Growth, Productivity, and Size Structure of Spirulina Strain Under Different Salinity Levels: Implications for Cultivation Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Experimental Treatments
2.3. Plate-Based Growth Experiments
2.4. Light and Dark Bottle Experiments
2.5. Tube-Based Growth Experiments
2.6. Data Analyses
3. Results
3.1. Variation in Growth
3.2. Variation in Productivity
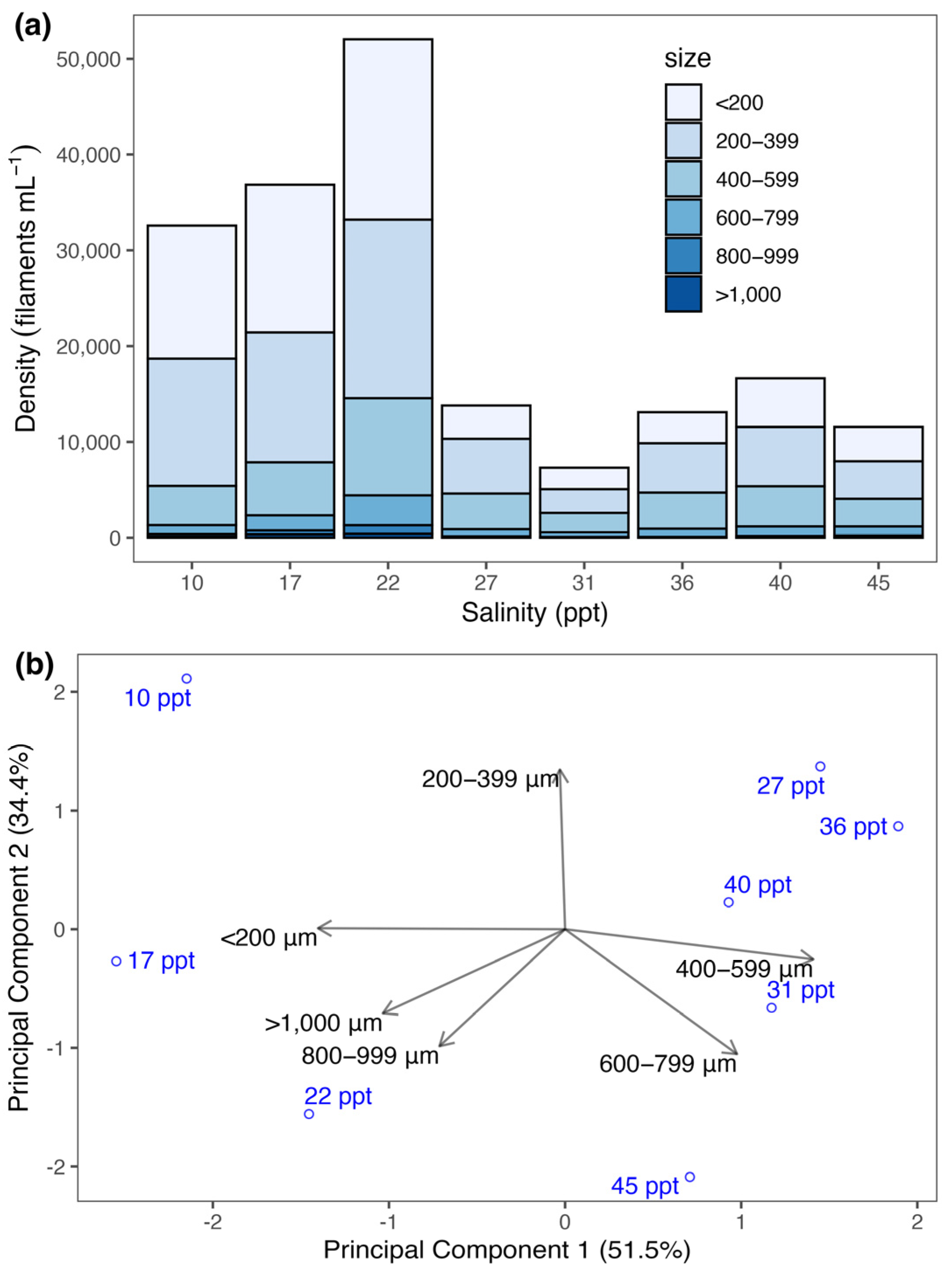
3.3. Change in Size Structure
4. Discussion
4.1. Salinity Modulates Growth of Spirulina
4.2. Salinity Alters Productivity of Spirulina
4.3. Salinity Shifts Size Structure of Spirulina
4.4. Putative Physiological Mechanisms Underlying Spirulina’s Response to Salinity
4.5. Caveats and Limitations
4.6. Implications for Optimization of Spirulina Cultivation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DO | Dissolved Oxygen |
| GLM | Generalized Linear Model |
| GPP | Gross Primary Productivity |
| HSD | Honestly Significant Difference |
| K | Carrying Capacity |
| L | Length |
| LD | Diagonal Length |
| OD | Optical Density |
| N | Population Size |
| NPP | Net Primary Productivity |
| PCA | Principal Component Analysis |
| PERMANOVA | Permutational Multivariate Analysis of Variance |
| R | Respiration |
| W | Width |
References
- Habib, M.A.B. (Ed.) A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; FAO Fisheries and Aquaculture Circular; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Nutritional and pharmaceutical properties of microalgal Spirulina. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 299–308. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I. Spirulina—An invaluable source of macro- and micronutrients with broad biological activity and application potential. Molecules 2024, 29, 5387. [Google Scholar] [CrossRef] [PubMed]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Gavilan-Figari, I.M.; Peña-Urdániga, G.V.; Naka, A.; Castro-Rosas, M.A. The role of Spirulina in food security: Nutritional and ecological implications in the context of climate change. CABI Rev. 2024, 19. [Google Scholar] [CrossRef]
- Ravelonandro, P.H.; Ratianarivo, D.H.; Joannis-Cassan, C.; Isambert, A.; Raherimandimby, M. Improvement of the growth of Arthrospira (Spirulina) platensis from Toliara (Madagascar): Effect of agitation, salinity and CO2 addition. Food Bioprod. Process 2011, 89, 209–216. [Google Scholar] [CrossRef]
- Mishra, P.; Prasad, S.M. Low dose UV-B radiation induced mild oxidative stress impact on physiological and nutritional competence of Spirulina (Arthrospira) species. Plant Stress 2021, 2, 100039. [Google Scholar] [CrossRef]
- González-Portela, R.E.; Romero-Villegas, G.I.; Kapoore, R.V.; Alammari, Z.M.; Malibari, R.A.; Al Shaikhi, A.; Al Hafedh, Y.; Aljahdali, A.H.; Banjar, R.E.; Mhedhbi, E.; et al. Cultivation of Limnospira maxima under extreme environmental conditions in Saudi Arabia: Salinity adaptation and scaling-up from laboratory culture to large-scale production. Bioresour. Technol. 2024, 406, 131089. [Google Scholar] [CrossRef]
- Yu, C.; Zheng, J.; Zhang, Y.; Hu, Y.; Luo, W.; Zhang, J.; Yu, J.; Liu, J.; Nixon, P.J.; Zhou, W.; et al. Towards sustainable Spirulina farming: Enhancing productivity and biosafety with a salinity-biostimulants strategy. Bioresour. Technol. 2025, 419, 132043. [Google Scholar] [CrossRef]
- Lassiter, A. Rising seas, changing salt lines, and drinking water salinization. Curr. Opin. Environ. Sustain. 2021, 50, 208–214. [Google Scholar] [CrossRef]
- Rai, S.V.; Rajashekhar, M. Effect of pH, salinity and temperature on the growth of six species of cyanobacteria isolated from Arabian Sea coast of Karnataka. Int. J. Biosci. Technol. 2016, 9, 1–6. [Google Scholar]
- Moisander, P.H.; McClinton, E.; Paerl, H.W. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb. Ecol. 2002, 43, 432–442. [Google Scholar] [CrossRef]
- van Eykelenburg, C. The ultrastructure of Spirulina platensis in relation to temperature and light intensity. Antonie Van Leeuwenhoek 1979, 45, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Zhao, Y. Morphological reversion of Spirulina (Arthrospira) platensis (Cyanophyta): From linear to helical. J. Phycol. 2005, 41, 622–628. [Google Scholar] [CrossRef]
- Shiraishi, H.; Sasase, M.; Nakashima, A.S. Helicoid morphology of Arthrospira platensis NIES-39 confers temperature compensation in the longitudinal movement velocity of its trichomes. Phycology 2024, 4, 104–116. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à L’étude D’une Cyanophycée: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthèse de Spirulina Maxima (Setch. et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies affecting the growth and cultivation of genus Spirulina: An investigative review on current trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef] [PubMed]
- Edullantes, B.; Low-Decarie, E.; Steinke, M.; Cameron, T. Comparison of thermal traits between non-toxic and potentially toxic marine phytoplankton: Implications to their responses to ocean warming. J. Exp. Mar. Biol. Ecol. 2023, 562, 151883. [Google Scholar] [CrossRef]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016, 6, 38828. [Google Scholar] [CrossRef]
- Pollina, T.; Larson, A.G.; Lombard, F.; Li, H.; Le Guen, D.; Colin, S.; de Vargas, C.; Prakash, M. PlanktoScope: Affordable modular quantitative imaging platform for citizen oceanography. Front. Mar. Sci. 2022, 9, 949428. [Google Scholar] [CrossRef]
- Lombard, F. PlanktoScope Protocol for Plankton Imaging. Protocols.io 2022. Available online: https://www.protocols.io/view/planktoscope-protocol-for-plankton-imaging-bp2l6bq3zgqe/v4 (accessed on 15 January 2023).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package (Version 2.5-7) [R package], 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 1 March 2025).
- Almahrouqi, H.A.; Sukumaran, P.; Naqqiuddin, M.A.; Alsabahi, J.N.; Hishamuddin; Omar, M.E.; Ismail, A. The effect of salinity on growth, biochemical composition and fatty acid profile of Spirulina (Arthrospira platensis) grown in sheltered outdoor conditions in Oman. J. Algal Biomass Util. 2015, 6, 61–67. [Google Scholar]
- Hongsthong, A.; Sirijuntarut, M.; Prommeenate, P.; Thammathorn, S.; Bunnag, B.; Cheevadhanarak, S.; Tanticharoen, M. Revealing differentially expressed proteins in two morphological forms of Spirulina platensis by proteomic analysis. Mol. Biotechnol. 2007, 36, 123–130. [Google Scholar] [CrossRef]
- Russo, N.P.; Ballotta, M.; Usai, L.; Torre, S.; Giordano, M.; Fais, G.; Casula, M.; Dessì, D.; Nieri, P.; Damergi, E.; et al. Mixotrophic cultivation of Arthrospira platensis (Spirulina) under salt stress: Effect on biomass composition, FAME profile and phycocyanin content. Mar. Drugs 2024, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Vonshak, A. Effects of salinity stress on photosystem II function in cyanobacterial Spirulina platensis cells. Physiol. Plant. 2002, 114, 405–413. [Google Scholar] [CrossRef]
- Vonshak, A.; Kancharaksa, N.; Bunnag, B.; Tanticharoen, M. Role of light and photosynthesis on the acclimation process of the cyanobacterium Spirulina platensis to salinity stress. J. Appl. Phycol. 1996, 8, 119–124. [Google Scholar] [CrossRef]
- Sudhir, P.-R.; Pogoryelov, D.; Kovacs, L.; Garab, G.; Murthy, S.D.S. The effects of salt stress on photosynthetic electron transport and thylakoid membrane proteins in the cyanobacterium Spirulina platensis. BMB Rep. 2005, 38, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Kougia, E.; Arapoglou, D.; Chentir, I.; Andreou, V.; Tzovenis, I. Production of Arthrospira platensis: Effects on growth and biochemical composition of long-term acclimatization at different salinities. Bioengineering 2023, 10, 233. [Google Scholar] [CrossRef]
- Jeppesen, E.; Beklioğlu, M.; Özkan, K.; Akyürek, Z. Salinization increase due to climate change will have substantial negative effects on inland waters: A call for multifaceted research at the local and global scale. Innovation 2020, 1, 100030. [Google Scholar] [CrossRef]
- Nosratimovafagh, A.; Esmaeili Fereidouni, A.; Krujatz, F. Effect of light spectrum, salinity, and glucose levels on Spirulina morphology. J. World Aquacult. Soc. 2023, 54, 1274–1286. [Google Scholar] [CrossRef]
- Wu, D.; Wang, S.; Liu, K.; Yu, X.; He, Y.; Wang, Z. Rapid measurement of morphological features of Spirulina microalgae filaments using microscopy and image processing algorithms. Biosyst. Eng. 2012, 112, 35–41. [Google Scholar] [CrossRef]
- Welsh, D.T. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiol. Rev. 2000, 24, 263–290. [Google Scholar] [CrossRef]
- Hong, D.D.; Hoang, T.M.H.; Le, T.T.; Nguyen, C.H.; Le, A.H.; Ngo, T.H.T.; Nguyen, C.; Tang, D.Y.Y.; Show, P.L. Transcriptome analysis of Spirulina platensis sp. at different salinity and nutrient compositions for sustainable cultivation in Vietnam. Sustainability 2023, 15, 11906. [Google Scholar] [CrossRef]
- Vetoshkina, D.; Balashov, N.; Ivanov, B.; Ashikhmin, A.; Borisova-Mubarakshina, M. Light harvesting regulation: A versatile network of key components operating under various stress conditions in higher plants. Plant Physiol. Biochem. 2023, 194, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; Lee, Y.J.; Kwon, J.-H. Relationship between harvesting efficiency and filament morphology in Arthrospira platensis Gomont. Microorganisms 2025, 13, 367. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Kupriyanova, E.V.; Los, D.A. Spirulina/Arthrospira/Limnospira—Three names of the single organism. Foods 2024, 13, 2762. [Google Scholar] [CrossRef] [PubMed]
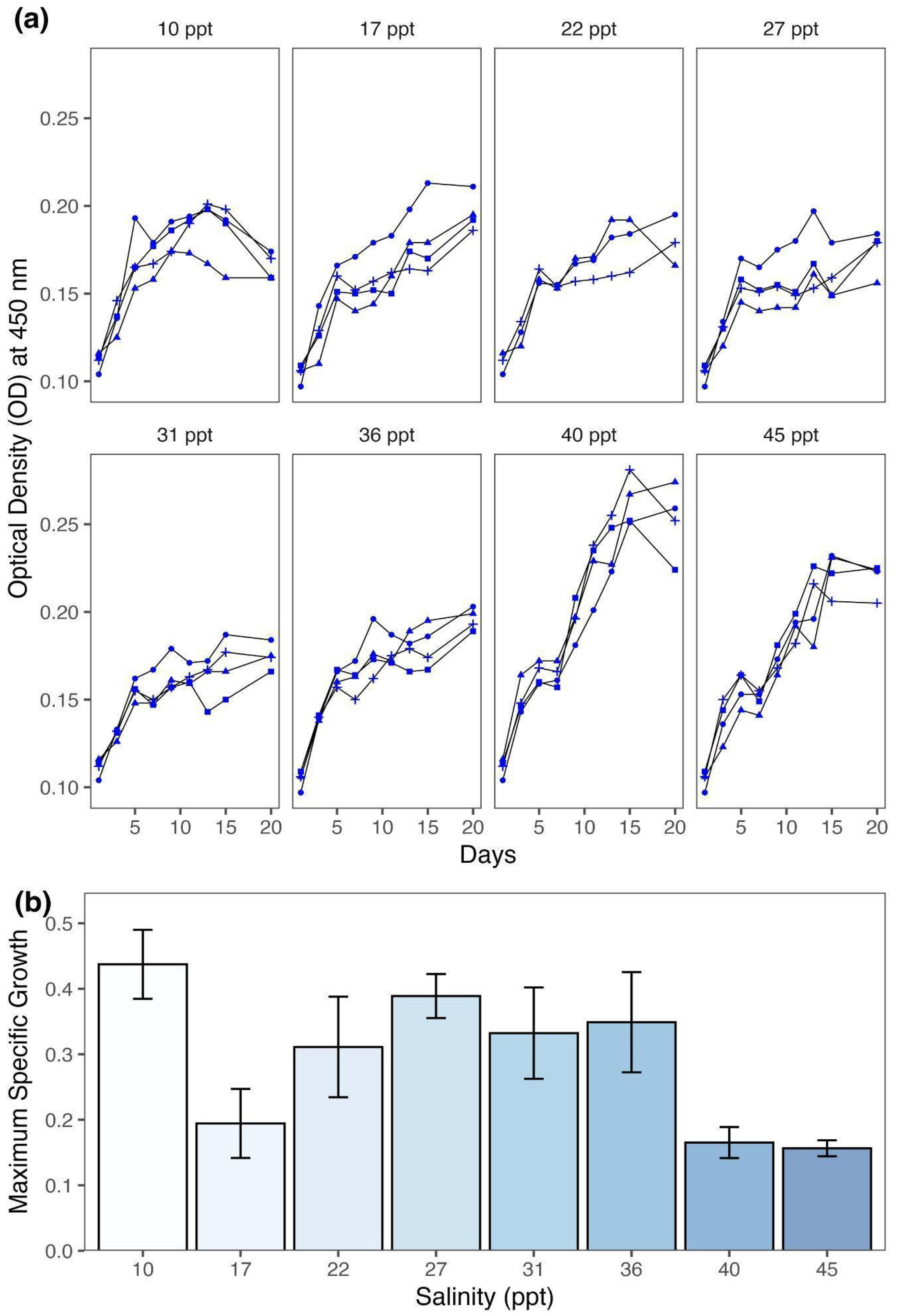

| Salinity Treatments | Growth Rate (r, d−1) | Carrying Capacity (K, a.u. 1) |
|---|---|---|
| 10 ppt | 0.44 ± 0.05 | 0.18 ± 0.01 |
| 17 ppt | 0.19 ± 0.05 | 0.20 ± 0.01 |
| 22 ppt | 0.31 ± 0.08 | 0.18 ± 0.01 |
| 27 ppt | 0.39 ± 0.03 | 0.16 ± 0.01 |
| 31 ppt | 0.33 ± 0.07 | 0.17 ± 0.01 |
| 36 ppt | 0.35 ± 0.08 | 0.19 ± 0.01 |
| 40 ppt | 0.17 ± 0.02 | 0.29 ± 0.01 |
| 45 ppt | 0.16 ± 0.01 | 0.24 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, I.K.M.; Edullantes, B. Growth, Productivity, and Size Structure of Spirulina Strain Under Different Salinity Levels: Implications for Cultivation Optimization. Phycology 2025, 5, 31. https://doi.org/10.3390/phycology5030031
Lao IKM, Edullantes B. Growth, Productivity, and Size Structure of Spirulina Strain Under Different Salinity Levels: Implications for Cultivation Optimization. Phycology. 2025; 5(3):31. https://doi.org/10.3390/phycology5030031
Chicago/Turabian StyleLao, Imma Krissalina M., and Brisneve Edullantes. 2025. "Growth, Productivity, and Size Structure of Spirulina Strain Under Different Salinity Levels: Implications for Cultivation Optimization" Phycology 5, no. 3: 31. https://doi.org/10.3390/phycology5030031
APA StyleLao, I. K. M., & Edullantes, B. (2025). Growth, Productivity, and Size Structure of Spirulina Strain Under Different Salinity Levels: Implications for Cultivation Optimization. Phycology, 5(3), 31. https://doi.org/10.3390/phycology5030031






