Abstract
Conservation units (CUs) play a fundamental role in maintaining and conserving biodiversity, and are important in preserving streams, reducing impacts from human activities and increasing water availability beyond the boundaries of the reserves. However, knowledge about the phytoplankton biodiversity of ecosystems in CUs is scarce. This study evaluated how environmental integrity alters microphytoplankton communities in extractive CUs and their surroundings in the southwestern Brazilian Amazon. Our results demonstrated that the streams exhibited distinct physicochemical and hydrological characteristics, representing spatially heterogeneous environments. Differences in habitat integrity values altered species composition in streams within and outside conservation units. Local beta diversity (LCBD) was negatively influenced by habitat integrity, indicating that sites with greater habitat integrity did not always present a greater number of unique species. The species Trachelomonas hispida, Gyrosigma scalproides and Spirogyra sp. were the ones that contributed the most to beta diversity. However, the phytoplankton species that contributed most to beta diversity were not always associated with streams with greater integrity, indicating that even environments that are less intact play a relevant role in maintaining species richness and beta diversity of microphytoplankton. Factors such as habitat integrity, pH, temperature and dissolved oxygen were the main influencers of microphytoplankton in the streams. Thus, the streams of both CUs and their surroundings, despite their physical–chemical and hydrological differences, effectively contribute to the high richness and beta diversity of regional microphytoplankton.
1. Introduction
Conservation units (CUs) are legally protected territorial spaces established with the aim of preserving the environment and ensuring the existence of viable habitats and ecosystems for different species [1,2]. These areas play an essential role in maintaining and conserving biodiversity, and the increase in the size of protected areas is directly associated with the growth of biological diversity [3,4,5]. Among the different types of CUs, extractive ones (Resex) stand out, which are characterized by the sustainable use of natural resources by traditional populations, reconciling the preservation of ecosystems with the maintenance of the ways of life of these communities [6]. Prioritizing areas for conservation requires an in-depth understanding of the interactions between biodiversity and ecosystem functioning [7].
Despite their invaluable support for biodiversity, rivers and streams are disappearing at an alarming rate throughout the world [8]. Streams are particularly vulnerable to human actions, since their small size makes it easy for them to be filled in, which in many cases causes their disappearance or profoundly alters their hydrological dynamics. These environments are highly influenced by the geological, morphological and surrounding landscape characteristics [9,10]. Streams have been significantly impacted by the degradation of riparian zones, the intensification of land use and mining activities, which alter the functioning regimes of these habitats [11,12]. These processes promote an increase in sediment load in water bodies, which compromises water quality and availability, in addition to negatively affecting ecological integrity and reducing biodiversity [13]. The environmental integrity of ecosystems is related to environmental heterogeneity, which consists of discontinuous distribution of biotic and abiotic factors in time and space [14,15].
The classic research of MacArthur (1960) [16] produced the hypothesis that heterogeneous environments will have an increase in species diversity. The greater the availability of habitats and resources that environments exhibit, the less competition there is between species and the more organisms can coexist [17]. The environmental integrity of Amazonian streams has been evaluated and is positively related to primary forest, canopy cover and riparian zone width, positively influencing species composition [18].
Knowledge of biodiversity in Brazilian protected areas is scarce [5]. For aquatic communities in streams within protected areas, there are some studies on insects and fish [19,20,21] and periphytic algae [22], but to date little is known about microphytoplankton in streams in protected areas. Microphytoplankton are composed of photosynthetic microorganisms with sizes ranging from 20 to 200 µm. These organisms are essential for the stability and functioning of aquatic ecosystems [23], and in small-order streams with moderate flows and hydrodynamic conditions with the presence of backwater areas, they can form microphytoplankton populations [24,25]. In these environments, in addition to the physical and chemical factors of the water, such as nutrients and temperature, the percentage of shading, channel width and water velocity are the main factors driving the dynamics of this community [25,26]. Furthermore, the availability of light in these ecosystems directly affects the survival of cells [27,28], and higher-order and wider channels tend to have higher primary productivity [29,30].
Among the ways to assess biodiversity, current measures such as the Local Beta Diversity Index (LCBD) and the contribution of individual species to beta diversity (SCBD [31]) have helped in understanding which environments have unique environmental conditions for maintaining regional biodiversity, and in determining the importance of certain species in global beta diversity [32]. It is known that beta diversity is positively driven by greater environmental heterogeneity [33,34,35]. Furthermore, generalist species with relatively broad niches and relatively high biovolumes or biomasses can contribute more to beta diversity, while rare species contribute less [36].
In accordance with the evidence that greater environmental heterogeneity leads to greater beta diversity [37,38] and greater species richness [39], this study sought to evaluate microphytoplankton communities in and around extractive CUs in the southwestern Brazilian Amazon. We specifically evaluated how the habitat integrity of Amazonian streams inside and outside CUs alters phytoplankton communities. We tested the following hypotheses: (i) Streams located inside CUs have a higher habitat integrity index when compared to streams outside the CUs. (ii) Habitat integrity (HII) alters the species composition of microphytoplankton, and streams with higher HII have a greater number of unique species when compared to streams with lower HII. (iii) Higher species biovolume values positively influence SCBD. (iv) Local beta diversity (LCBD) is positively influenced by HII. (v) Streams with higher HII values have greater species richness and lower biovolume.
2. Materials and Methods
2.1. Study Area
The study was conducted in 24 small-order streams located in the Cazumbá-Iracema and Chico Mendes extractive CUs in the southwestern Brazilian Amazon, and in adjacent areas (Figure 1). We investigated streams of orders 1 to 3 according to the classification of Vannote et al. (1980) [40]. The vegetation of these CUs is characterized by their forest typologies, with a predominance of dense ombrophilous forest and open ombrophilous forest [41]. The climate of the region has an “AFI” classification according to Köppen [42], influenced by rainy periods (November to June), where the maximum precipitation occurs in the summer with 285.9 mm, and dry periods (July to October), when the minimum precipitation is in the winter with 40.4 mm; temperatures vary from 15 °C in the autumn to 34.3 °C in the spring [43].
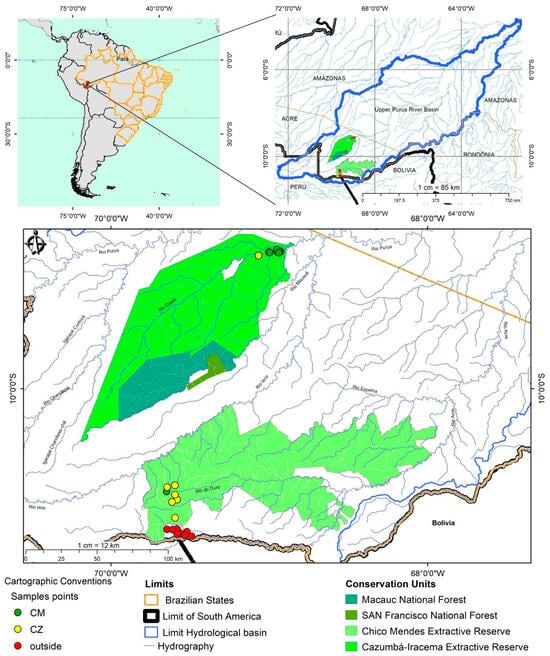
Figure 1.
Location map of the streams sampled in the Chico Mendes (CM) and Cazumbá (CZ) conservation units and in the surroundings of the CUs (outside).
2.2. Sampling
2.2.1. Environmental Variables
The sampling took place in August 2019 and August 2022, both during the dry season to avoid seasonal influences on the sampling. Moran’s spatial independence analysis [44] was applied to avoid spatial autocorrelation between the sampling points. We measured 4 limnological variables in the streams using a multiparameter probe (Horiba-U22): pH, temperature (°C), conductivity (µS/cm) and dissolved oxygen concentrations (mg/L O2—DO). Physical habitat variables such as channel morphology (length, channel depth, water flow), type of land use, riparian forest conservation, sediment retention and substrate characterization were also evaluated for each stream using the US Environmental Protection Agency assessment protocol [45]. These variables were used to calculate the habitat integrity index (HII). This index ranges from 0 to 1, with values close to 1 reflecting a more physically intact environment, while values close to 0 indicate environments with little physical integrity. This environmental integrity index allows us to measure the environmental heterogeneity of streams by quantifying the structural elements that make up the streams [18].
2.2.2. Microphytoplankton
Samples for analysis of the microphytoplankton community were collected from each of the 24 streams in the subsurface of the water in the limnetic region, 16 within the CUs and 8 in the surroundings. Since phytoplankton in streams is scarce, water samples from the streams were concentrated by filtering 100 L with a 20 µm plankton net and preserved with Transeau solution. Thus, in this study, only microplankton (20–200 µm) were analyzed, and the species composition did not include smaller organisms, such as small unicellular cyanobacteria and nanophytoplankton. Even when evaluating concentrated samples, the populations presented low population densities. In the laboratory, communities were quantified using random fields and their densities estimated according to the Utermöhl method [46] using an inverted microscope with 400× magnification and 2 to 5 mL sedimentation chambers. The sedimentation time was at least three hours for each centimeter of chamber height [47]. To estimate the density of microphytoplankton, the filtered volume of the initial sample was included in the estimate, and the final value of the count calculation was divided by 1000 (mL) and the result was expressed in ind/mL [48].
The biovolume of the species (mm3/L) was estimated by calculating the biovolume using geometric approximations [49]. To identify the species, we followed the classification system of van-de-Hock et al. (1995) [50] and used identification keys from specialized bibliographies on microalgae.
2.2.3. Data Analysis
To calculate the local contribution of each stream to beta diversity (LCBD) and which taxa contributed the most to beta diversity, we calculated the Species Contribution to Beta Diversity (SCBD) measure. To do this, the species biovolume matrix was first transformed by the Hellinger distance and then the total beta diversity (BDtotal) was calculated. The LCBD values for each stream and SCBD for each species were estimated from the decomposition of BDtotal. LCBD is a measure of the ecological uniqueness of the environment based on the community composition, providing a relative measure of the contribution of each site to beta diversity BDtotal, so that the sum of the LCBD indices results in 1. The contribution of each species to BDtotal is represented by the SCBD values showing the degree of variation of each species for all environments [31].
To identify patterns of variation in physical–chemical and hydrological parameters and in the habitat integrity index (HII), and to assess whether habitat integrity is greater in streams located in CUs (referring to our first hypothesis), we performed the following three steps: (1) First, we performed Multivariate Analysis of Variance (MANOVA). This technique was used due to the multivariate nature of the data, allowing us to simultaneously test the effect of the “stream” factor on a set of dependent environmental variables, verifying whether the streams are different from each other. (2) Next, to identify which environmental variables contributed significantly to the differences detected by MANOVA, ANOVAs were performed for each dependent variable separately (physical–chemical and hydrological) considering the streams as a categorical independent variable with more than two levels (Chico Mendes, Cazumbá, and outside the CUs). Before performing the test, we assessed the homogeneity of variance of the residuals using Levene’s test. (3) Subsequently, to visualize the spatial patterns of the environmental variables, we performed a principal component analysis (PCA) with the previously standardized data. The PCA included physical–chemical variables (temperature, dissolved oxygen, conductivity and pH), hydrological variables (depth and length) and HII.
To determine whether habitat integrity (HII) alters the species composition of microphytoplankton, referring to our second hypothesis, we performed a PERMANOVA [51] with the species biovolume matrix. We combined a PCoA to identify patterns of similarity between streams in terms of microphytoplankton composition. In these analyses, we used the habitat integrity index as the predictor and species composition as the response variable. For these analyses, the taxon biovolume matrix was previously standardized by the Hellinger method [52] and transformed into a Bray–Curtis distance matrix.
We performed a beta regression analysis [53] to verify whether the highest SCBD values are positively influenced by species biovolume and whether LCBD is positively influenced by HII, referring to our third and fourth hypotheses. This analysis is indicated for results with values in the range of 0 to 1. As explanatory habitat variables, we used the habitat integrity index (HII).
Finally, regarding our fifth hypothesis, to determine how species richness is affected by HII values and how HII affects biovolume, we used generalized linear models—GLMs. In addition, we modeled richness and biovolume as a function of temperature, dissolved oxygen, conductivity, pH, depth and width. In the error distribution of the models for biovolume, we used the Gaussian distribution (with log-transformed data), and for richness, the Poisson distribution [54] with the log-link function [55]. Before the beta regression and GLM analyses, we verified whether the assumptions were met through the patterns in the residuals (homogeneity and independence) and the multicollinearity between the explanatory variables. The best models were those with the lowest Akaike Information Criterion—AIC. The global Nagelkerke test was used to quantify the global chi-square and p-value values.
All analyses were performed using the R program, version 4.0.2 [56]. We used the “pca” function from the “vegan” package for PCA [57] and “manova” for MANOVA and “aov” for ANOVA. To calculate the distance matrix of environmental variables, we used the “vegdist” function and the “euclidian” method, and for the distance matrix of species, we used the same function with the “bray” method. To calculate the PCoA, we used the “pcoa” function from the “vegan” package [52]. For the Permanova analysis [58], we used the “adonis2” function and for BETADISPER [57], the “betadisper” function, both from the vegan package. The LCBD and SCBD indices were calculated using the “beta.div” function available from the “adespatial” package. To calculate beta regression, we used the “betareg” package [53]. GLM analyses were performed using the “glm” function from the stats package. The “nagelkerke” function was from the “rcompanion” package.
3. Results
3.1. Spatial Structure of Environmental Variables
The streams present average integrity index values of 0.535 (max = 0.875; min = 0.111). The streams are shallow with an average depth of 20 cm (max = 40 cm; min = 12 cm) and narrow with an average length of 1.7 m (max = 4.7 m; min = 0.6 m). Regarding the physical-chemical characteristics of the water, average pH values were found around 7.75 (max = 9; min = 6), indicating neutral-to-basic waters, and average temperatures of 21 °C (max = 30 °C; min = 14 °C). Low values of electrical conductivity of water with an average of 0.12 µS/cm (max = 0.362 µS/cm; min = 0.013 µS/cm) and dissolved oxygen concentrations with an average of 7 mg/L O2 (max = 11 mg/L O2; min = 2 mg/L O2) (see Table S1, Supplementary Materials).
Principal component analysis (PCA) revealed a clear separation of points based on environmental parameters, evidencing gradients associated with the habitat integrity index (HII) and physical–chemical and hydrological parameters (Figure 2). Axis 1 summarizes 33.5% of the total variation and is strongly correlated with conductivity, pH and depth, indicating that these parameters play significant roles in stream organization. Axis 2 summarizes 27.1% of the variation and is more associated with temperature and dissolved oxygen. Streams related to higher HII values (shades closer to blue in the gradient, Figure 2) were generally associated with higher temperatures (1 °C difference) and low conductivity. Streams with lower HII (purple tones) clustered according to high conductivity, depth and pH (Figure 2).
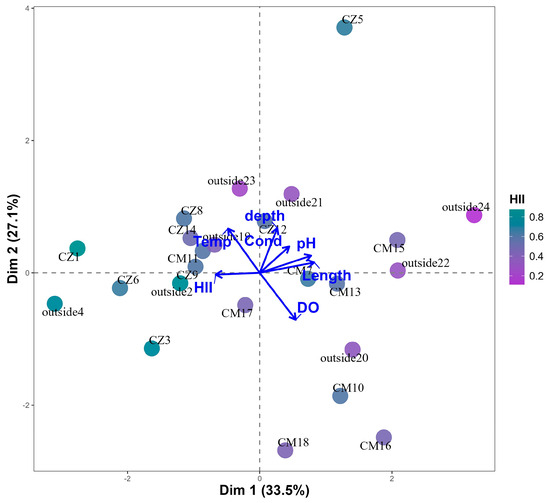
Figure 2.
Principal component analysis of the physical–chemical variables (temperature—Temp, dissolved oxygen—DO, conductivity—Cond, and pH), hydrological (depth–depth and width–length) and habitat integrity (HII) of the streams sampled in the Chico Mendes (CM) and Cazumbá (CZ) RESEX and surroundings.
The MANOVA results indicated statistically significant overall differences between the streams in relation to HII and the physica–-chemical and hydrological variables (Figure 3). Deeper streams were found outside the CUs (df = 21.951, p < 0.001). On the other hand, the streams located in the Cazumbá CU had the highest HII values (df = 21.951, p < 0.001), which also presented higher temperature values (df = 21.982, p = 0.001), while the Chico Mendes CU recorded the highest dissolved oxygen values (df = 22.972, p = 0.001).
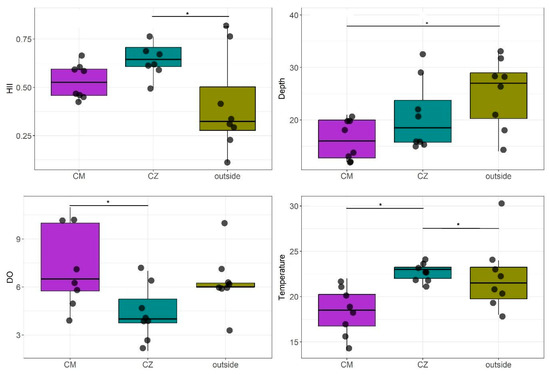
Figure 3.
Box-plots demonstrate the environmental variables that differed between streams sampled in the Chico Mendes (CM) and Cazumbá (CZ) RESEX and the surroundings (outside). Asterisk (*) represents statistically significant (α < 0.005).
3.2. Spatial Distribution of the Phytoplankton Community in Streams
We identified 67 phytoplankton taxa distributed among nine taxonomic classes. Zygnematophyceae was the main contributor to the total biovolume, representing 57% of the total observed, followed by Euglenophyceae with 30%. The other classes, Bacillariophyceae (12%), Chlorophyceae (0.69%), Coscinodiscophyceae (0.42%), Cyanobacteria (0.14%), Mediophyceae (0.07%), Trebouxiophyceae (0.01%) and Eustigmatophyceae (0.009%), had low contributions.
Regarding taxon richness, the Bacillariophyceae class presented the highest values with 22 taxa observed. The other classes presented the following species richness: Zygnematophyceae, with 19 taxa; Euglenophyceae, with 18 taxa; Cyanophyceae, with 10 taxa; and Chlorophyceae, with 5 taxa. Less representative classes, such as Coscinodiscophyceae, Mediophyceae and Trebouxiophyceae, recorded only 1 taxon each. The complete list of species can be found in the Supplementary Materials (Table S2).
Habitat integrity (HII) altered the species composition of microphytoplankton in streams within and outside CUs (PERMANOVA; R2 = 0.088; p = 0.035). The value of R2 = 0.088 indicates that habitat integrity explains 8.8% of the variation in microphytoplankton composition, suggesting that other environmental factors may also play an important role in structuring these communities.
Principal Coordinate Analysis (PCoA) applied to the species matrix demonstrated the distribution of streams inside and outside the CUs in a two-dimensional space based on species composition. Axes 1 and 2 summarize, respectively, 28.60% and 19.94% of the total variation. The PCoA results showed a separation between streams, with streams with high HII values (in green) separated from streams with low HII values (in purple, Figure 4). This indicates a relationship between HII and species composition, with sixteen species being exclusive to streams with HII greater than 0.51, nine species exclusive to streams with HII less than 0.50 and ten species present in both locations. Thus, the groups observed in the PCoA and the statistically significant differences in the PERMANOVA confirm that species composition was influenced by the habitat integrity gradient (Figure 4).
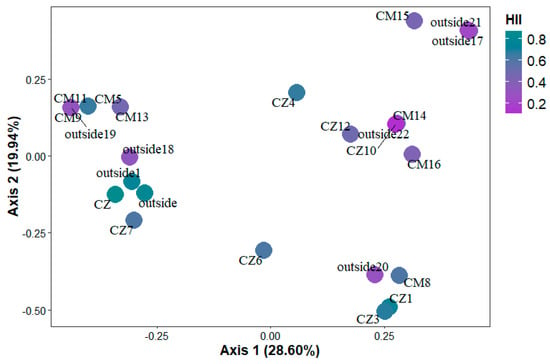
Figure 4.
Principal Coordinate Analysis (PCoA) showing the differences in the composition of microphytoplankton species between streams with different habitat integrity (HII) values. The color of the sample points represents the HII, ranging from purple tones (lower integrity) to green (higher integrity).
3.3. Contribution of Streams and Taxa to Beta Diversity
Total beta diversity (BDtotal) was high in our study (0.835 out of 1), with local contributions from sampling units (LCBD) ranging from 0.015 to 0.069. We found a negative influence of the habitat integrity index on LCBD (Figure 5). In addition, we observed a positive influence of water temperature, pH, conductivity and dissolved oxygen on LCBD values, highlighting the importance of these environmental variables in determining the local contribution to phytoplankton beta diversity.
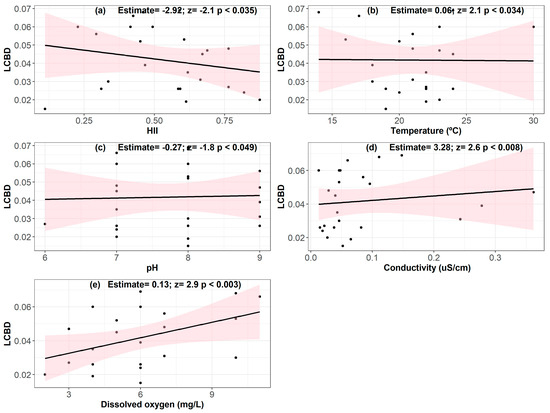
Figure 5.
Relationships between beta diversity (LCBD) and habitat integrity—HII (a). Other physical–chemical variables of the water: temperature (b), pH (c), conductivity (d) and dissolved oxygen (e). The black line indicates the central tendency of the relationship between the variables.
Regarding the contribution of species to beta diversity (SCBD), only three species presented values above the global average of all species (global average = 0.008; average of the three species = 0.164). These species belong to the following classes: Euglenophyceae (Trachelomonas hispida var. hispida), Bacillariophyceae (Gyrosigma scalproides (Rabenhorst) Cleve) and Zygnematophyceae (Spirogyra sp. Link, nom. cons.) (Figure 6a). These species present distinct distribution patterns and do not always occur in streams with the highest habitat integrity index (HII). T. hispida was recorded in 11 of the 24 streams studied, occurring in both high- and low-integrity streams. Spirogyra sp., in turn, was found in only three streams, all with a high integrity index. G. scalproides occurred in 12 streams, covering a wide range of integrity indices, from low to high. Regarding biovolume, we found a positive relationship between SCBD values and biovolume (Figure 6c). Spirogyra sp. is among the taxa with the greatest representation, occurring exclusively in the Chico Mendes CU. G. scalproides, with a lower biovolume compared to Spirogyra sp., also occurred exclusively, but in the Cazumbá CU. On the other hand, T. hispida was recorded in both CUs, presenting a slightly lower biovolume than G. scalproides (Figure 6b).
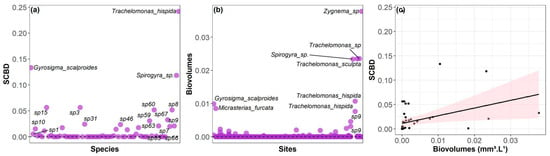
Figure 6.
Contributions of species to beta diversity (SCBD), highlighting the name of the species with the greatest contribution (a). Biovolumes of species between environments (b). Influence of biovolumes on SCBD (c). The black line indicates the central tendency of the relationship between the variables.
The interactions of environmental conditions on the phytoplankton community demonstrated by GLM indicated that microphytoplankton richness was negatively influenced by HII (Stimate = −1.619; z = −2.199 p = 0.027), while biovolumes were not influenced by physical–chemical and hydrological variables.
4. Discussion
Our results demonstrate that the physicochemical and hydrological differences between the streams of the CUs and their surroundings reflected the environmental heterogeneity of these areas, directly influencing the habitat integrity indices (HIIs). Streams with lower environmental integrity exhibited distinct physicochemical characteristics compared to those with higher HII, reinforcing the importance of these variables in distinguishing the analyzed environments. Habitat integrity was a determining factor for the differences in species composition, and streams with higher values presented lower LCBD and species richness. However, the species that contributed most to beta diversity (SCBD) were not always associated with streams with high HII, indicating that less intact environments also played a relevant role in maintaining species richness and phytoplankton beta diversity. Species with moderate biovolume levels were the ones that contributed most to SCBD. Thus, the streams of both CUs and their surroundings effectively contribute to high phytoplankton richness and beta diversity. Species composition was strongly influenced by habitat integrity.
4.1. Environmental Heterogeneity
We found spatial variability in the physical–chemical and hydrological conditions of the streams studied, with streams located within CUs presenting a higher habitat integrity index, confirming our first hypothesis. Furthermore, streams with greater habitat integrity showed higher levels of oxygenation, while those with lower integrity presented higher temperatures and depths. The highest HII values are related to the degree of preservation of their natural hydrological, physical and chemical characteristics, based on a reference environment, since this index evaluates environmental indicators such as canopy cover, bed material and interaction with organisms and disturbances due to human activities, among others [45]. Thus, the highest HII values of streams located within CUs reinforce the important role of preserved areas in stream conservation [59]. The highest dissolved oxygen values in streams with greater habitat integrity are good indicators of water quality [60]. High oxygen consumption in water or low dissolved oxygen values in streams occur when physical oxygenation processes, such as rapids and waterfalls, are absent. Under these conditions, slow water flow and high temperatures intensify the decomposition of organic matter by heterotrophic microorganisms, reducing the concentration of dissolved oxygen [61,62]. Oxygen consumption is higher in degraded streams with low habitat integrity levels, where there is a greater ingress of sediments, compromising water flow; this consumption tends to increase under conditions of contamination by domestic sewage and agricultural waste [63,64,65]. In our study, streams with lower integrity did indeed present higher temperatures and greater depths. The balance of the physical and chemical conditions of the water depends, to a large extent, on the conservation of riparian forests [66]. Canopy cover acts as a thermal regulator by shading the watercourse [67,68]; thus, streams that have had their marginal vegetation removed tend to present higher temperatures [69,70]. On the other hand, the greater depth of some streams, especially those located outside the CUs, may indicate preserved morphological conditions. This is because the decrease in depth in Amazonian streams is usually related to sediment deposition, often caused by the greater erodibility of the soils in the sedimentary basin and by intensive land use [71]. Thus, even deeper streams located outside the CUs can maintain good structural conditions of the drainage channel. Previous studies in Amazonian streams have reported similar patterns, where higher temperatures and depths were associated with areas with greater anthropic influence. These variables have been highlighted as determinants of environmental differences between preserved and altered streams [19,72].
4.2. Phytoplankton Community of Amazonian Streams
Variations in the habitat integrity index (HII) alter the species composition of microphytoplankton, and streams with higher HII have a greater number of exclusive species when compared to streams with lower HII, corroborating our second hypothesis. Streams with higher integrity have greater vegetation cover and higher oxygen values, while streams with lower HII have higher temperatures. These variables play a determining role in the structuring of phytoplankton communities [26,73]. We revealed that streams with lower habitat integrity indexes shared more species with other streams, while more intact environments contained exclusive species. These results reinforce that more intact environments tend to support more stable and specialized communities, while degraded environments may favor generalist or opportunistic species [74,75]. In our study, Trachelomonas hispida was a more frequent species. This species is a generalist in freshwater environments, less demanding of light and can survive on the bottom and in less oxygenated environments [76,77].
Despite this, streams with lower environmental integrity play a relevant role in maintaining beta diversity by contributing unique or sporadic species [24], such as Leibleinia gracilis, which presented low abundance and occurrence and which, according to the literature, is not widely distributed (SpeciesLink, accessed on 20 February 2025; http://www.splink.org.br). These results highlight the importance of preserving both environments with high integrity and those that are more impacted, as both contribute in a complementary way to regional biodiversity.
4.3. Contribution of Streams and Species to Beta Diversity
Regarding the SCBD results, higher values were positively influenced by the biovolumes of microphytoplankton species, confirming our third hypothesis. This indicates that species with higher biovolumes are contributing more to beta diversity, as also observed by other authors [32,36,78]. In our study, for example, Trachelomonas hispida var. hispida, Gyrosigma scalproides and Spirogyra sp., with high occurrence and intermediate biovolumes, were the taxa with higher SCBD values. This occurs because species that have high biovolume and vary between sites increase their dissimilarities in composition between communities and contribute more to beta diversity [36]. Microalgae species may indicate specific environmental conditions, such as light availability and hydrodynamics [79,80]. If these conditions vary between the sampled points, as in the present study, the species with higher biovolume may also vary, reinforcing beta diversity [21,81]. T. hispida, G. scalproides and Spirogyra sp. occurred in streams with higher habitat integrity indices, shallow and narrow, but which were sites with low LCBD. However, the occurrence of these taxa in streams with low contribution to beta diversity highlights that the structuring of beta diversity does not depend exclusively on the most abundant or dominant species, but also on the occurrence of rare species and unique combinations of taxa in certain sites [31,82,83].
The results related to our fourth hypothesis demonstrated that HII negatively influenced local beta diversity (LCBD), not corroborating our hypothesis. We expected that more intact environments would present a unique species composition that was different from other locations [31]. Similar studies indicate that high forest cover and HII were responsible for reductions in LCBD for diatom communities [24] and in the total beta diversity of periphytic algae in streams [84]. This suggests that site-specific conditions may play a crucial role in the high uniqueness of species. Streams with high levels of environmental integrity tend to have biological communities formed by well-adapted species with similar ecological niches. This reduces the uniqueness of species composition compared to less intact streams, where stressors can create unique conditions that favor the establishment of opportunistic or rare species [85]. On the other hand, streams with low habitat integrity often support generalist species that are tolerant to adverse conditions, often exclusive to these environments, which can increase LCBD values in these locations, even with lower environmental integrity [86,87]. Furthermore, lightly impacted environments, such as streams inside and outside CUs, can exhibit intermediate diversity, as proposed by the intermediate disturbance hypothesis [88], where moderate levels of disturbance can increase habitat heterogeneity and allow the coexistence of specialized and generalist species. Degraded streams can also present greater environmental heterogeneity, resulting from anthropogenic impacts, such as erosion, sediment deposition or pollution, which can create distinct microhabitats and support unique communities, contributing to increased LCBD [89,90]. Finally, in addition to local integrity, regional factors such as hydrological connectivity and metacommunity processes such as invasive species can obscure the relationship between habitat integrity and LCBD [91]. Although these factors favor species exchange between streams, reducing the influence of local conditions on beta diversity [92,93], we found significant influences of temperature, pH, conductivity and dissolved oxygen on LCBD. These explanations indicate that, although streams with high integrity are crucial for conservation, their contribution to beta diversity may not be exclusively associated with habitat quality, but also with complex ecological processes and interactions between local and regional factors.
4.4. Environmental Variables and Phytoplankton Communities
Our fifth hypothesis that streams with higher HII values have higher species richness and lower biovolumes was not confirmed. HII negatively affected species richness, and we found no association for biovolumes. The negative effect of HII on species richness can be explained by the fact that these environments have higher canopy cover [18], which in turn reduces light input [94]. Light energy is the main controller of photosynthetic efficiency for phytoplankton [95,96], and headwater streams are influenced by riparian vegetation that reduces autotrophic production [40], while in wider streams or streams where canopy cover is reduced, photosynthetic efficiency reaches 100% [97]. Thus, species richness is reduced because many phytoplankton species require light. In these streams, there was a high frequency of taxa physiologically adapted to low radiance, such as T. armata, which predominates in streams with 87% canopy cover.
Another important component of HII that may have contributed to this negative effect on phytoplankton richness is water velocity. More intact streams generally have higher current velocities, since the presence of riparian vegetation helps to contain the entry of sediments, and the water flow is maintained. When this vegetation is removed, which is reflected in lower HII values, there is an increase in sedimentation, which in turn reduces current velocity. The slope of the channel causes an increase in water flow and the drag force of the sediments causes abrasion capable of removing most of the plankton downstream, with the intensity of river discharge [98] and water turbulence [99] being determining components of phytoplankton groups. Thus, environments with higher current velocities did not favor the continuing presence of many phytoplankton species.
5. Conclusions
In this study, we contribute to the advancement of knowledge about how phytoplankton algae respond to environmental factors in streams of CUs. The streams sampled in the Amazon biome located in the Chico Mendes and Cazumbá Extractive CUs and their surroundings showed high spatial variability in their physical–chemical and hydrological conditions, indicating high environmental heterogeneity. This was reflected in the phytoplankton composition, beta diversity and species richness. We conclude that environmental integrity was an important variable in the distribution of species. Even streams with low habitat integrity values, mostly located outside the CUs, provide high local and species contribution to phytoplankton beta diversity. We emphasize that both types of streams, inside and outside the CUs, have high conservation value. It is important to highlight that these streams are poorly studied environments that are currently rapidly disappearing due to anthropogenic pressures. Thus, management strategies that prioritize ecological conservation and the restoration of degraded habitats are essential to ensure the maintenance of ecosystem services provided by phytoplankton, such as oxygen production and maintenance of the trophic chain, and by other aquatic organisms in conservation units.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/phycology5030030/s1, Table S1: Enviromental variability of: habitat integrity index (HII), Delpth conductivity, pH, dissolved oxygen and water temperature; Table S2: Complete list of taxa and their total biomass found in the streams of the Chico Mendes and Cazumbá Conservation Units (UCs) and in the streams outside them. Ordered from largest to smallest biomass.
Author Contributions
Methodology, I.G.d.S. and L.P.; Formal Analysis, I.G.d.S. and E.G.A.T.; Review and Editing, B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (funding number: 428961/2018-5) through the project “Diminuindo as lacunas Lineanas e Wallaceanas da biota aquática na Amazônia”; by Programa Nacional de Cooperação Acadêmica na Amazônia—PROCAD Amazônia (funding number: 88887.200518/2018-00), through the project “Efeito do uso e cobertura do solo sobre a biodiversidade e funções ecossistêmicas na Amazônia Sul-Ocidental”; and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001 and (funding number: 88881.145918/2017-01) through the project “Adaptação Climática e Conservação da Biodiversidade Brasileira Baseada em Modelos Climáticos Regionais”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank the Postgraduate Program in Ecology at the Federal University of Pará and the Primary Producer Ecology Laboratory—ECOPRO and the Aquatic Ecology and Tropical Aquaculture Laboratory at the Federal Rural University of the Amazon for the infrastructure and support. We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 (CAPES), which granted scholarships to Idelina Gomes da Silva and Leandra Palheta. We thank the Norsk Hydro company for financing the master’s research scholarship for Ellen Trindade. We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROCAD—Amazônia 2018. funding number: 88887.200518/2018-00) for financial support. We also thank ICMBio and all landowners and farmers for giving us permission and support to collect data in RESEX Chico Mendes and on their properties. We are grateful for the logistical and operational support provided by UFAC in the field.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The Biodiversity of Species and Their Rates of Extinction, Distribution, and Protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hoek, Y. The Potential of Protected Areas to Halt Deforestation in Ecuador. Environ. Conserv. 2017, 44, 124–130. [Google Scholar] [CrossRef]
- Montag, L.F.A.; Leão, H.; Benone, N.L.; Monteiro-Júnior, C.S.; Faria, A.P.J.; Nicacio, G.; Ferreira, C.P.; Garcia, D.H.A.; Santos, C.R.M.; Pompeu, P.S.; et al. Contrasting Associations between Habitat Conditions and Stream Aquatic Biodiversity in a Forest Reserve and Its Surrounding Area in the Eastern Amazon. Hydrobiologia 2019, 826, 263–277. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Alexandre, R.J.R.; Pena, S.A.; Correia, L.L.; Vieira, T.B. Conservation Gaps for Brazilian Bats, Limited Protection across Conservation Units and the Importance of the Indigenous Lands. Sci. Rep. 2024, 14, 23183. [Google Scholar] [CrossRef]
- Oliveira, U.; Soares-Filho, B.S.; Paglia, A.P.; Brescovit, A.D.; De Carvalho, C.J.B.; Silva, D.P.; Rezende, D.T.; Leite, F.S.F.; Batista, J.A.N.; Barbosa, J.P.P.P.; et al. Biodiversity Conservation Gaps in the Brazilian Protected Areas. Sci. Rep. 2017, 7, 9141. [Google Scholar] [CrossRef]
- Brasil Lei No 9.985; Lei n° 9.985 de 18 de Julho de 2000; Sistema Nacional de Unidades de Conservação: Brasília, Brasil, 2000.
- Gatiso, T.T.; Kulik, L.; Bachmann, M.; Bonn, A.; Bösch, L.; Freytag, A.; Heurich, M.; Wesche, K.; Winter, M.; Ordaz-Németh, I.; et al. Sustainable Protected Areas: Synergies between Biodiversity Conservation and Socioeconomic Development. People Nat. 2022, 4, 893–903. [Google Scholar] [CrossRef]
- Messager, M.L.; Lehner, B.; Cockburn, C.; Lamouroux, N.; Pella, H.; Snelder, T.; Tockner, K.; Trautmann, T.; Watt, C.; Datry, T. Global Prevalence of Non-Perennial Rivers and Streams. Nature 2021, 594, 391–397. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Katz, M.E.; Knoll, A.H.; Quigg, A.; Raven, J.A.; Schofield, O.; Taylor, F.J.R. The Evolution of Modern Eukaryotic Phytoplankton. Science 2004, 305, 354–360. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Bazova, M.M.; Dinu, M.I.; Gashkina, N.A.; Kudryavtseva, L.P. Changes in the Geochemistry of Land Waters at Climate Warming and a Decrease in Acid Deposition: Recovery of the Lakes or Their Evolution? Geochem. Int. 2022, 60, 685–701. [Google Scholar] [CrossRef]
- Ferreira, V.R.S.; Cunha, E.J.; Calvão, L.B.; Luiza-Andrade, A.; de Resende, B.O.; de Carvalho, F.G.; Bomfim, F.d.F.; Fares, A.L.B.; Cabral, G.S.; Lima, M.; et al. Amazon Streams Impacted by Bauxite Mining Present Distinct Local Contributions to the Beta Diversity of Aquatic Insects, Fish, and Macrophytes. Sci. Total Environ. 2024, 955, 177292. [Google Scholar] [CrossRef]
- Bomfim, F.F.; Fares, A.L.B.; Melo, D.G.L.; Vieira, E.; Michelan, T.S. Land Use Increases Macrophytes Beta Diversity in Amazon Streams by Favoring Amphibious Life Forms Species. Community Ecol. 2023, 24, 159–170. [Google Scholar] [CrossRef]
- de Paiva, C.K.S.; de Faria, A.P.J.; Calvão, L.B.; Juen, L. Effect of Oil Palm on the Plecoptera and Trichoptera (Insecta) Assemblages in Streams of Eastern Amazon. Environ. Monit. Assess. 2017, 189, 393. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Reynolds, J.F. FORUM and Quantification on Definition. Oikos 1995, 73, 280–284. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity Organisation, Spatial Extent and Dispersal in Aquatic Systems: Patterns, Processes and Prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- MacArthur, R. On the Relative Abundance of Species. Am. Nat. 1960, 94, 25–36. [Google Scholar] [CrossRef]
- Cardinale, B.J. Biodiversity Improves Water Quality through Niche Partitioning. Nature 2011, 472, 86–91. [Google Scholar] [CrossRef]
- Nessimian, J.L.; Venticinque, E.M.; Zuanon, J.; De Marco, P.; Gordo, M.; Fidelis, L.; D’arc Batista, J.; Juen, L. Land Use, Habitat Integrity, and Aquatic Insect Assemblages in Central Amazonian Streams. Hydrobiologia 2008, 614, 117–131. [Google Scholar] [CrossRef]
- Espinosa, A.C.E.; Cunha, E.J.; Shimano, Y.; Rolim, S.; Mioli, L.; Juen, L.; Dunck, B. Functional Diversity of Mayflies (Ephemeroptera, Insecta) in Streams in Mining Areas Located in the Eastern Amazon. Hydrobiologia 2023, 850, 929–945. [Google Scholar] [CrossRef]
- Alves-Martins, F.; Calatayud, J.; Medina, N.G.; De Marco, P.; Juen, L.; Hortal, J. Drivers of Regional and Local Diversity of Amazonian Stream Odonata. Insect Conserv. Divers. 2019, 12, 251–261. [Google Scholar] [CrossRef]
- Leão, H.; Siqueira, T.; Raiol, N.; Fogaça, L.; Montag, D.A. Ecological Uniqueness of Fi Sh Communities from Streams in Modi Fi Ed Landscapes of Eastern Amazonia. Ecol. Indic. 2020, 111, 106039. [Google Scholar] [CrossRef]
- França, A.A.; Dunck, B.; Rodrigues, L.; Fonseca, B.M.; Felisberto, S.A. Periphytic Diatoms (Bacillariophyta) in Streams from Three Conservation Units of Central Brazil: Pinnularia Ehrenberg. Hoehnea 2017, 44, 524–538. [Google Scholar] [CrossRef]
- Kruk, C. Classification Schemes for Phytoplankton: A Local Validation of a Functional Approach to the Analysis of Species Temporal Replacement. J. Plankton Res. 2002, 24, 901–912. [Google Scholar] [CrossRef]
- Schneck, F.; Bini, L.M.; Melo, A.S.; Petsch, D.K.; Saito, V.S.; Wengrat, S.; Siqueira, T. Catchment Scale Deforestation Increases the Uniqueness of Subtropical Stream Communities. Oecologia 2022, 199, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Xing, R.; Huang, B.; Cheng, X.; Shi, W.; Liu, S. Phytoplankton in Headwater Streams: Spatiotemporal Patterns and Underlying Mechanisms. Front. Plant Sci. 2023, 14, 1276289. [Google Scholar] [CrossRef]
- Piirsoo, K.; Vilbaste, S.; Truu, J.; Pall, P.; Trei, T.; Tuvikene, A.; Viik, M. Origin of Phytoplankton and the Environmental Factors Governing the Structure of Microalgal Communities in Lowland Streams. Aquat. Ecol. 2007, 41, 183–194. [Google Scholar] [CrossRef]
- Borics, G.; Tóthmérész, B.; Lukács, B.A.; Várbíró, G. Functional Groups of Phytoplankton Shaping Diversity of Shallow Lake Ecosystems. Hydrobiologia 2012, 698, 251–262. [Google Scholar] [CrossRef]
- Schwaderer, A.S.; Yoshiyama, K.; De Tezanos Pinto, P.; Swenson, N.G.; Klausmeier, C.A.; Litchman, E. Eco-Evolutionary Differences in Light Utilization Traits and Distributions of Freshwater Phytoplankton. Limnol. Oceanogr. 2011, 56, 589–598. [Google Scholar] [CrossRef]
- Lampert, W.; Sommer, U. Limnoecology: The Ecology of Lakes and Streams. J. Plankton Res. 2008, 30, 489–490. [Google Scholar] [CrossRef]
- Reynolds, C.S. Hydroecology of River Plankton the Role of Variability in Channel Flow. Hydrol. Process. 2000, 14, 3119–3132. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta Diversity as the Variance of Community Data: Dissimilarity Coefficients and Partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Heino, J.; Grönroos, M. Exploring Species and Site Contributions to Beta Diversity in Stream Insect Assemblages. Oecologia 2017, 183, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rolls, R.J.; Deane, D.C.; Johnson, S.E.; Heino, J.; Anderson, M.J.; Ellingsen, K.E. Biotic Homogenisation and Differentiation as Directional Change in Beta Diversity: Synthesising Driver—Response Relationships to Develop Conceptual Models across Ecosystems. Biol. Rev. 2023, 98, 1388–1423. [Google Scholar] [CrossRef] [PubMed]
- Olden, J.D. Biotic Homogenization: A New Research Agenda for Conservation Biogeography. J. Biogeogr. 2006, 33, 2027–2039. [Google Scholar] [CrossRef]
- Petsch, D.K. Causes and Consequences of Biotic Homogenization in Freshwater Ecosystems. Int. Rev. Hydrobiol. 2016, 101, 113–122. [Google Scholar] [CrossRef]
- Brito, M.T.d.S.; Heino, J.; Pozzobom, U.M.; Landeiro, V.L. Ecological Uniqueness and Species Richness of Zooplankton in Subtropical Floodplain Lakes. Aquat. Sci. 2020, 82, 43. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The Metacommunity Concept: A Framework for Multi-Scale Community Ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M.; Bozelli, R.L. Floods Increase Similarity among Aquatic Habitats in River-Floodplain Systems. Hydrobiologia 2007, 579, 1–13. [Google Scholar] [CrossRef]
- Ortega, J.C.G.; Thomaz, S.M.; Bini, L.M. Experiments Reveal That Environmental Heterogeneity Increases Species Richness, but They Are Rarely Designed to Detect the Underlying Mechanisms. Oecologia 2018, 188, 11–22. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- IBGE. Vegetação Por Estado Acre. Available online: https://www.ibge.gov.br/geociencias/informacoes-ambientais/vegetacao/22460-vegetacao-por-estado.html (accessed on 7 April 2025).
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classificatio. Permafr. Periglac. Process. 2007, 13, 1633–1644. [Google Scholar] [CrossRef]
- INPE. Instituto Nacional de Pesquisas Espaciais—INPE. Available online: https://www.gov.br/inpe/pt-br (accessed on 10 December 2024).
- Moran, P.A.P. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef]
- Peck, D.V.; Herlihy, A.T.; Hill, B.H.; Hughes, R.M.; Kaufmann, P.R.; Klemm, D.J.; Lazorchak, J.M.; McCormick, F.H.; Peterson, S.A.; Ringold, P.L.; et al. Environmental Monitoring and Assessment Program-Surface Waters Western Pilot Study: Field Operations Manual for Wadeable Streams; United States Environmental Protection Agency: Washington, DC, USA, 2006.
- Utermöhl, H. Zur Vervollkommnung Der Quantitativen Phytoplankton-Methodik. Int. Ver. Theor. Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Lund, J.; Kipling, C.; Le Cren, E. The Inverted Microscope Method of Estimating Algal Numbers and the Statistical Basis of Estimations by Counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- APHA Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association; American Water Works Association: Washington, DC, USA, 2005.
- Hillebrand, H. Biovolume Calculation for Palagic and Benthic Microalgae. J. Phycol. 1999, 424, 403–424. [Google Scholar] [CrossRef]
- van den Hoek, C.; Mann, D.G. Algae. An Introduction to Phycology; Cambridge University Press: Cambridge, UK, 1995; ISBN 0-521-30419-9. [Google Scholar]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Nielsen, S.N. Numerical Ecology. Legendre P. and Legendre L., second ed., Elsevier, Amsterdam, p. 853, 1998. Ecol. Modell. 2000, 132, 303–304. [Google Scholar] [CrossRef]
- Zeileis, A.; Cribari-Neto, F.; Gruen, B.; Kosmidis, I.; Simas, A.B.; Rocha, A.V. Betareg: Beta Regression. 2013. Available online: https://cran.r-project.org/web/packages/betareg/index.html (accessed on 20 February 2022).
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Effect of Boundary Layer Conductance on the Response of Stomata to Humidity. Ecol. Modell. 2002, 8, 89–100. [Google Scholar] [CrossRef]
- Sackett, L.C. Does the Host Matter? Variable Influence of Host Traits on Parasitism Rates. Int. J. Parasitol. 2018, 48, 27–39. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020.
- Oksanen, J. Vegan: Ecological Diversity. R Proj. 2013, 368, 1–11. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- de Souza, J.C.; Cana Verde, B.S.; de Santana, R.O.; da Silva, D.M.L. Importance of Conservation Units in the Biogeochemistry of Cerrado Streams. J. South Am. Earth Sci. 2024, 135, 104803. [Google Scholar] [CrossRef]
- Brasil. Resolução CONAMA No 357. 2005; pp. 58–63. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450 (accessed on 10 February 2022).
- Wang, F.; Tian, S.; Yan, W. Unveiling the Temporal Variability of Gas Transfer Coefficients of Streams Based on High-Frequency Dissolved Oxygen Measurements. Environ. Res. 2024, 262, 119939. [Google Scholar] [CrossRef]
- Sø, J.S.; Kragh, T.; Sand-Jensen, K.; Martinsen, K.T. Environmental Drivers and Sources of Stream Oxygen Consumption in an Agricultural Lake Catchment. Ecol. Eng. 2022, 176, 106516. [Google Scholar] [CrossRef]
- Cooper, C.M. Biological Effects of Agriculturally Derived Surface Water Pollutants on Aquatic Systems—A Review. J. Environ. Qual. 1993, 22, 402–408. [Google Scholar] [CrossRef]
- Kumaraswamy, T.R.; Javeed, S.; Javaid, M.; Naika, K. Impact of Pollution on Quality of Freshwater Ecosystems. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 69–81. [Google Scholar] [CrossRef]
- Sierra, M.V.; Gomez, N. Structural Characteristics and Oxygen Consumption of the Epipelic Biofilm in Three Lowland Streams Exposed to Different Land Uses. Water Air Soil Pollut. 2007, 186, 115–127. [Google Scholar] [CrossRef]
- Castello, L.; Mcgrath, D.G.; Hess, L.L.; Coe, M.T.; Lefebvre, P.A.; Petry, P.; Macedo, M.N.; Ren, V.F.; Arantes, C.C. The Vulnerability of Amazon Freshwater Ecosystems. Conserv. Lett. 2013, 217–229. [Google Scholar] [CrossRef]
- Blinn, D.W.; Bailey, P.C.E. Land-Use Influence on Stream Water Quality and Diatom Communities in Victoria, Australia: A Response to Secondary Salinization. Hydrobiologia 2001, 466, 231–244. [Google Scholar] [CrossRef]
- Allan, J.D.; Arbor, A.; Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2012, 35, 257–284. [Google Scholar] [CrossRef]
- Bunn, S.E.; Davies, P.M.; Mosisch, T.D. Ecosystem Measures of River Health and Their Response to Riparian and Catchment Degradation. Freshw. Biol. 1999, 41, 333–345. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Pedersen, N.L. Differences in Temperature, Organic Carbon and Oxygen Consumption among Lowland Streams. Freshw. Biol. 2005, 50, 1927–1937. [Google Scholar] [CrossRef]
- Larkin, Z.T.; Ralph, T.J.; Tooth, S.; Fryirs, K.A.; Carthey, A.J.R. Identifying Threshold Responses of Australian Dryland Rivers to Future Hydroclimatic Change. Sci. Rep. 2020, 10, 6653. [Google Scholar] [CrossRef]
- Silva, K.; Joás, S.; Brito, S.; Martins, G.; Rafael, C.; Bastos, C.; Camilo, C.; Penagos, M.; Silva, E.; Montag, L.; et al. Odonata Diversity and Ecological Thresholds in Protected Areas of the Brazilian Amazon. Neotrop. Entomol. 2025, 54, 1–14. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.L.; Kim, H.S. Phytoplankton Functional Groups as Indicators of Environmental Changes in Weir and Non-Weir Sections of the Lower Nakdong River, Republic of Korea. Heliyon 2024, 10, e22966. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Bini, L.M. Reconceptualising the Beta Diversity-Environmental Heterogeneity Relationship in Running Water Systems. Freshw. Biol. 2015, 60, 223–235. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater Biodiversity Conservation: Recent Progress and Future Challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a Functional Classification of the Freshwater Phytoplankton. J. Plankt. Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Crossetti, L.O.; Bicudo, C.E.d.M. Adaptations in Phytoplankton Life Strategies to Imposed Change in a Shallow Urban Tropical Eutrophic Reservoir, Garças Reservoir, over 8 Years. Hydrobiologia 2008, 614, 91–105. [Google Scholar] [CrossRef]
- De, K.; Dey, D.; Shruti, M.; Uniyal, V.P.; Adhikari, B.S.; Johnson, J.A.; Hussain, S.A. β-Diversity of Odonate Community of the Ganga River: Partitioning and Insights from Local and Species Contribution. Wetl. Ecol. Manag. 2023, 31, 899–912. [Google Scholar] [CrossRef]
- Amorim, C.A.; Moura, A.d.N. Ecological Impacts of Freshwater Algal Blooms on Water Quality, Plankton Biodiversity, Structure, and Ecosystem Functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef]
- Fernández-González, C.; Tarran, G.A.; Schuback, N.; Woodward, E.M.S.; Arístegui, J.; Marañón, E. Phytoplankton Responses to Changing Temperature and Nutrient Availability Are Consistent across the Tropical and Subtropical Atlantic. Commun. Biol. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Victorero, L.; Robert, K.; Robinson, L.F.; Taylor, M.L.; Huvenne, V.A.I. Species Replacement Dominates Megabenthos Beta Diversity in a Remote Seamount Setting. Sci. Rep. 2018, 8, 4152. [Google Scholar] [CrossRef]
- Perez Rocha, M.; Morris, T.J.; Cottenie, K.; Schwalb, A.N. Limitations of Beta Diversity in Conservation Site Selection. Ecol. Indic. 2023, 154, 110732. [Google Scholar] [CrossRef]
- Hill; Wood, P.J.; White, J.C.; Thornhill, I.; Fairchild, W.; Williams, P.; Nicolet, P.; Biggs, J. Environmental Correlates of Aquatic Macroinvertebrate Diversity in Garden Ponds: Implications for Pond Management. Insect Conserv. Divers. 2024, 17, 374–385. [Google Scholar] [CrossRef]
- Trindade, E.G.A.; Dunck, B. Environmental Preservation Leads to Greater Beta Diversity of Periphytic Algae in Amazonian Streams. Limnologica 2025, 110, 126221. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide Decline of Specialist Species: Toward a Global Functional Homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response Diversity, Ecosystem Change, and Resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Schindler, D.W. Experimental Perturbations of Whole Lakes as Tests of Hypotheses Concerning Ecosystem Structure and Function. Oikos 1990, 57, 25. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Agra, J.; Ligeiro, R.; Heino, J.; Macedo, D.R.; Castro, D.M.P.; Linares, M.S.; Callisto, M. Anthropogenic Disturbances Alter the Relationships between Environmental Heterogeneity and Biodiversity of Stream Insects. Ecol. Indic. 2021, 121, 107079. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Brian, D.; Sparks, R.E.; Stromberg, J.C.; Poff, N.L.; Allan, J.D.; et al. A Paradigm for River Conservation and Restoration. Bioscience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Gavioli, A.; Milardi, M.; Soininen, J.; Soana, E.; Lanzoni, M.; Castaldelli, G. How Does Invasion Degree Shape Alpha and Beta Diversity of Freshwater Fish at a Regional Scale? Ecol. Evol. 2022, 12, e9493. [Google Scholar] [CrossRef]
- Larned, S.T.; Datry, T.; Arscott, D.B.; Tockner, K. Emerging Concepts in Temporary-River Ecology. Freshw. Biol. 2010, 55, 717–738. [Google Scholar] [CrossRef]
- Lopes, P.M.; Bini, L.M.; Declerck, S.A.J.; Farjalla, V.F.; Vieira, L.C.G.; Bonecker, C.C.; Lansac-Toha, F.A.; Esteves, F.A.; Bozelli, R.L. Correlates of Zooplankton Beta Diversity in Tropical Lake Systems. PLoS ONE 2014, 9, e109581. [Google Scholar] [CrossRef]
- Kirk, J.T.O. The Upwelling Light Stream in Natural Waters. Limnol. Oceanogr. 1989, 34, 1410–1425. [Google Scholar] [CrossRef]
- Portalier, S.M.J.; Cherif, M.; Zhang, L.; Fussmann, G.F.; Loreau, M. Size-Related Effects of Physical Factors on Phytoplankton Communities. Ecol. Modell. 2016, 323, 41–50. [Google Scholar] [CrossRef]
- Dantas, Ê.W.; Bittencourt-Oliveira, M.d.C.; Moura, A.d.N. Dynamics of Phytoplankton Associations in Three Reservoirs in Northeastern Brazil Assessed Using Reynolds’ Theory. Limnologica 2012, 42, 72–80. [Google Scholar] [CrossRef]
- Hill, W.R.; Fanta, S.E.; Roberts, B.J. Quantifying Phosphorus and Light Effects in Stream Algae. Limnol. Oceanogr. 2009, 54, 368–380. [Google Scholar] [CrossRef]
- Pannard, A.; Minaudo, C.; Leitao, M.; Abonyi, A.; Moatar, F.; Gassama, N. Meroplanktic Phytoplankton Play a Crucial Role in Responding to Peak Discharge Events in the Middle Lowland Section of the Loire River (France). Hydrobiologia 2023, 851, 869–895. [Google Scholar] [CrossRef]
- Margalef, R. Life-Forms of Phytoplankton as Survival Alternatives in an Unstable Environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).