Productivity and Carbon Utilization of Three Green Microalgae Strains with High Biotechnological Potential Cultivated in Flat-Panel Photobioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Maintenance Conditions
2.2. Algae Pre-Culture for Photobioreactor Inoculation
2.3. Flat-Panel Photobioreactors
2.4. Gas-Air Mixture Supply
2.5. Temperature Control System
2.6. Growth Characteristics
- The productivity (P) was estimated by dry weight (g DW L−1 d−1):
- The specific growth rate (μ) was estimated by the change in the culture OD (d−1):
- The total biomass productivity of each strain was calculated as the difference between final and initial biomass concentrations divided by the total cultivation period.
2.7. pH Measurements
2.8. Carbon Dioxide Utilization Efficiency
2.9. Biochemical Composition
- [Chl a]—chlorophyll a content,
- [Chl b]—chlorophyll b content,
- [Car]—total carotenoid content.
2.10. Algae Biomass Post-Processing and Storage
2.11. Statistics
3. Results
3.1. Growth Characteristics of Algae Under Intensive Cultivation Regime
3.2. Protein and Carbohydrate Composition of the Algae
3.3. Pigment Content
4. Discussion
4.1. Intensive Cultivation in Flat-Panel Photobioreactors
4.2. Biochemical Composition of Cells
4.3. Application Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CUE | cardon dioxide utilization efficiency |
| g DW | gram of dry weight |
| GAM | gas-air mixture |
| IPP RAS | Institute of Plants Physiology of the Russian Academy of Sciences |
| LED | light-emitting diode |
| Iave | average illumination level |
| RGAM | gas-air mixture flow rate |
| nCO2 | CO2 concentration |
| VCO2 | total volume of CO2 passed through the photobioreactor |
| PBR | photobioreactor |
| FP-5 PBR | flat-panel photobioreactor with a 5-L working volume |
| P | biomass productivity |
| vvm | volume of sparged gas per unit volume of growth medium per minute |
| DWW | diluted dairy wastewater |
References
- Diaz, C.J.; Douglas, K.J.; Kang, K.; Kolarik, A.L.; Malinovski, R.; Torres-Tiji, Y.; Molino, J.V.; Badary, A.; Mayfield, S.P. Developing algae as a sustainable food source. Front. Nutr. 2023, 9, 1029841. [Google Scholar] [CrossRef]
- Khan, A.Z.; Zhao, X.Q.; Bai, F.W.; Mustafa, H.H.; Liu, C.G. Cyanobacteria Biotechnology: Challenges and Prospects. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Mehmood, M.A., Verma, P., Shah, M.P., Betenbaugh, M.J., Eds.; Springer: Cham, Switzerland, 2024; pp. 325–343. [Google Scholar] [CrossRef]
- Lobus, N.V.; Knyazeva, M.A.; Popova, A.F.; Kulikovskiy, M.S. Carbon Footprint Reduction and Climate Change Mitigation: A Review of the Approaches, Technologies, and Implementation Challenges. C 2023, 9, 120. [Google Scholar] [CrossRef]
- Kao, C.Y.; Chen, T.Y.; Chang, Y.B.; Chiu, T.W.; Lin, H.Y.; Chen, C.D.; Chang, J.S.; Lin, C.S. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour. Technol. 2014, 166, 485–493. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Haider, M.N.; Tahir, F.; Musharraf, S.G.; Jabeen, F.; Malik, S. Cost-Effective Cultivation of Cyanobacteria for Biotechnological Applications. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Mehmood, M.A., Verma, P., Shah, M.P., Betenbaugh, M.J., Eds.; Springer: Cham, Switzerland, 2024; pp. 113–131. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables Governing Photosynthesis and Growth in Microalgae Mass Cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 2, 87–96. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef]
- Krivina, E.S.; Bobrovnikova, L.A.; Temraleeva, A.D.; Markelova, A.G.; Gabrielyan, D.A.; Sinetova, M.A. Description of Neochlorella semenenkoi gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a Novel Chlorella-like Alga with High Biotechnological Potential. Diversity 2023, 15, 513. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of the biotechnological potential of cyanobacterial and microalgal strains from IPPAS culture collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- Bobrovnikova, L.A.; Pakholkova, M.S.; Sidorov, R.A.; Sinetova, M.A. Starch and triacylglycerol accumulation in the cells of the stain Chlorella sp. IPPAS C-1210. Issues Mod. Algol. 2021, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lobus, N.V.; Glushchenko, A.M.; Osadchiev, A.A.; Maltsev, Y.I.; Kapustin, D.A.; Konovalova, O.P.; Kulikovskiy, M.S.; Krylov, I.N.; Drozdova, A.N. Production of Fluorescent Dissolved Organic Matter by Microalgae Strains from the Ob and Yenisei Gulfs (Siberia). Plants 2022, 11, 3361. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, S.; Gao, Y.; Pan, X.; Wang, X.; Deng, R.; Liu, P. Study on physicochemical properties and antioxidant activity of polysaccharides from Desmodesmus armatus. J. Food Biochem. 2020, 44, e13243. [Google Scholar] [CrossRef]
- Yuan, S.; Ye, S.; Yang, S.; Luo, G. Purification of potato wastewater and production of byproducts using microalgae Scenedesmus and Desmodesmus. J. Water Process Eng. 2021, 43, 102237. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Shen, D.D.; Gao, B.Y.; Huang, L.D.; Li, A.F.; Zhang, C.W. Cultivation of a Starch-rich Microalga Desmodesmus insignis in Dairy Wastewater and Removal of Nitrogen and Phosphorus. Plant Sci. J. 2016, 34, 446–459. [Google Scholar] [CrossRef]
- Purba, L.D.A.; Susanti, H.; Vadiveloo, A.; Anam, K.; Susilaningsih, D. Optimizing biomass and metabolite recovery from municipal wastewater using locally isolated microalgae strains. Int. J. Environ. Sci. Technol. 2025, 22, 10453–10468. [Google Scholar] [CrossRef]
- Solovchenko, A.; Gorelova, O.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Baulina, O.; Lobakova, E. Desmodesmus sp. 3Dp86E-1-a novel symbiotic chlorophyte capable of growth on pure CO2. Mar. Biotechnol. 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Gabel, B.V.; Sinetova, M.A.; Gabrielian, A.K.; Markelova, A.G.; Shcherbakova, N.V.; Los, D.A. Optimization of CO2 Supply for the Intensive Cultivation of Chlorella sorokiniana IPPAS C-1 in the Laboratory and Pilot-Scale Flat-Panel Photobioreactors. Life 2022, 12, 1469. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabel, B.V.; Gabrielian, A.K.; Markelova, A.G.; Rodionova, M.V.; Bedbenov, V.S.; Shcherbakova, N.V.; Los, D.A. Cultivation of Chlorella sorokiniana IPPAS C-1 in Flat-Panel Photobioreactors: From a Laboratory to a Pilot Scale. Life 2022, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabrielian, A.K.; Bobrovnikova, L.A.; Bedbenov, V.S.; Starikov, A.Y.; Zorina, A.A.; Gabel, B.V.; Los, D.A. Laboratory System for Intensive Cultivation of Microalgae and Cyanobacteria. Russ. J. Plant Physiol. 2023, 70, 20. [Google Scholar] [CrossRef]
- Hase, E.; Morimura, Y.; Tamiya, H. Some data on the growth physiology of Chlorella studied by the technique of synchronous culture. Arch. Biochem. Biophys. 1957, 69, 149–165. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Solovchenko, A.E.; Gorelova, O.A.; Baulina, O.I.; Selyakh, I.O.; Semenova, L.R.; Chivkunova, O.B.; Scherbakov, P.N.; Lobakova, E.S. Physiological plasticity of symbiotic Desmodesmus (Chlorophyceae) isolated from taxonomically distant white sea invertibrates. Russ. J. Plant Physiol. 2015, 62, 653–663. [Google Scholar] [CrossRef]
- Pandey, S.; Vinayagam, R.; Selvaraj, R.; Selvaraj, R.; Varadavenkatesan, T. Optimizing growth conditions of Desmodesmus armatus NCIM 5583 for enhanced biodiesel production using CaO bionanocatalyst. Discov. Appl. Sci. 2025, 7, 331. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ziganshina, E.; Bulynina, S.; Ziganshin, A. Semi-continuous cultivation of indigenous Chlorella sorokiniana for biomass and pigment production. In Proceedings of the XI International Scientific and Practical Conference Innovative Technologies in Environmental Science and Education (ITSE-2023), Divnomorskoe Village, Russia, 11 November 2023; Rudoy, D.V., Olshevskaya, A.V., Odabashyan, M.Y., Eds.; E3S Web of Conferences. EDP Sciences: Les Ulis, France, 2023; Volume 431. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Zhu, X.; Chang, H.; Ou, S.; Wang, H. Role of Bioreactors in Microbial Biomass and Energy Conversion. In Bioreactors for Microbial Biomass and Energy Conversion; Liao, Q., Chang, J.S., Herrmann, C., Xia, A., Eds.; Springer: Singapore, 2018; pp. 39–78. [Google Scholar]
- Klementev, S.V.; Budenkova, E.A.; Kulikova, Y.V.; Sirotkin, A.S. Utilization of the aqueous phase of the hydrothermal liquefaction process as a substrate for microalgae cultivation. Proc. Univ. Appl. Chem. Biotechnol. 2024, 14, 537–547. (In Russian) [Google Scholar] [CrossRef]
- Sriram, S.; Seenivasan, R. Biophotonic perception on Desmodesmus sp. VIT growth, lipid and carbohydrate content. Bioresour. Technol. 2015, 198, 626–633. [Google Scholar] [CrossRef]
- Vesenick, D.C.; Paula, N.A.; Niwa, A.M.; Mantovani, M.S. Evaluation of the effects of chlorophyllin on apoptosis induction, inhibition of cellular proliferation and mRNA expression of CASP8, CASP9, APC and b-catenin. Curr. Res. J. Biol. Sci. 2012, 4, 315–322. [Google Scholar]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Ang, W.S.; Kee, P.E.; Lan, J.C.W.; Chen, W.H.; Chang, J.S.; Khoo, K.S. Unveiling the rise of microalgae-based foods in the global market: Perspective views and way forward. Food Biosci. 2025, 66, 105390. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Pinto, F.R.; Silva, F.; Teixeira, P.; Mendes, S.; Gil, M.M. Application of microalgae as natural colorant for pastry and confectionary products. Food Sci. Nutr. 2024, 12, 9479–9492. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Xie, Q.; Lin, S.; Xu, W.; Cheung, P.C.K. Microalgae-derived astaxanthin: Bioactivities, biotechnological approaches and industrial technologies for its production. Crit. Rev. Food Sci. Nutr. 2025, 24, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 10, 3487–3515. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.G.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. Adv. Biochem. Eng. Biotechnol. 2016, 153, 37–58. [Google Scholar] [CrossRef]
- Six, A.; Dauvillée, D.; Lancelon-Pin, C.; Dimitriades-Lemaire, A.; Compadre, A.; Dubreuil, C.; Alvarez, P.; Sassi, J.-F.; Li-Beisson, Y.; Putaux, J.-L.; et al. From raw microalgae to bioplastics: Conversion of Chlorella vulgaris starch granules into thermoplastic starch. Carbohydr. Polym. 2024, 342, 122342. [Google Scholar] [CrossRef]
- Di Caprio, F.; Amenta, S.; Francolini, I.; Altimari, P.; Pagnanelli, F. Microalgae Biorefinery: Optimization of Starch Recovery for Bioplastic Production. ACS Sustain. Chem. Eng. 2023, 11, 16509–16520. [Google Scholar] [CrossRef]
- Gifuni, I.; Olivieri, G.; Russo Krauss, I.; D’Errico, G.; Pollio, A.; Marzocchella, A. Microalgae as new sources of starch: Isolation and characterization of microalgal starch granules. Chem. Eng. Trans. 2017, 57, 1423–1428. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vitová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review. Starch-Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Petkov, G.; Ivanova, A.; Iliev, I.; Vaseva, I. A critical look at the microalgae biodiesel. Eur. J. Lipid Sci. Technol. 2012, 114, 103–111. [Google Scholar] [CrossRef]
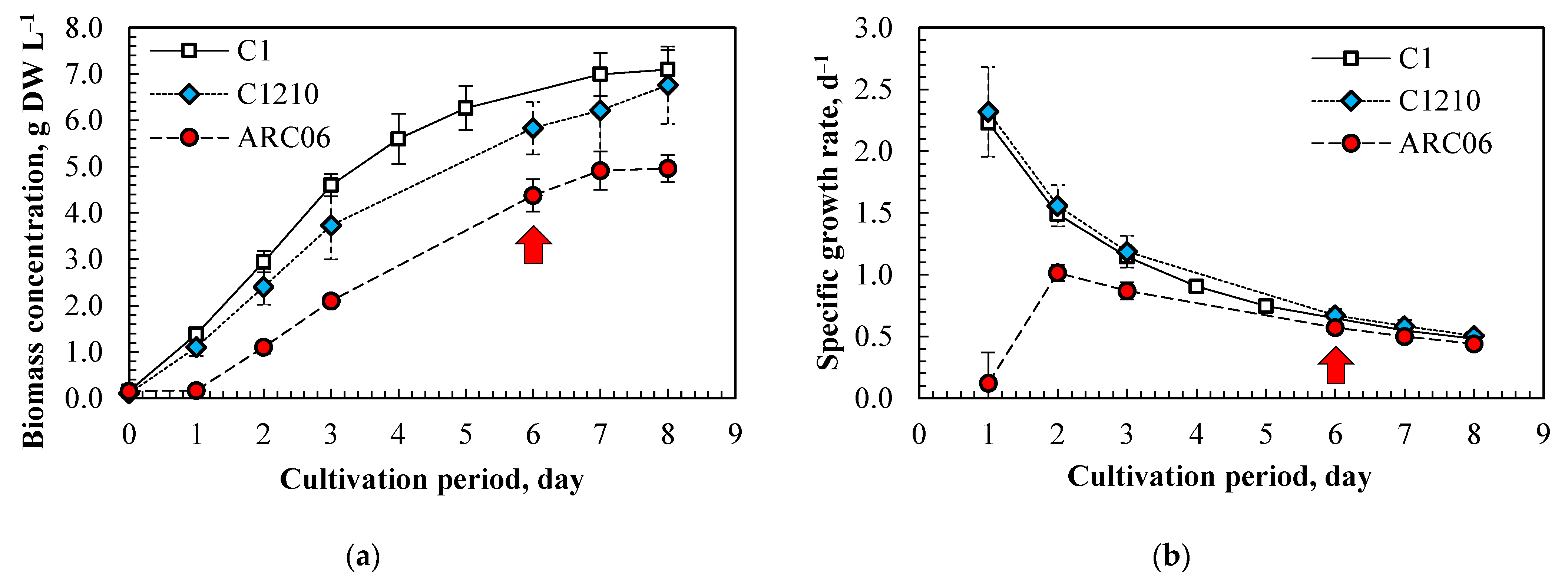
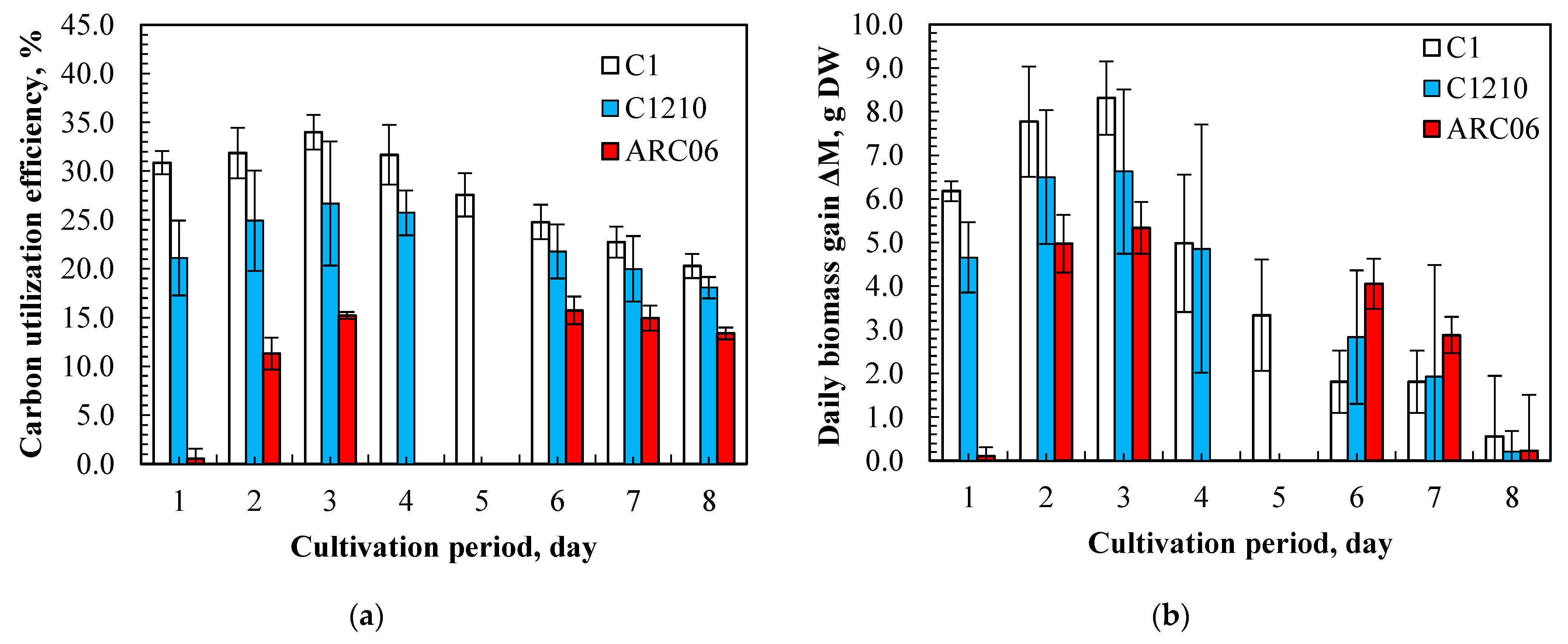
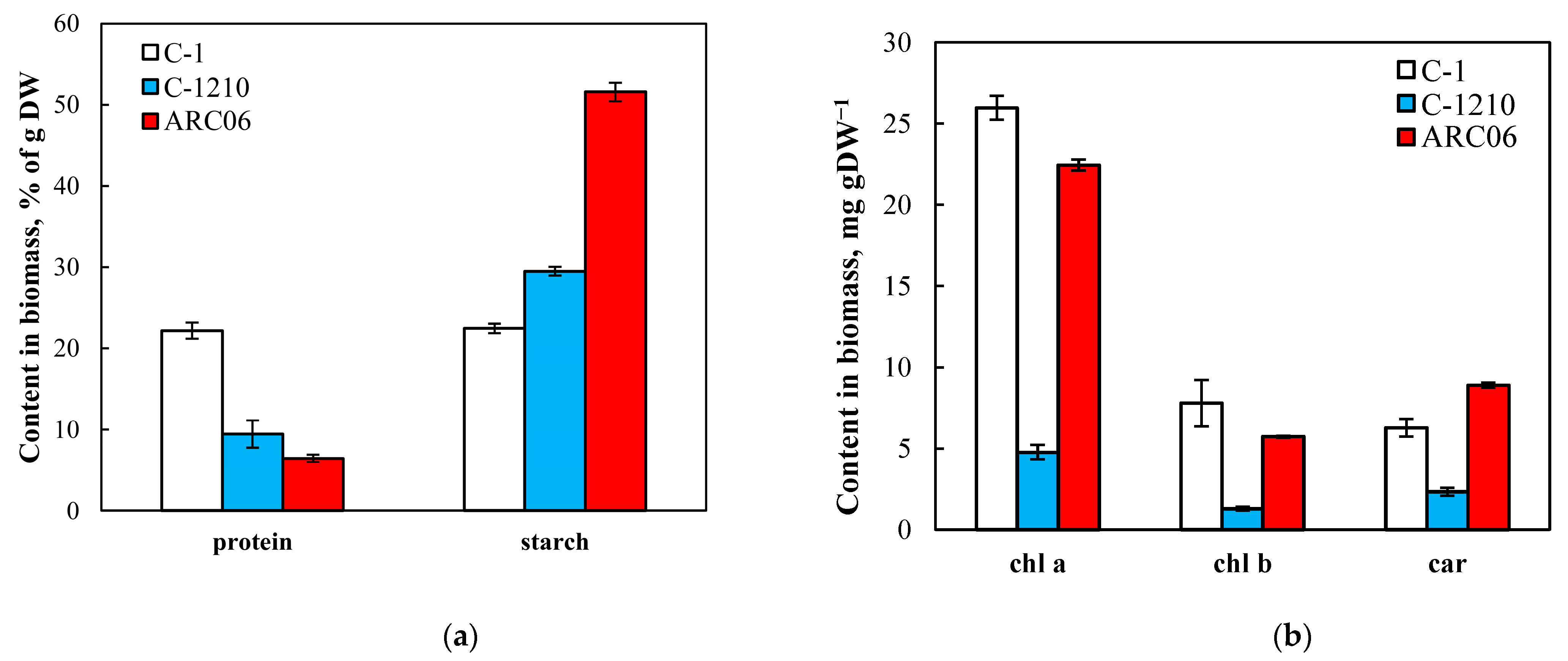
| Parameters | Chlorella sorokiniana C-1 | Neochlorella semenenkoi C-1210 |
|---|---|---|
| Starting biomass concentration, g DW L−1 | 0.15 ± 0.01 | 0.11 ± 0.02 |
| Biomass concentration at the 3rd day, g DW L−1 | 4.60 ± 0.24 | 3.73 ± 0.73 |
| Final biomass concentration (day 8), g DW L−1 | 7.10 ± 0.41 | 6.76 ± 0.84 |
| Specific growth rate (max), day−1 | 2.23 ± 0.05 | 2.32 ± 0.36 |
| pH starting level | 6.28 ± 0.01 | 6.29 ± 0.06 |
| pH maximum level | 8.33 ± 0.03 | 8.34 ± 0.09 |
| The productivity during days 0–3, g DW L−1 d−1 | 1.48 ± 0.08 | 1.20 ± 0.25 |
| Total productivity (8 days), g DW L−1 d−1 | 0.87 ± 0.05 | 0.80 ± 0.06 |
| Total biomass yield, g DW | 34.73 ± 2.02 | 33.22 ± 4.22 |
| Parameters | Desmodesmus armatus ARC-06 |
|---|---|
| Starting biomass concentration, g DW L−1 | 0.15 ± 0.01 |
| Biomass concentration at the 3rd day, g DW L−1 | 2.10 ± 0.03 |
| Final biomass concentration (day 8), g DW L−1 | 4.96 ± 0.30 |
| Specific growth rate (max), day−1 | 1.00 ± 0.05 |
| pH starting level | 6.74 ± 0.03 |
| pH maximum level | 7.99 ± 0.06 |
| The productivity during days 0–3, g DW L−1 d−1 | 0.68 ± 0.01 |
| Total productivity (8 days), g DW L−1 d−1 | 0.61 ± 0.04 |
| Total biomass yield, g DW | 24.05 ± 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrielyan, D.A.; Sinetova, M.A.; Savinykh, G.A.; Zadneprovskaya, E.V.; Goncharova, M.A.; Markelova, A.G.; Gabrielian, A.K.; Gabel, B.V.; Lobus, N.V. Productivity and Carbon Utilization of Three Green Microalgae Strains with High Biotechnological Potential Cultivated in Flat-Panel Photobioreactors. Phycology 2025, 5, 43. https://doi.org/10.3390/phycology5030043
Gabrielyan DA, Sinetova MA, Savinykh GA, Zadneprovskaya EV, Goncharova MA, Markelova AG, Gabrielian AK, Gabel BV, Lobus NV. Productivity and Carbon Utilization of Three Green Microalgae Strains with High Biotechnological Potential Cultivated in Flat-Panel Photobioreactors. Phycology. 2025; 5(3):43. https://doi.org/10.3390/phycology5030043
Chicago/Turabian StyleGabrielyan, David A., Maria A. Sinetova, Grigoriy A. Savinykh, Elena V. Zadneprovskaya, Maria A. Goncharova, Alexandra G. Markelova, Alexander K. Gabrielian, Boris V. Gabel, and Nikolay V. Lobus. 2025. "Productivity and Carbon Utilization of Three Green Microalgae Strains with High Biotechnological Potential Cultivated in Flat-Panel Photobioreactors" Phycology 5, no. 3: 43. https://doi.org/10.3390/phycology5030043
APA StyleGabrielyan, D. A., Sinetova, M. A., Savinykh, G. A., Zadneprovskaya, E. V., Goncharova, M. A., Markelova, A. G., Gabrielian, A. K., Gabel, B. V., & Lobus, N. V. (2025). Productivity and Carbon Utilization of Three Green Microalgae Strains with High Biotechnological Potential Cultivated in Flat-Panel Photobioreactors. Phycology, 5(3), 43. https://doi.org/10.3390/phycology5030043








