Morphology and Reproduction of Acanthophora spicifera (Ceramiales: Rhodophyta)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Morphological Description
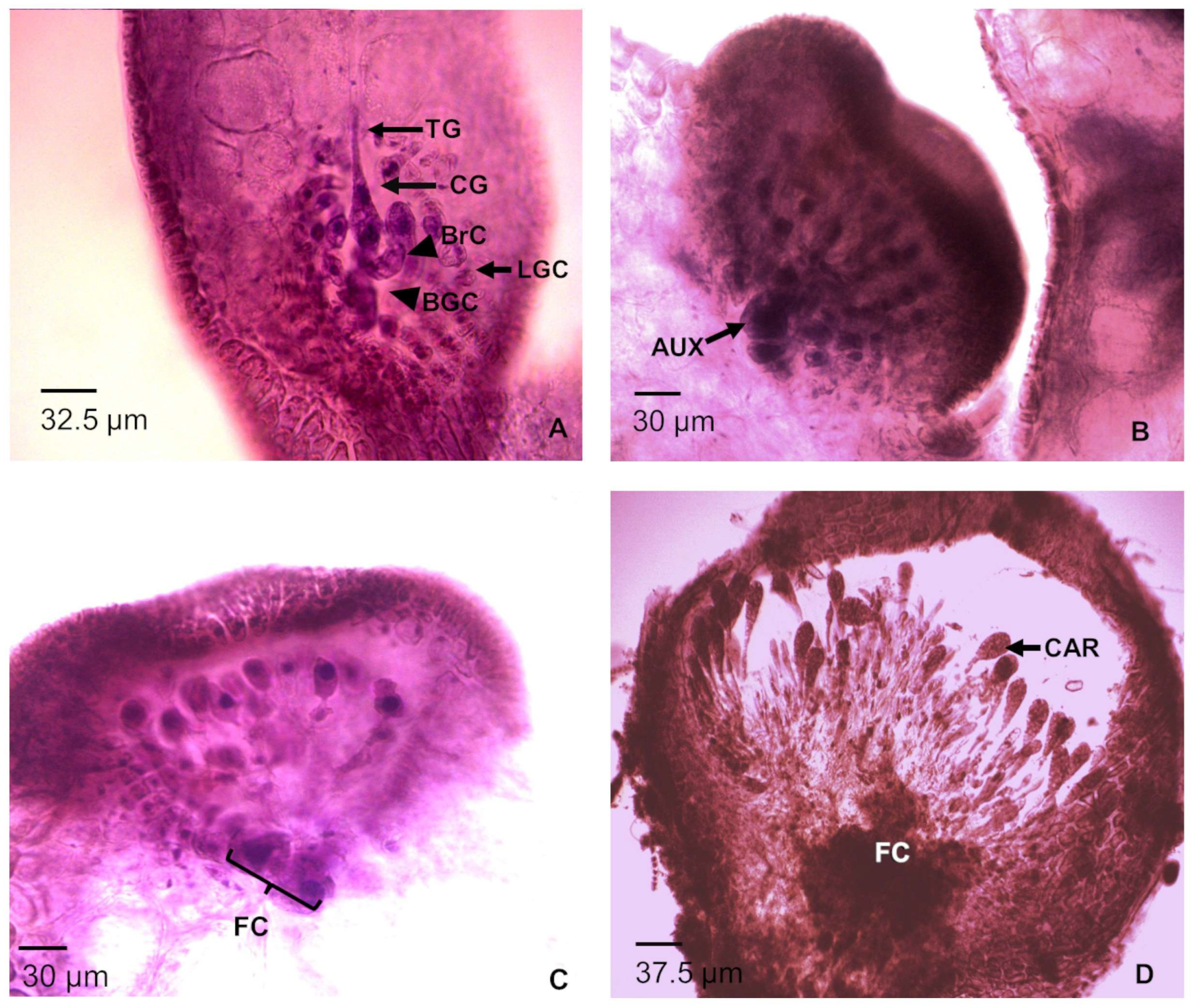
3.1.1. Male Gametophyte
3.1.2. Female Gametophyte with Carposporophyte
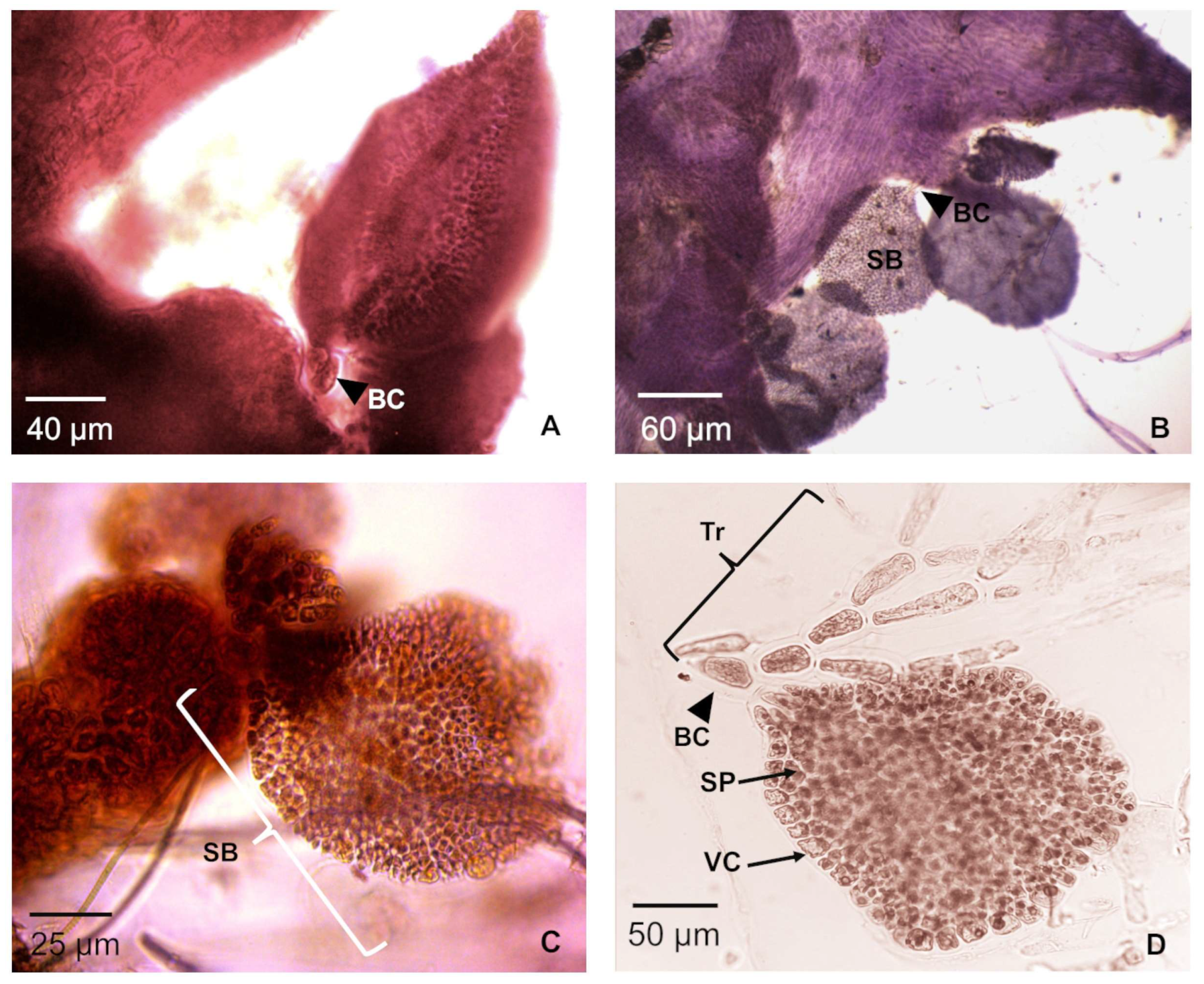
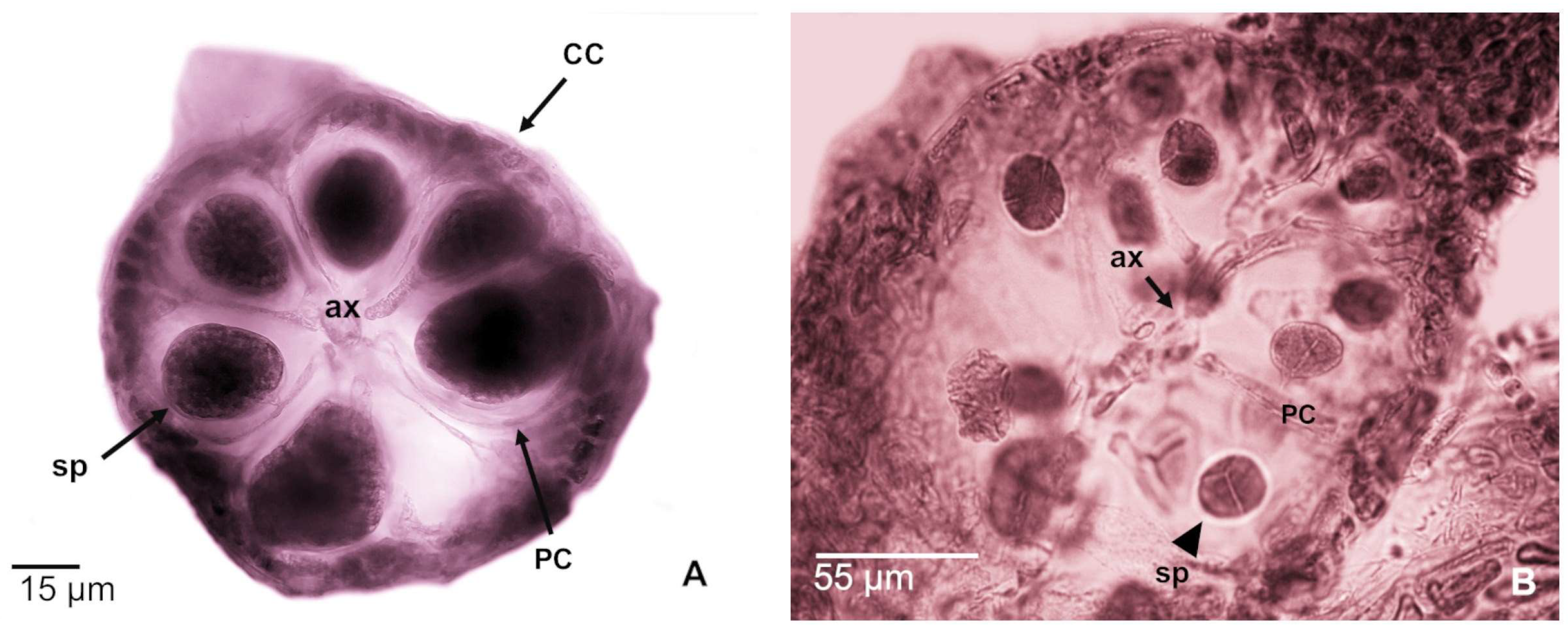
3.1.3. Tetrasporophytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Saunders, G.W.; Hommersand, M.H. Assessing red algal supraordinal diversity and taxonomy in the context of contemporary systematic data. Am. J. Bot. 2004, 91, 1494–1507. [Google Scholar] [CrossRef]
- Yoon, H.S.; Müller, K.M.; Sheath, R.G.; Ott, F.D.; Bhattacharya, D. Defining the Major Lineages of Red Algae (Rhodophyta). J. Phycol. 2006, 42, 482–492. [Google Scholar] [CrossRef]
- Graham, L.E.; Graham, J.M.; Wilcox, L.W. Algae; Benjamin Cummings: San Francisco, CA, USA, 2009. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase Version 4.2. World-Wide Electronic Publication. National University of Ireland, Galway. 2025. Available online: http://www.algaebase.org (accessed on 20 May 2025).
- Yang, E.; Boo, S.; Bhattacharya, D.; Sauders, G.W.; Knoll, A.H.; Fredericq, S.; Graf, L.; Yoon, H.S. Divergence time estimates and the evolution of major lineages in the florideophyte red algae. Sci. Rep. 2016, 6, 21361. [Google Scholar] [CrossRef]
- Scagel, R.F. A morphological study of some dorsiventral Rhodomelaceae. Univ. Calif. Publ. Bot. 1953, 27, 1–108. [Google Scholar]
- Hommersand, M.H. The morphology and classification of some Ceramiaceae and Rhodomelaceae. Univ. Calif. Publ. Bot. 1963, 35, 165–366. [Google Scholar]
- Wynne, M.J. Checklist of benthic marine algae of the tropical and subtropical Western Atlantic: Fifth revision. Nova Hedwig. Beih. 2022, 153, 180. [Google Scholar]
- Kylin, H. Entwicklungsgescllichtliche Florideenstudien. Ibid 1928, 24, 127. [Google Scholar]
- Kylin, H. 6ber die Entwicklungsgescllichte der FIorideen. Ibid 1930, 26, 104. [Google Scholar]
- Papenfus, G.F. Structure and taxonomy of Taenioma; including a discussion on the phylogeny of the Ceramiales. Madroño 1994, 7, 193–214. [Google Scholar]
- Choi, H.G.; Kraft, G.T.; Lee, I.K.; Saunders, G.W. Phylogenetic analyses of anatomical and nuclear SSU rDNA sequence data indicate that the Dasyaceae and Delesseriaceae (Ceramiales, Rhodophyta) are polyphyletic. Eur. J. Phycol. 2002, 37, 551–569. [Google Scholar] [CrossRef]
- Fritsch, F.E. Present-day classification of algae. Bot. Rev. 1944, 10, 233–277. [Google Scholar] [CrossRef]
- Kim, M.S.; Maggs, E.A.; Mc Ivor, L.; Guiry, M.D. Reappraisal of the type species of Polysiphonia (Rhodomelaceae, Rhodophyta). Eur. J. Phycol. 2000, 35, 83–92. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, E.J.; Endo, H.; Kitade, Y.; Saga, N. Inheritance pattern of chloroplast and mitochondrial genomes in artificial hybrids of Porphyra yezoensis (Rhodophyta). Fish. Sci. 2008, 74, 822–829. [Google Scholar] [CrossRef]
- Sentíes, A.; Dreckmann, K.M. Biodiversidad de las macroalgas marinas de la familia Rhodomelaceae (Rhodophyta) en México. Rev. Mex. Biodivers. 2014, 85, S62–S68. [Google Scholar] [CrossRef]
- Nam, K.W.; Saito, Y.; Sohn, C.H. Vegetative structure and reproduction of Laurencia nipponica Yamada (Rhodomelaceae, Rhodophyta). Korean J. Phycol. 1991, 6, 83–92. [Google Scholar]
- Furnari, G.; Serio, D. The reproductive structures of the Mediterranean alga Laurencia pelagosae (Ceramiales, Rhodophyta). Eur. J. Phycol. 1993, 28, 141–143. [Google Scholar] [CrossRef]
- Nam, K.W.; Saito, Y. Vegetative and reproductive anatomy of some Laurencia (Ceramiales, Rhodophyta) species with a description of L. maris-rubri sp. nov. from the Red Sea. Phycologia 1995, 34, 157–165. [Google Scholar] [CrossRef]
- Nam, K.W.; Choi, H.G.; Lee, S.; Park, E.J.; Kang, K.H.; Kim, Y.S. Vegetative and reproductive development of Laurencia venusta (Ceramiales, Rhodophyta). Cryptogam. Algae 2000, 21, 97–110. [Google Scholar] [CrossRef]
- Kyaw, S.P.P.; Soe-Htun, U. Studies on the developmental morphology and life history in culture of Laurencia sp. 1 (Ceramiales, Rhodophyta) from Myanmar. J. Aquac. Mar. Biol. 2018, 7, 246–251. [Google Scholar] [CrossRef]
- Böker-Tôrres, M.; Horta, P.A.; Salles, J.; Ouriques, L.; Fujii, M.T.; Bouzon, Z. Morfologia e reprodução de Chondria curvilineata F. S. Collins & Hervey (Rhodomelaceae, Rhodophyta), uma adição à flora brasileira. Acta Bot. Bras. 2009, 23, 73–78. [Google Scholar] [CrossRef]
- Williams, T.M.; Stacy, A.; Krueger-Hadfield, K.; Hill-Spanik, K.M.; Kosaki, R.K.; Stoeckel, S.; Spalding, H.L. The reproductive system of the cryptogenic alga Chondria tumulosa (Florideophyceae) at Manawai, Papahānaumokuākea Marine National Monument. Phycologia 2024, 63, 36–44. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, I.K. Morphology and reproduction of Polysiphonia yendoi Segi (Rhodomelaceae, Rhodophyta) in Korea. Algae 1997, 12, 73–81. [Google Scholar]
- Rindi, F.; Guiry, M.D.; Cinelli, F. Morphology and reproduction of the adventive Mediterranean rhodophyte Polysiphonia setacea. Hydrobiologia 1999, 398/399, 91–100. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, I.K.; Guiry, M. Vegetative and Reproductive Morphology of Polysiphonia lanosa (Rhodomelaceae, Rhodophyta) from Ireland. Bot. Mar. 2002, 45, 293–302. [Google Scholar] [CrossRef]
- Aguilar-Rosas, R.; Aguilar-Rosas, L.E.; Pedroche, F.F. Descripción de talos espermatangiales y combinación de fases en Polysiphonia confusa (Rhodomelaceae, Rhodophyta). Rev. Mex. Biodivers. 2006, 77, 1–6. [Google Scholar]
- Buchan-Antalan, T.; Trono, G.C. The morphology, growth and seasonality in the reproductive states of Acanthophora spicifera (Vahl) Boergesen in Bacoor Bay. Nat. Appl. Sci. Bull. 1983, 35, 18–27. [Google Scholar]
- Kilar, J.A.; McLachlan, J.L. Ecological studies of the algae, Acanthophora spicifera (Vahl) Boerg, (Ceramiales: Rhodophyta): Vegetative fragmentation. J. Exp. Mar. Biol. Ecol. 1986, 104, 1–21. [Google Scholar] [CrossRef]
- De Jong, Y.S.D.M.; Hitipew, C.; Prud’homme van Reine, W.F. A taxonomic, phylogenetic and biogeographic study of the genus Acanthophora (Rhodomelaceae, Rhodophyta). Blumea 1999, 44, 217–249. [Google Scholar]
- García-Soto, G.C.; López-Bautista, J.M. Acanthophora dendroides Harvey (Rhodomelaceae), a new record for the Atlantic and Pacific oceans. Check List 2019, 15, 509–514. [Google Scholar] [CrossRef]
- Ávila, E.; Méndez-Trejo, M.; Riosmena-Rodríguez, R.; López-Vivas, J.; Sentíes, A. Epibiotic traits of the invasive red seaweed Acanthophora spicifera in la paz bay, south Baja California (Eastern pacific). Mar. Ecol. 2012, 33, 470–480. [Google Scholar] [CrossRef]
- Mendoza-Becerril, M.A.; Pedroche, F.F.; Estrada-González, M.C.; Serviere-Zaragoza, E. Records of the non-native alga Acanthophora spicifera (Rhodophyta) and their colonial epibionts in La Paz Bay, Gulf of California. Biodivers. Data J. 2023, 11, e114262. [Google Scholar] [CrossRef]
- Mendez-Trejo, C.; Riosmena-Rodríguez, R.; Ávila, E.; López-Vivas, J.M.; Sentíes, A. Evaluación de la invasión de Acanthophora spicifera (Rhodophyta) sobre la epifauna en Bahía de La Paz, B.C.S. In Especies Invasoras Acuáticas: Casos de estudio en Ecosistemas de México; Low-Pfeng, A.M., Quijón, P.A., Peters-Recagno, E.M., Eds.; Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT): Mexico City, Mexico, 2012; pp. 433–456. [Google Scholar]
- Schnöller, V.C.G. Caracterización química del alga roja Acanthophora spicifera (M. Vahl) Børgesen (Ceramiales: Rhodophyta) en La Bahía De La Paz, Baja California Sur, México. Cienc. Mar. 2016, 46, 165–176. [Google Scholar] [CrossRef]
- O’Doherty, D.C.; Sherwood, A.R. Genetic population structure of the Hawaiian alien invasive seaweed Acanthophora spicifera (Rhodophyta) as revealed by DNA Sequencing and ISSR Analyses. Pac. Sci. 2007, 61, 223–234. [Google Scholar] [CrossRef]
- Ávila-Ortiz, A.; Mateo-Cid, L.E.; Mendoza-González, A.C. Caracterización morfológica de Padina boergesenii (Dictyotaceae, Phaeophyceae) en la costa mexicana del Golfo de México y mar Caribe. Polibotánica 2011, 31, 1–20. [Google Scholar]
- Borgesen, F. Some new or little known West Indian Florideae. II. Botanisk Tidsskrift 1910, 30, 177–207. [Google Scholar]
- Santelices, B. Ecología de Algas Marinas Bentónicas. Efectos de factores ambientales. Ediciones Universidad Católica de Chile.; Universidad Católica de Chile: Santiago, Chile, 1977; 488p. [Google Scholar]
- Mateo-Cid, L.E.; Mendoza-González, A.C. Algas marinas bénticas de la Isla Cozumel, Quintana Roo, México. Acta Bot. Mex. 1991, 16, 57–87. [Google Scholar] [CrossRef]
- Cecere, E.; Saracino, O.D.; Fanelli, M.; Petrocelli, A. Phenology of two Acanthophora najadiformis (Rhodophyta, Ceramiales) populations in the Ionian Sea (Mediterranean Sea). Bot. Mar. 2000, 43, 109–117. [Google Scholar] [CrossRef]
- Magalhães, L.A.; de Castro-Nunes, J.M. Aportación al conocimiento fenológico de las rodofíceas marinas de la playa de Guarajuba (Camaçari, Bahía) Brasil. Bot. Complut. 2002, 26, 17–34. [Google Scholar]
- Mateo-Cid, L.E.; Mendoza-González, A.C.; Ávila-Ortiz, A.G.; Díaz Martínez, S. Algas marinas bentónicas del litoral de Campeche, México. Acta Bot. Mex. 2013, 104, 53–92. [Google Scholar] [CrossRef]
- Mateo-Cid, L.E.; Mendoza-González, A.C.; Hernández-Casas, C.M. Diversity of brown algae (Ochrophyta, Phaeophyceae) of Sian Ka’an reserve Biosphere, Mexican Caribbean. Pak. J. Bot. 2019, 51, 361–366. [Google Scholar] [CrossRef]
- Ardito, S.; Gómez, S. Patrón fenológico de una población de Gelidium serrulatum J. Agardh (Rhodophyta, Gelidiales) en la localidad de Taguao, Estado Vargas, Venezuela. Acta Bot. Venez. 2005, 28, 101–111. [Google Scholar]
- Mateo-Cid, L.E.; Mendoza-González, A.C.; García-López, D.Y.; Hernández-Casas, C.M.; Méndez-Guzmán, I. Diversidad de algas marinas bentónicas del litoral de Veracruz, México. Acta Bot. Mex. 2024, 131, e2316. [Google Scholar] [CrossRef]
- Cecere, E.; Perrone, C.; Petrocelli, A. Acanthophora nayadiformis (Ceramiales, Rhodophyta) in the Gulf of Taranto. Giorn. Bot. Ital. 1994, 128, 1082–1084. [Google Scholar]
- Lee, Y.P.; Yoon, S.Y. Taxonomy of Chondria (Rhodophyta) in Korea. Algae 1996, 11, 107–139. [Google Scholar]
- Bustamante, D.E.; Ramírez, M.E. El género Polysiphonia sensu lato, en la costa norte y centro-sur de Chile (18–41°S) (Rhodophyta, Rhodomelaceae). Bol. Mus. Nac. Hist. Nat. Chile 2009, 58, 31–50. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Casas, C.M.; Pineda-Mendoza, R.M.; Mendoza-González, Á.C.; Zúñiga, G.; Mateo-Cid, L.E. Morphology and Reproduction of Acanthophora spicifera (Ceramiales: Rhodophyta). Phycology 2025, 5, 45. https://doi.org/10.3390/phycology5030045
Hernández-Casas CM, Pineda-Mendoza RM, Mendoza-González ÁC, Zúñiga G, Mateo-Cid LE. Morphology and Reproduction of Acanthophora spicifera (Ceramiales: Rhodophyta). Phycology. 2025; 5(3):45. https://doi.org/10.3390/phycology5030045
Chicago/Turabian StyleHernández-Casas, Cynthia Mariana, Rosa María Pineda-Mendoza, Ángela Catalina Mendoza-González, Gerardo Zúñiga, and Luz Elena Mateo-Cid. 2025. "Morphology and Reproduction of Acanthophora spicifera (Ceramiales: Rhodophyta)" Phycology 5, no. 3: 45. https://doi.org/10.3390/phycology5030045
APA StyleHernández-Casas, C. M., Pineda-Mendoza, R. M., Mendoza-González, Á. C., Zúñiga, G., & Mateo-Cid, L. E. (2025). Morphology and Reproduction of Acanthophora spicifera (Ceramiales: Rhodophyta). Phycology, 5(3), 45. https://doi.org/10.3390/phycology5030045






