]
- Darini, M.; Amalia, F. Growth Response and Antioxidant Content of Aloe Vera L. Plant Under Different types and Dosage of Green Mulch in Sandy Soil. Int. J. Adv. Sci. Eng. Technol. 2017, 5, 59–63. [Google Scholar]
- Ge, Y.; Atefi, A.; Zhang, H.; Miao, C.; Ramamurthy, R.K.; Sigmon, B.; Yang, J.; Schanble, J.C. High-throughput analysis of leaf physiological and chemical traits with VIS–NIR–SWIR spectroscopy: A case study with a maize diversity panel. Plant Methods 2019, 15, 66. [Google Scholar] [CrossRef]
- Association of Official Seed Analysts, 1st ed.; Seed Vigor Testing Handbook; AOSA: East Lasing, MI, USA, 1983; Volume 88.
- Hossain, M.A.; Arefin, M.K.; Khan, B.M.; Rahman, M.A. Effects of seed treatments on germination and seedling growth attributes of Horitaki (Terminalia chebula Retz.) in the nursery. Res. J. Agric. Biol. Sci. 2005, 1, 135–141. [Google Scholar]
- Noriyuki, O. Height-dependent changes in shoot structure and tree allometry in relation to maximum height in four deciduous tree species. Funct. Ecol. 2011, 25, 777–786. [Google Scholar] [CrossRef]
- Machado, C.B.; Marsh, R.; Hargreaves, J.K.; Oxenford, H.A.; Maddix, G.M.; Webber, D.F.; Webber, M.; Tonon, T. Changes in holopelagic Sargassum spp. biomass composition across an unusual year. Proc. Natl. Acad. Sci. USA 2024, 121, e2312173121. [Google Scholar] [CrossRef]
- Kaveh, H.; Nemati, H.; Farsi, M.; Jartoodeh, S.V. How salinity affect germination and emergence of tomato lines. J. Biol. Environ. Sci. 2011, 5, 159–163. [Google Scholar]
- Konuşkan, Ö.; Gözübenli, H.; Atiş, I.; Atak, M. Effects of salinity stress on emergence and seedling growth parameters of some maize genotypes (Zea mays L.). Turk. J. Agric.-Food Sci. Technol. 2017, 5, 1668–1672. [Google Scholar] [CrossRef]
- da Silva, M.G.; de Oliveira Gondim, A.R.; Feitosa Lêdo, E.R.; Francilino, A.H.; da Silva, Y.A.; Gheyi, H.R. Response of two pepper species (Capsicum chinense Jacq. and Capsicum frutescens L.) to salt stress at germination stage in Northeast Brazil. Rev. Cienc. Agric. 2021, 38, 75–88. [Google Scholar] [CrossRef]
- Kaladharan, P.; Sathianandan, T.V.; Edison, S.J.; Shahana, T.S.; Vysakhan, P. Effects of basal application of mulch and foliar spray of Sargassum wightii extract on certain vegetable crops. Fish. Technol. 2019, 56, 44–48. [Google Scholar]
- Kaladharan, P.; Subramanniyan, S.; Pushparaj, A.; Thulasidharan, A.; Vysakhan, P. Mulching brown seaweed Sargassum wightii during transplant on the growth and yield of paddy. J. Mar. Biol. Ass. India 2021, 63, 117–121. [Google Scholar]
- Agricultural Analytical Services Lab (AASL). Interpretive Nutrient Levels for Plant Analysis. Penn State College of Agricultural Sciences. Available online: https://agsci.psu.edu/aasl/plant-analysis/plant-tissue-total-analysis/interpretive-nutrient-levels-for-plant-analysis (accessed on 9 April 2025).
- Food and Agriculture Organizations of the United Nations (FAO); World Health Organization. General Standards for Contaminants and Toxins in Food and Feed. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 9 April 2025).
- Hanaoka, K.; Yosida, K.; Tamano, M.; Kuroiwa, T.; Kaise, T.; Maeda, S. Arsenic in the prepared edible brown alga hijiki, Hizikia fusiforme. Appl. Organomet. Chem. 2001, 15, 561–565. [Google Scholar] [CrossRef]
- Ortega-Flores, P.A.; Gobert, T.; Méndez-Rodríguez, L.C.; Serviere-Zaragoza, E.; Connan, S.; Robledo, D.; Freile-Pelegrín, Y.; Montañez, A.; Waeles, M. Inorganic arsenic in holopelagic Sargassum spp. Stranded in the Mexican Caribbean: Seasonal variations and comparison with international regulations and guidelines. Aquat. Bot. 2023, 188, 103674. [Google Scholar] [CrossRef]
| Code | Rank | Description |
|---|---|---|
| 0 | Dead | Completely dead plant. |
| 1 | Worst | Disease-ridden, drooping, heavy senescence, dead leaves, and pests. |
| 2 | Bad | Moderate pests, mild flaccidity, moderate leaf-yellowing. or other abnormalities. |
| 3 | OK | Fair health, no leaf discolouration, moderately turgid, and minor abnormalities. |
| 4 | Good | No abnormalities, turgid plant, no signs of disease, minor scarring, or holes in leaves (<2 mm). |
| 5 | Best | Plant in pristine condition. |
| Plant | Treatment | Imbibition Period (Days) | Rate of Emergence (RE) | Germination Percentage (GP, %) | Germination Period (Days) | Seed Vigour (SV) | Germination Index (GI) | Germination Value (GV) |
|---|---|---|---|---|---|---|---|---|
| Corn | CON | 2 | 61.54 | 26 | 10 | 84.62 | 4.67 | 9.81 |
| RC | 2 | 85.71 | 21 | 4 | 80.95 | 4.00 | 11.53 | |
| URC | 4 | 35.48 | 31 | 10 | 80.65 | 4.96 | 13.47 | |
| RM | 4 | 30.77 | 26 | 8 | 76.92 | 4.17 | 7.75 | |
| URM | 6 | 0.00 | 2 | 0 | −300.00 | 0.67 | 0.22 | |
| Tomato | CON | 4 | 57.14 | 49 | 8 | 91.84 | 11.08 | 30.67 |
| RC | 2 | 50.55 | 91 | 10 | 95.60 | 19.58 | 129.36 | |
| URC | 2 | 82.35 | 68 | 12 | 94.12 | 15.36 | 67.02 | |
| RM | 4 | 3.53 | 85 | 8 | 95.29 | 16.00 | 49.38 | |
| URM | 4 | 12.50 | 64 | 10 | 93.75 | 6.57 | 37.24 | |
| Pepper | CON | 6 | 0.00 | 85 | 4 | 92.94 | 9.33 | 91.34 |
| RC | 4 | 15.66 | 83 | 8 | 95.18 | 10.17 | 91.62 | |
| URC | 4 | 6.98 | 86 | 10 | 95.35 | 7.64 | 76.41 | |
| RM | 4 | 1.27 | 79 | 8 | 94.94 | 6.83 | 77.29 | |
| URM | 6 | 0.00 | 90 | 6 | 93.33 | 11.17 | 77.05 |
| Treatment | Health Rank | Number of Plants | ||
|---|---|---|---|---|
| Corn | Pepper | Tomato | ||
| CON | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | |
| 2 | 7 | 0 | 0 | |
| 3 | 21 | 6 | 7 | |
| 4 | 26 | 43 | 31 | |
| 5 | 0 | 16 | 27 | |
| RC | 0 | 0 | 2 | 0 |
| 1 | 0 | 5 | 0 | |
| 2 | 3 | 2 | 4 | |
| 3 | 20 | 3 | 5 | |
| 4 | 32 | 29 | 32 | |
| 5 | 0 | 24 | 24 | |
| URC | 0 | 0 | 0 | 0 |
| 1 | 2 | 0 | 0 | |
| 2 | 17 | 2 | 1 | |
| 3 | 24 | 7 | 2 | |
| 4 | 11 | 56 | 51 | |
| 5 | 1 | 0 | 11 | |
| RM | 0 | 2 | 0 | 0 |
| 1 | 5 | 0 | 0 | |
| 2 | 8 | 4 | 2 | |
| 3 | 21 | 7 | 2 | |
| 4 | 19 | 51 | 47 | |
| 5 | 0 | 3 | 14 | |
| URM | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | |
| 2 | 19 | 0 | 0 | |
| 3 | 17 | 5 | 5 | |
| 4 | 19 | 53 | 38 | |
| 5 | 0 | 7 | 22 | |
| Samples | Na | Mg | Ca | K | P | As |
|---|---|---|---|---|---|---|
| COMPOST | ||||||
| CON | 0 ± 0 | 6737.64 ± 126.99 | 13,565.14 ± 239.53 | 2504.51 ± 73.36 | 886.51 ± 23.05 | 0.35 ± 0.02 |
| RC | 6287.58 ± 495.79 | 5115.69 ± 195.94 | 28,139.35 ± 1385.82 | 9433.86 ± 123.42 | 441.41 ± 5.13 | 13.72 ± 0.84 |
| URC | 6569.59 ± 199.02 | 5464.63 ± 209.12 | 19,033.67 ± 1799.16 | 12,362.34 ± 489.01 | 677.11 ± 79.54 | 14.74 ± 0.16 |
| Tomato | ||||||
| Roots CON | 5737.72 ± 107.06 | 6853.75 ± 620.66 | 11,034.8 ± 322.96 | 29,802.29 ± 3681.9 | 2990.32 ± 179.41 | 4.79 ± 2.48 |
| Roots RC | 9825.17 ± 130.13 | 5430.79 ± 218.93 | 11,868.86 ± 710.55 | 36,597.79 ± 3329.35 | 1736.88 ± 58.24 | 9.95 ± 0.45 |
| Roots URC | 6472.17 ± 917.64 | 4928.29 ± 818.76 | 14,616.28 ± 3042.71 | 53,596.9 ± 5325.7 | 2330.37 ± 511.95 | 208.22 ± 34.59 |
| Leaves CON | 0 ± 0 | 14,938.4 ± 1329.05 | 23,261.51 ± 2668.73 | 38,461.82 ± 4015.5 | 3464.41 ± 305.09 | 0.5 ± 0.14 |
| Leaves RC | 4821.16 ± 179.03 | 15,783.24 ± 3354.12 | 37,767.17 ± 8313.52 | 40,880 ± 7046.44 | 2055.85 ± 131.3 | 0.8 ± 0.15 |
| Leaves URC | 5022.91 ± 296.24 | 18,294.38 ± 1441.35 | 20,292.91 ± 2227.15 | 57,872.53 ± 11,076.32 | 2405.37 ± 456.32 | 8.33 ± 0.37 |
| Fruits CON | 0 ± 0 | 1820.8 ± 91.49 | 801.84 ± 101.47 | 45,817.8 ± 1508.95 | 4804.26 ± 175.71 | 0.13 ± 0.12 |
| Fruits RC | 0 ± 0 | 1680.86 ± 90.63 | 740.45 ± 56.59 | 45,386.75 ± 1900.01 | 3964.58 ± 293.67 | 0.22 ± 0.11 |
| Fruits URC | 0 ± 0 | 1769.91 ± 48.86 | 723.05 ± 81 | 46,494.77 ± 606.71 | 4163.13 ± 302.15 | 0.26 ± 0.13 |
| Pepper | ||||||
| Roots CON | 0 ± 0 | 2946.93 ± 315.86 | 12,578.93 ± 295.54 | 29,041.02 ± 2224.43 | 1290.63 ± 241.89 | 2.2 ± 0.29 |
| Roots RC | 0 ± 0 | 3042.5 ± 136.52 | 14,441.91 ± 1041.56 | 37,737.33 ± 4158.85 | 2193.25 ± 366.99 | 19.67 ± 0.57 |
| Roots URC | 0 ± 0 | 3188.09 ± 256.8 | 15,125.09 ± 3361.17 | 33,247.99 ± 3613.03 | 1791.74 ± 50.92 | 67.79 ± 10.24 |
| Leaves CON | 0 ± 0 | 15,961.95 ± 1168.78 | 18,580.24 ± 844.42 | 58,442.25 ± 3849.49 | 2227.8 ± 225.43 | 0.14 ± 0.09 |
| Leaves RC | 0 ± 0 | 20,346.98 ± 1927.17 | 18,432.68 ± 1152.14 | 70,201.64 ± 5661.76 | 2203.56 ± 145.79 | 3.3 ± 0.38 |
| Leaves URC | 0 ± 0 | 20,303.2 ± 1587.75 | 19,338.63 ± 867.78 | 60,893.84 ± 9081.94 | 2294.67 ± 65.22 | 6.21 ± 1.76 |
| Fruits CON | 0 ± 0 | 2640.77 ± 138.95 | 1276.19 ± 153.58 | 49,882.08 ± 3335.62 | 3805.86 ± 86.19 | 0.37 ± 0.19 |
| Fruits RC | 0 ± 0 | 3443.12 ± 143.82 | 1697.73 ± 88.49 | 51,819.74 ± 1580.51 | 4652.24 ± 264.92 | 0.57 ± 0.06 |
| Fruits URC | 0 ± 0 | 2637.79 ± 156.6 | 1283.19 ± 89.45 | 44,895.51 ± 1729.13 | 4379.28 ± 111.84 | 0.39 ± 0.29 |
| MULCH | ||||||
| CON | 0 ± 0 | 6737.64 ± 126.99 | 13,565.14 ± 239.53 | 2504.51 ± 73.36 | 886.51 ± 23.05 | 0.35 ± 0.02 |
| RM | 11,913.48 ± 624.95 | 7874.47 ± 190.03 | 32,729.1 ± 2258.58 | 28,105.74 ± 2075.41 | 753.33 ± 53.64 | 35.27 ± 2.57 |
| URM | 10,620.25 ± 1057.47 | 7640.91 ± 282.46 | 25,087.65 ± 1688.73 | 26,580.07 ± 2777.63 | 1110.74 ± 76.79 | 22.81 ± 2.62 |
| Tomato | ||||||
| Roots CON | 5737.72 ± 107.06 | 6853.75 ± 620.66 | 11,034.8 ± 322.96 | 29,802.29 ± 3681.9 | 2990.32 ± 179.41 | 4.79 ± 2.48 |
| Roots RM | 6870.21 ± 574.84 | 6064.47 ± 271.89 | 15,148.93 ± 1059.11 | 34,932.64 ± 3927.26 | 1650.21 ± 100.65 | 14.51 ± 1.53 |
| Roots URM | 5152.31 ± 43.95 | 5440.41 ± 660.31 | 13,013.04 ± 1584.11 | 43,031.55 ± 5241.84 | 2415.35 ± 518.29 | 120.56 ± 35.54 |
| Leaves CON | 0 ± 0 | 14,938.4 ± 1329.05 | 23,261.51 ± 2668.73 | 38,461.82 ± 4015.5 | 3464.41 ± 305.09 | 0.5 ± 0.14 |
| Leaves RM | 4876.52 ± 206.56 | 16,727.82 ± 2905.49 | 35,807.57 ± 8695.1 | 38,110.25 ± 7032.65 | 2864.4 ± 320.78 | 1.08 ± 0.35 |
| Leaves URM | 3128.94 ± 164.63 | 17,136.93 ± 3919.61 | 23,604.73 ± 6124.41 | 43,251.27 ± 2699.34 | 3204.86 ± 543.39 | 4.8 ± 1.29 |
| Fruits CON | 0 ± 0 | 1820.8 ± 91.49 | 801.84 ± 101.47 | 45,817.8 ± 1508.95 | 4804.26 ± 175.71 | 0.13 ± 0.12 |
| Fruits RM | 0 ± 0 | 1820.46 ± 61.47 | 677.93 ± 77.54 | 48,090.75 ± 2088.81 | 4772.41 ± 231.83 | 0.3 ± 0.14 |
| Fruits URM | 0 ± 0 | 2080.94 ± 60.95 | 854.1 ± 116.51 | 52,679.35 ± 3496.67 | 4705.52 ± 400.76 | 0.28 ± 0.16 |
| Pepper | ||||||
| Roots CON | 0 ± 0 | 2946.93 ± 315.86 | 12,578.93 ± 295.54 | 29,041.02 ± 2224.43 | 1290.63 ± 241.89 | 2.2 ± 0.29 |
| Roots RM | 0 ± 0 | 3508.51 ± 480.06 | 14,926.5 ± 770.07 | 30,142.31 ± 638.25 | 1269.25 ± 18.85 | 6.46 ± 2.45 |
| Roots URM | 0 ± 0 | 4226.3 ± 158.35 | 13,579.65 ± 759.08 | 29,243.6 ± 2721.59 | 1546.43 ± 206.17 | 84.84 ± 15.46 |
| Leaves CON | 0 ± 0 | 15,961.95 ± 1168.78 | 18,580.24 ± 844.42 | 58,442.25 ± 3849.49 | 2227.8 ± 225.43 | 0.14 ± 0.09 |
| Leaves RM | 0 ± 0 | 17,094.21 ± 959.4 | 22,949.54 ± 2352.6 | 63,012.6 ± 2591 | 2072.26 ± 102.44 | 0.86 ± 0.11 |
| Leaves URM | 0 ± 0 | 22,295.39 ± 2131.13 | 18,001.31 ± 1333.99 | 67,683.4 ± 8470.58 | 2188.31 ± 179.26 | 5.21 ± 1.66 |
| Fruits CON | 0 ± 0 | 2640.77 ± 138.95 | 1276.19 ± 153.58 | 49,882.08 ± 3335.62 | 3805.86 ± 86.19 | 0.37 ± 0.19 |
| Fruits RM | 0 ± 0 | 2699.53 ± 244.3 | 2027.75 ± 427.29 | 44,018.21 ± 543.79 | 4465.28 ± 354.47 | 0 ± 0 |
| Fruits URM | 0 ± 0 | 2806.92 ± 63.08 | 1193.73 ± 193.65 | 51,596.17 ± 3545.29 | 4257.17 ± 333.29 | 0.77 ± 0.1 |
| Tomato | |||||||
| Elemental parameters | AASL | CAC | Control | Rinsed compost | Unrinsed compost | Rinsed mulch | Unrinsed mulch |
| P | 3000 | - | 4804.26 ± 175.71 | 3964.58 ± 293.67 | 4163.13 ± 302.15 | 4772.41 ± 231.83 | 4705.52 ± 400.76 |
| K | 23,000 | - | 45,817.8 ± 1508.95 | 45,386.75 ± 1900.01 | 46,494.77 ± 606.71 | 48,090.75 ± 2088.81 | 52,679.35 ± 3496.67 |
| Ca | 10,000 | - | 801.84 ± 101.47 | 740.45 ± 56.59 | 723.05 ± 81 | 677.93 ± 77.54 | 854.1 ± 116.51 |
| Mg | 5500 | - | 1820.8 ± 91.49 | 1680.86 ± 90.63 | 1769.91 ± 48.86 | 1820.46 ± 61.47 | 2080.94 ± 60.95 |
| Mn | 30 | - | 9.55 ± 0.2 | 9.69 ± 1.24 | 9.23 ± 1.31 | 8.87 ± 0.33 | 8.05 ± 1.52 |
| Fe | 40 | - | 52.13 ± 12.35 | 81.52 ± 39.18 | 70.39 ± 23.38 | 125.41 ± 50.76 | 72.24 ± 8.88 |
| Cu | 10 | - | 4.69 ± 0.75 | 6.25 ± 1.59 | 6.81 ± 0.34 | 5.75 ± 0.17 | 4.51 ± 0.72 |
| Zn | 25 | - | 32.22 ± 1.45 | 27.96 ± 1.97 | 31.48 ± 3.09 | 35.62 ± 1.73 | 33.7 ± 1.84 |
| As | - | 0.2 | 0.55 ± 0.12 | 0.74 ± 0.13 | 0.97 ± 0.15 | 0.8 ± 0.08 | 0.72 ± 0.08 |
| Cd | - | 0.05 | 0.09 ± 0.03 | 0.18 ± 0.06 | 0.21 ± 0.05 | 0.12 ± 0.03 | 0.14 ± 0.03 |
| Pb | - | 0.1 | 0.04 ± 0.03 | 0.08 ± 0.06 | 0.08 ± 0.03 | 0.07 ± 0.05 | 0.05 ± 0.05 |
| Pepper | |||||||
| Elemental parameters | AASL | CAC | Control | Rinsed compost | Unrinsed compost | Rinsed mulch | Unrinsed mulch |
| P | 3000 | - | 3805.86 ± 86.19 | 4652.24 ± 264.92 | 4379.28 ± 111.84 | 4465.28 ± 354.47 | 4257.17 ± 333.29 |
| K | 25,000 | - | 49,882.08 ± 3335.62 | 51,819.74 ± 1580.51 | 44,895.51 ± 1729.13 | 44,018.21 ± 543.79 | 51,596.17 ± 3545.29 |
| Ca | 6000 | - | 1276.19 ± 153.58 | 1697.73 ± 88.49 | 1283.19 ± 89.45 | 2027.75 ± 427.29 | 1193.73 ± 193.65 |
| Mg | 3000 | - | 2640.77 ± 138.95 | 3443.12 ± 143.82 | 2637.79 ± 156.6 | 2699.53 ± 244.3 | 2806.92 ± 63.08 |
| Mn | 30 | - | 22.99 ± 0.49 | 29.71 ± 1.59 | 16.23 ± 1.2 | 11.09 ± 5.86 | 18.05 ± 2.87 |
| Fe | 30 | - | 65.92 ± 9.75 | 69.53 ± 6.85 | 70.18 ± 6.7 | 57.58 ± 33.14 | 59.4 ± 7.71 |
| Cu | 5 | - | 4.54 ± 0.84 | 6.03 ± 0.52 | 6.54 ± 1.04 | 6.18 ± 3.1 | 4.3 ± 0.98 |
| Zn | 25 | - | 24.62 ± 0.95 | 30.28 ± 3.27 | 25.29 ± 1.75 | 40.03 ± 14.2 | 24.76 ± 1.67 |
| As | - | 0.2 | 0.37 ± 0.19 | 0.57 ± 0.06 | 0.39 ± 0.29 | 0 ± 0 | 0.77 ± 0.1 |
| Cd | - | 0.05 | 0.14 ± 0.03 | 0.3 ± 0.06 | 0.24 ± 0.04 | 0.2 ± 0.1 | 0.15 ± 0.05 |
| Pb | - | 0.1 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.04 ± 0 | 0.06 ± 0.03 | 0.01 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haye, D.; Machado, C.B.; Young, R.; Webber, D.; Chinthapalli, B.R.; Tonon, T.; Webber, M. Influence of Pelagic Sargassum spp. On Soil Amelioration for Seed Germination and Seedling Growth of Corn (Zea mays), Scotch Bonnet Pepper (Capsicum chinense), and Tomato (Solanum lycopersicum). Phycology 2025, 5, 44. https://doi.org/10.3390/phycology5030044
Haye D, Machado CB, Young R, Webber D, Chinthapalli BR, Tonon T, Webber M. Influence of Pelagic Sargassum spp. On Soil Amelioration for Seed Germination and Seedling Growth of Corn (Zea mays), Scotch Bonnet Pepper (Capsicum chinense), and Tomato (Solanum lycopersicum). Phycology. 2025; 5(3):44. https://doi.org/10.3390/phycology5030044
Chicago/Turabian StyleHaye, Dannielle, Carla Botelho Machado, Robyn Young, Dale Webber, Bhaskar Rao Chinthapalli, Thierry Tonon, and Mona Webber. 2025. "Influence of Pelagic Sargassum spp. On Soil Amelioration for Seed Germination and Seedling Growth of Corn (Zea mays), Scotch Bonnet Pepper (Capsicum chinense), and Tomato (Solanum lycopersicum)" Phycology 5, no. 3: 44. https://doi.org/10.3390/phycology5030044
APA StyleHaye, D., Machado, C. B., Young, R., Webber, D., Chinthapalli, B. R., Tonon, T., & Webber, M. (2025). Influence of Pelagic Sargassum spp. On Soil Amelioration for Seed Germination and Seedling Growth of Corn (Zea mays), Scotch Bonnet Pepper (Capsicum chinense), and Tomato (Solanum lycopersicum). Phycology, 5(3), 44. https://doi.org/10.3390/phycology5030044






