Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes
Abstract
:1. Introduction
2. Materials and Methods
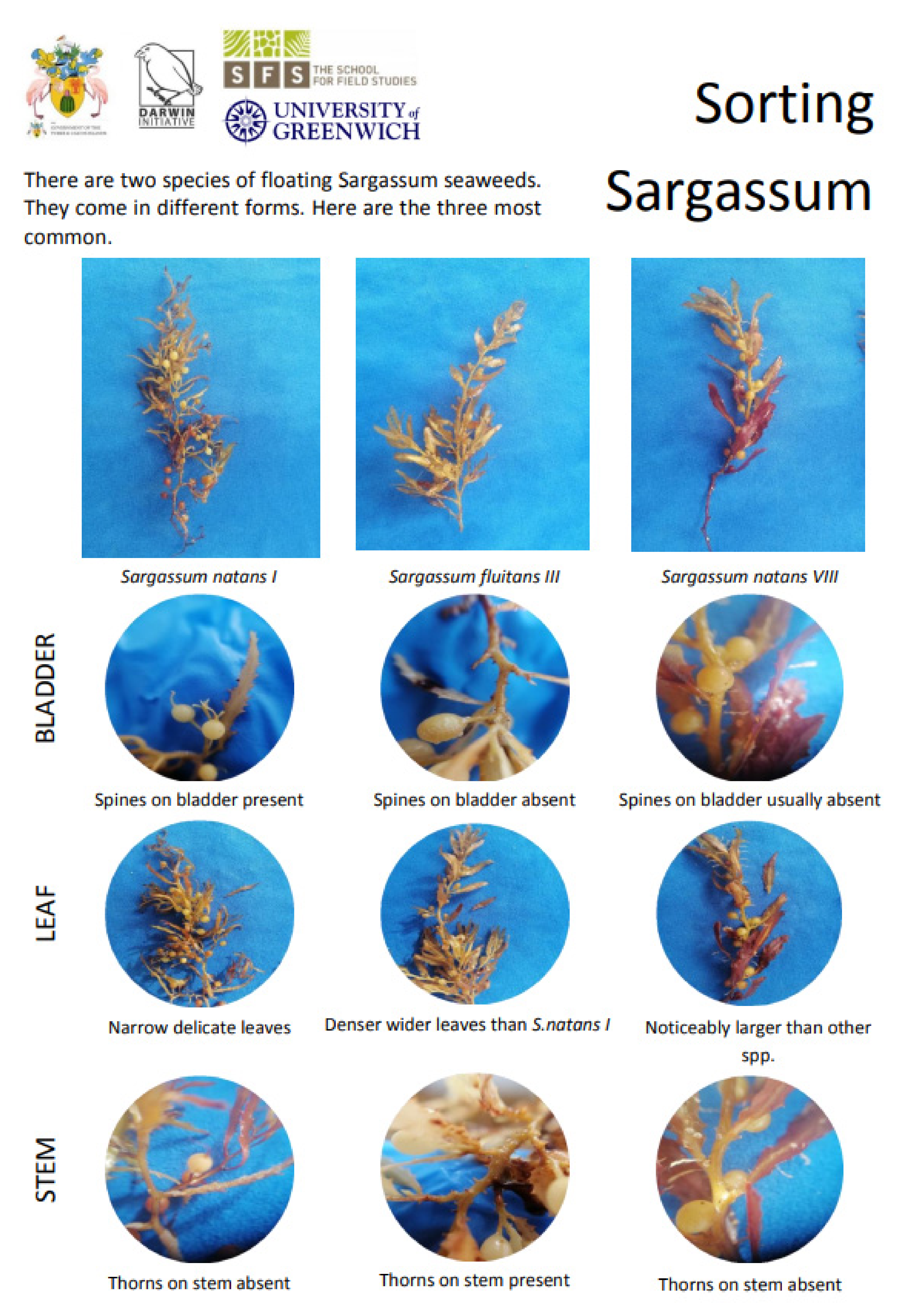
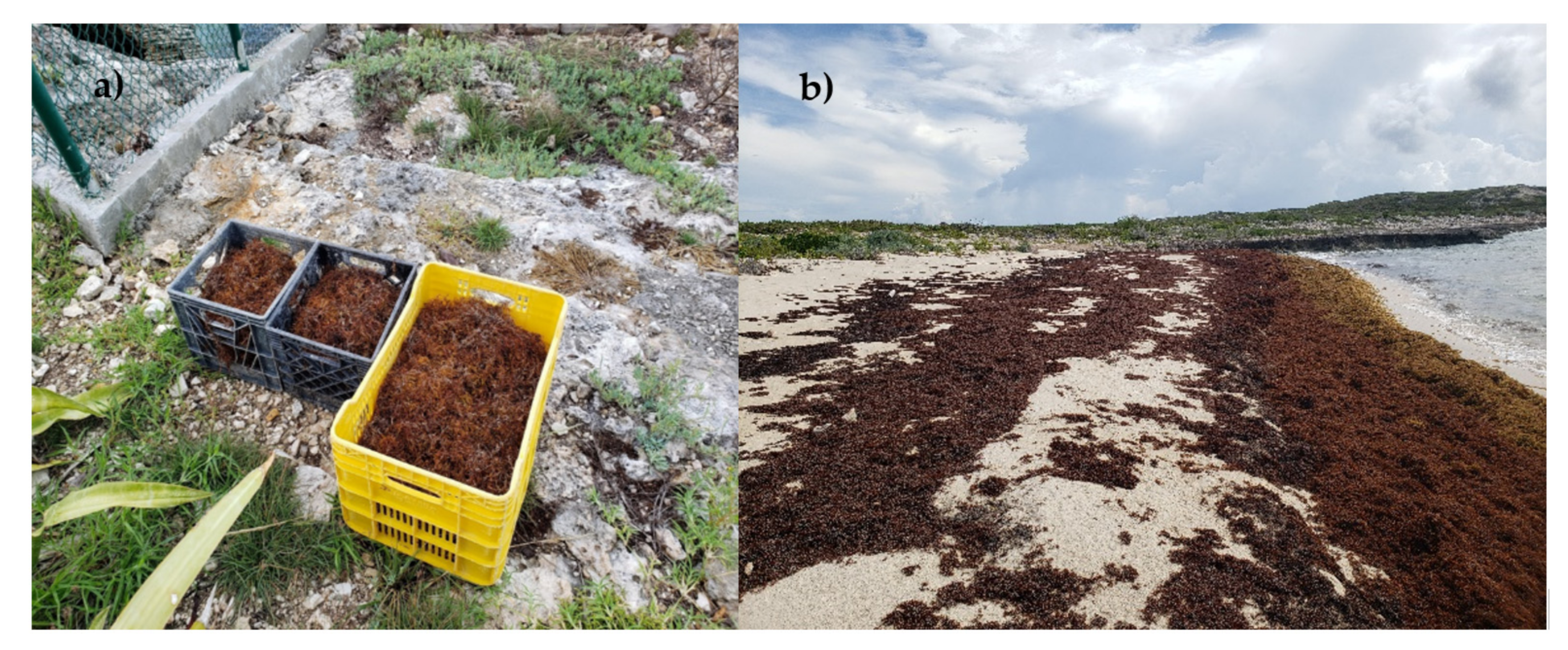
2.1. Sample Collection and Preparation
2.2. Compositional Analyses
2.2.1. Moisture
2.2.2. Ash
2.2.3. Carbon, Hydrogen, Nitrogen (CHN) and Protein Content
2.2.4. Higher Heating Value
2.2.5. Total Lipid Content
2.2.6. Phenolic Content
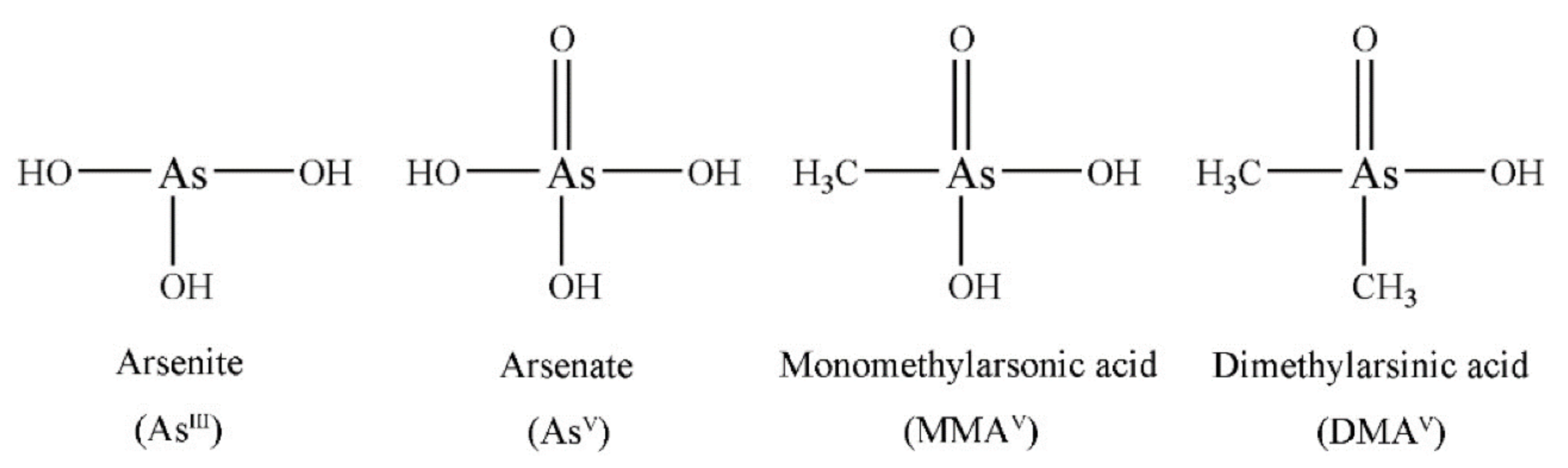
2.2.7. Arsenic and Heavy Metals
2.3. Methane Potential
2.3.1. Theoretical Methane Potential
2.3.2. Methane Potential Determination
2.3.3. Statistical Analysis
3. Results
3.1. Composition
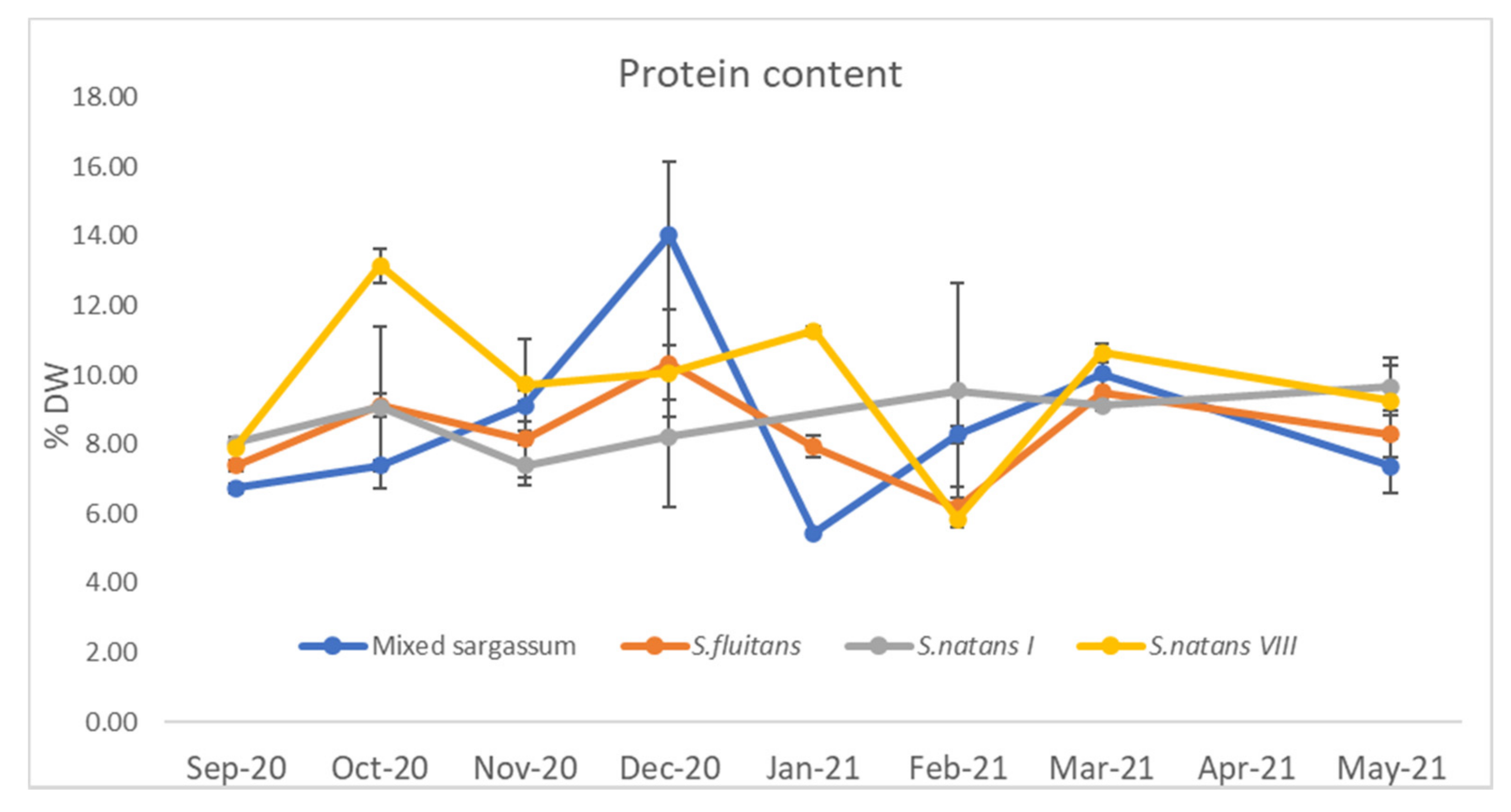
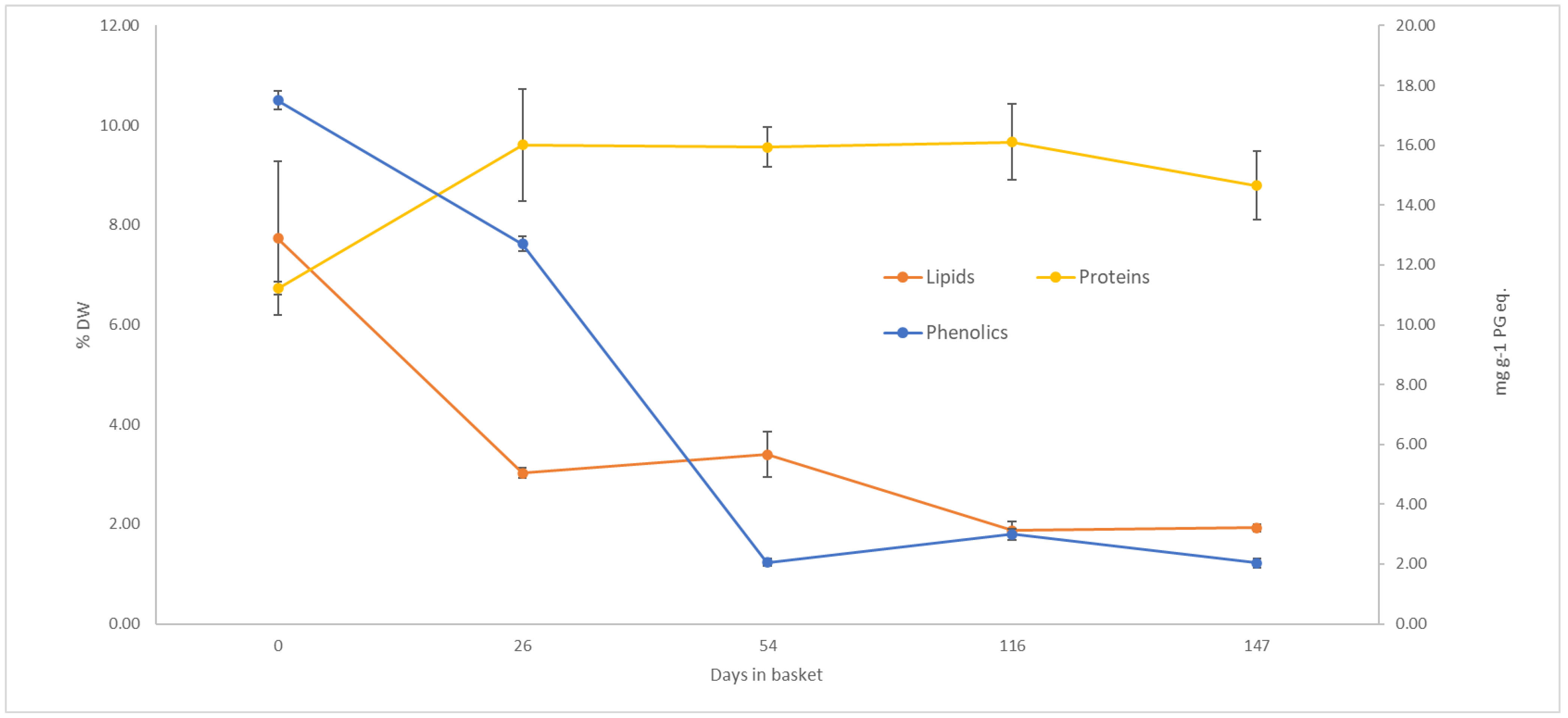
3.1.1. Protein Content
3.1.2. Lipid Content
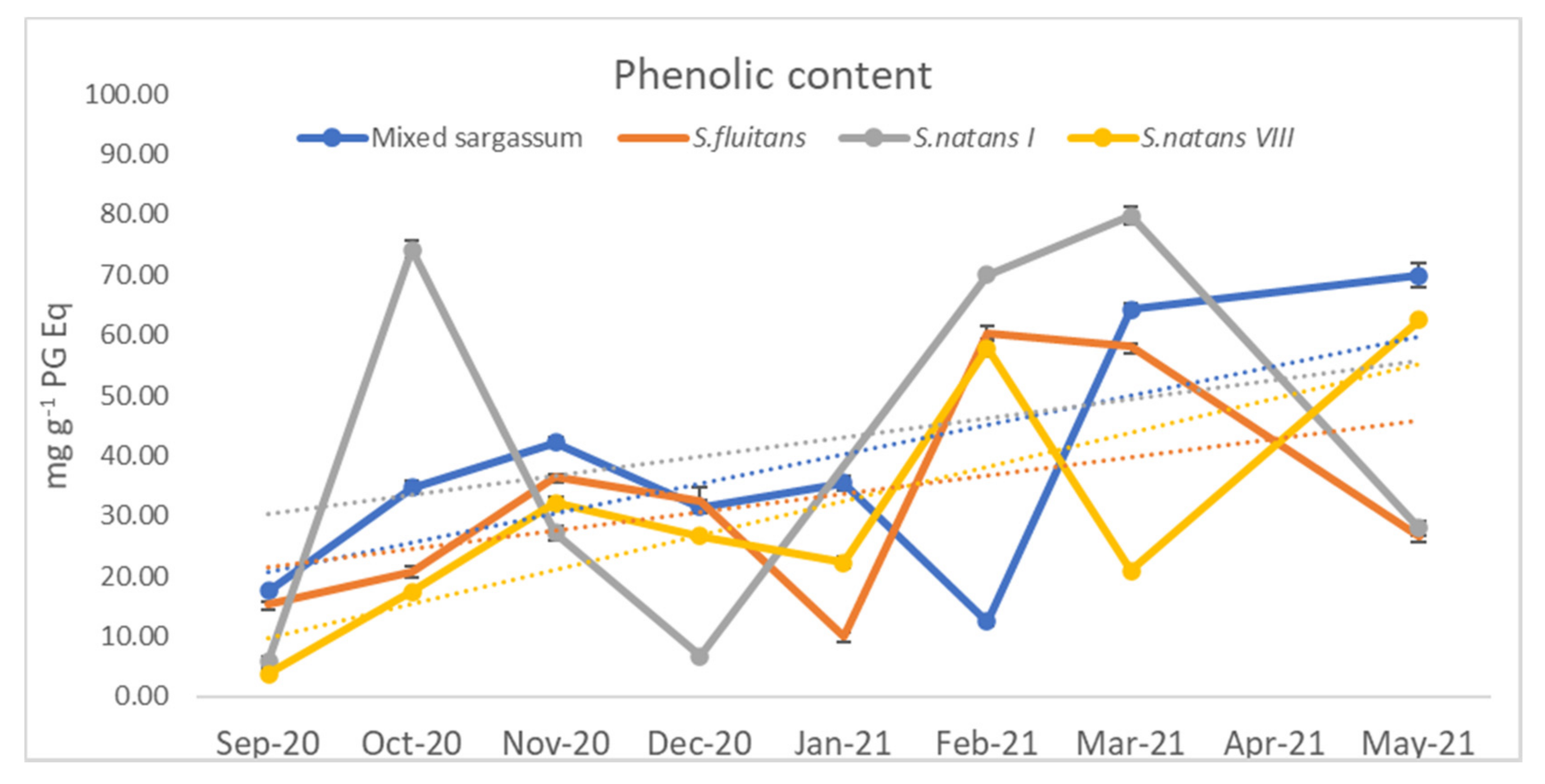
3.1.3. Phenolic Content
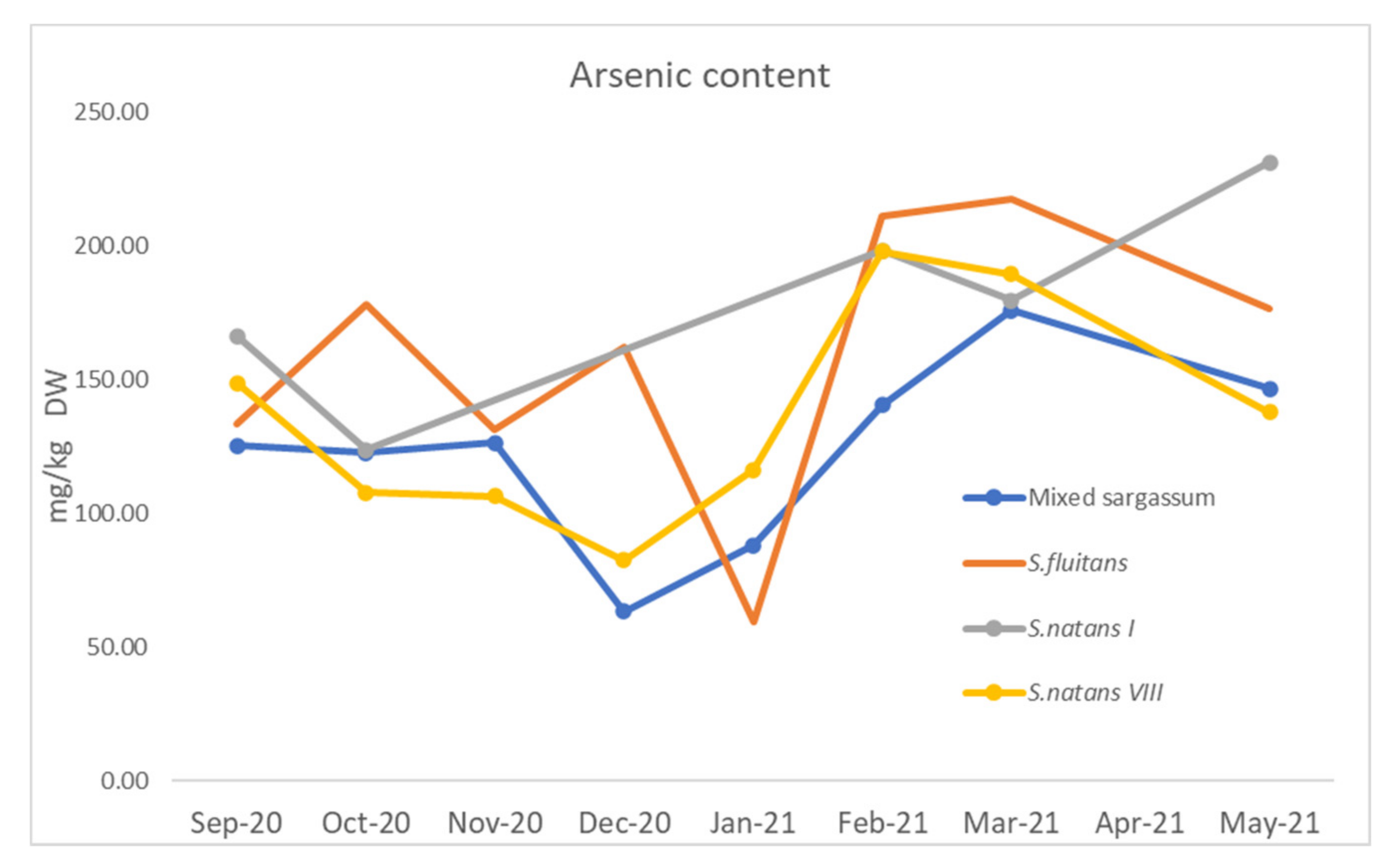
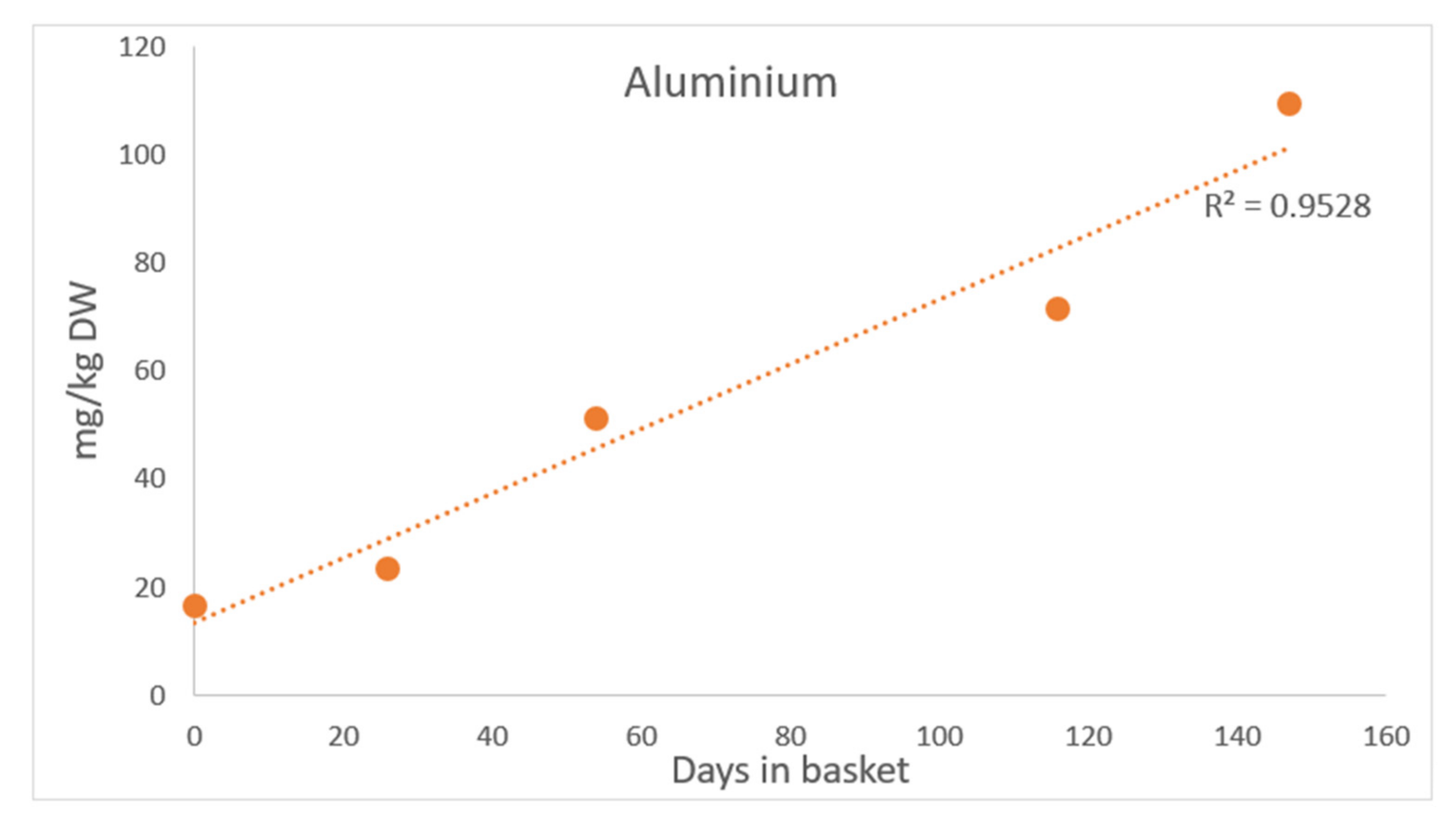
3.1.4. Arsenic and Heavy Metals
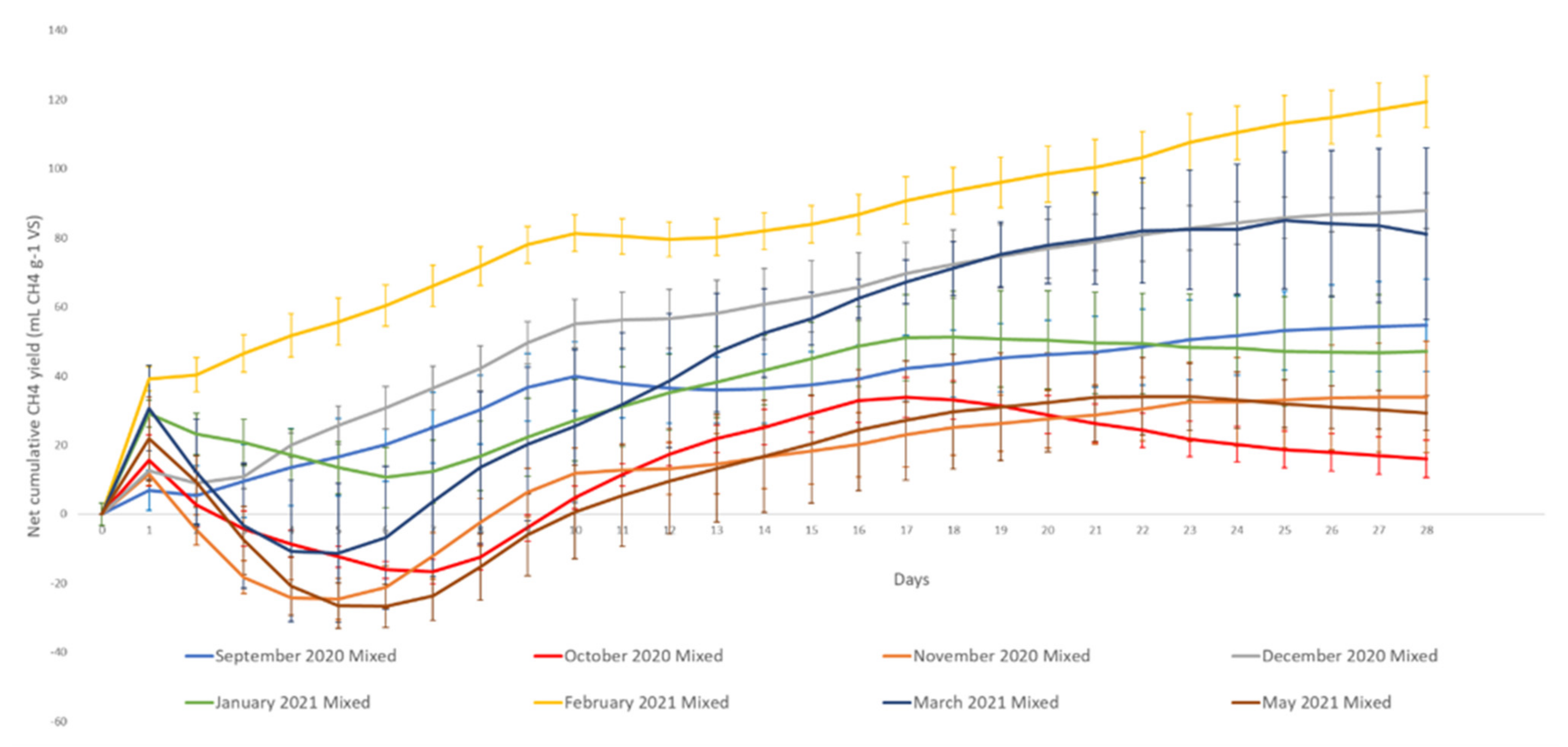
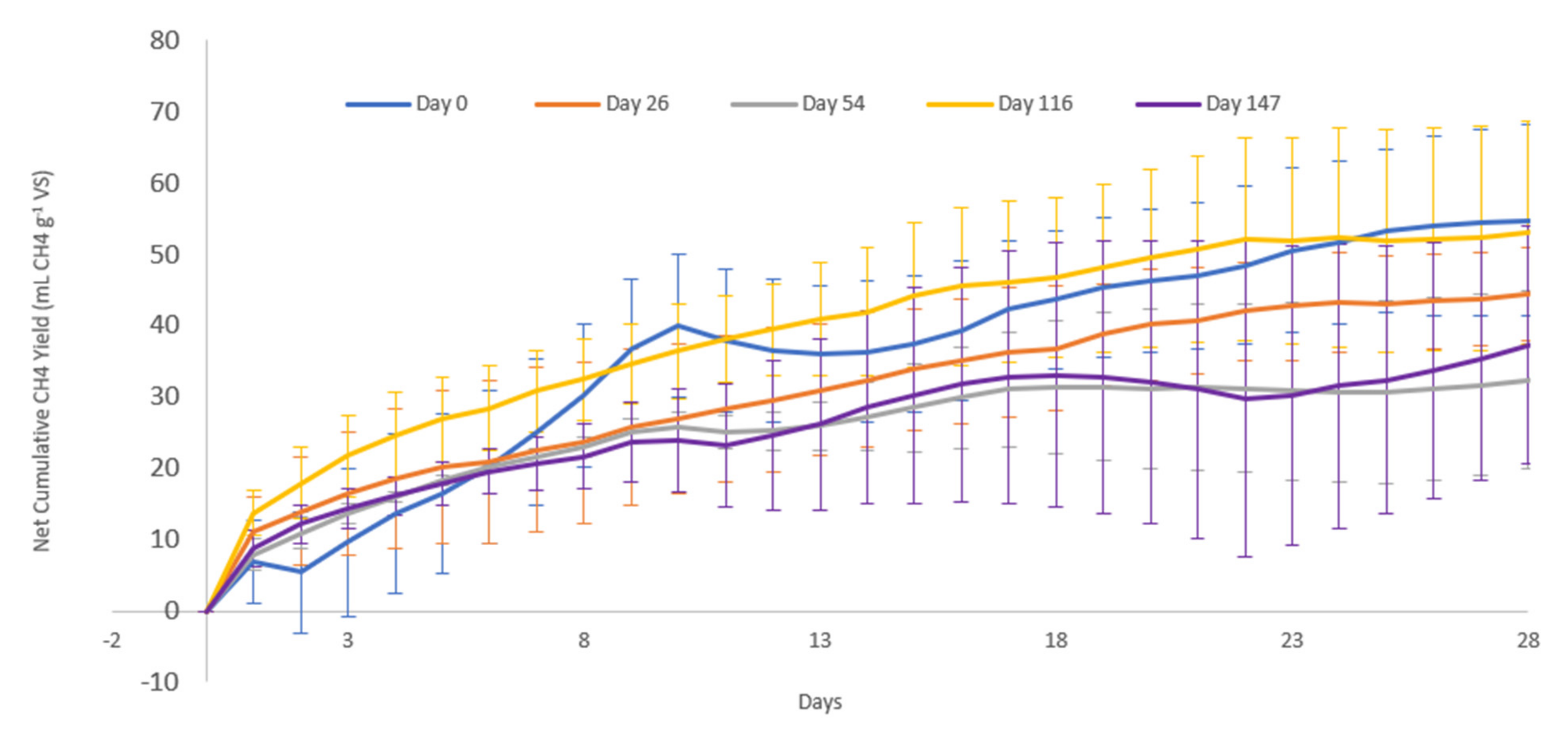
3.2. Methane Potential
4. Discussion
4.1. Composition
4.1.1. Protein Content
4.1.2. Higher Heating Values
4.1.3. Lipid Content
4.1.4. Phenol Content
4.1.5. Arsenic and Heavy Metals
4.2. Methane Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oxenford, H.A.; Cox, S.-A.; van Tussenbroek, B.I.; Desrochers, A. Challenges of turning the sargassum crisis into gold: Current constraints and implications for the Caribbean. Phycology 2021, 1, 27–48. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.-A.; Oxenford, H.A.; van Tussenbroek, B. Sargassum Uses guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers. Report Prepared for the Climate Change Adaptation in the Eastern Caribbean Fisheries Sector (CC4FISH) Project of the Food and Agriculture Organization (FAO) and the Global Environment Facility (GEF); Centre for Resource Management and Environmental Studies (CERMES), University of the West Indies, Cave Hill Campus: Bridgetown, Barbados, 2020. [Google Scholar]
- Oxenford, H.A. Sargassum moss: Ecological aspects and source of influx. In Proceedings of the Sargassum Symposium, UWI, Cave Hill, Barbados, 17 August 2018. [Google Scholar]
- Laffoley, D.d.A.; Roe, H.S.J.; Angel, M.V.; Ardron, J.; Bates, N.R.; Boyd, L.L.; Brooke, S.; Buck, K.N.; Carlson, C.A.; Causey, B.; et al. The Protection and Management of the Sargasso Sea: The Golden Floating Rainforest of the Atlantic Ocean: Summary Science and Supporting Evidence Case; Sargasso Sea Alliance: Hamilton, Bermuda, 2011; 44p. [Google Scholar]
- Milledge, J.J.; Harvey, P. Golden tides: Problem or golden opportunity? The valorisation of sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Williams, A.; Feagin, R. Sargassum as a natural solution to enhance dune plant growth. Environ. Manag. 2010, 46, 738–747. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tussenbroek, B.I.; Hernandez Arana, H.A.; Rodriguez-Martinez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; Gonzalez-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, R.; Wabnitz, C.; Eyzaguirre, J. The Great Sargassum Disaster of 2018. Available online: https://essa.com/the-great-sargassum-disaster-of-2018/ (accessed on 25 March 2019).
- Langin, K. Seaweed masses assault Caribbean islands. Science 2018, 360, 1157–1158. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Hendy, I.W.; Woolford, K.; Vincent-Piper, A.; Burt, O.; Schaefer, M.; Cragg, S.M.; Sanchez-Navarro, P.; Ragazzola, F. Climate-driven golden tides are reshaping coastal communities in Quintana Roo, Mexico. Clim. Chang. Ecol. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Bartlett, D.; Elmer, F. The impact of Sargassum inundations on the Turks and Caicos islands. Phycology 2021, 1, 83–104. [Google Scholar] [CrossRef]
- Resiere, D.; Valentino, R.; Nevière, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabié, A.; Lebrun, T.; Mégarbane, B.; Guerrier, G.; et al. Sargassum seaweed on Caribbean islands: An international public health concern. Lancet 2018, 392, 2691. [Google Scholar] [CrossRef] [Green Version]
- Willoughby, S. Sargassum and the fishing industry. In Proceedings of the Sargassum Symposium, UWI, Cave Hill, Barbados, 17 August 2015. [Google Scholar]
- Resiere, D.; Mehdaoui, H.; Florentin, J.; Gueye, P.; Lebrun, T.; Blateau, A.; Viguier, J.; Valentino, R.; Brouste, Y.; Kallel, H.; et al. Sargassum seaweed health menace in the Caribbean: Clinical characteristics of a population exposed to hydrogen sulfide during the 2018 massive stranding. Clin. Toxicol. 2020, 59, 215–223. [Google Scholar] [CrossRef]
- Pan American Health Organisation. Potential health effects of Sargassum. In Proceedings of the 71st Session of the Regional Committee of WHO for the Americas, Washington, DC, USA, 30 September–4 October 2019. [Google Scholar]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.; et al. Massive influx of pelagic Sargassum spp. on the coasts of the Mexican Caribbean 2014–2020: Challenges and opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Thompson, T.M.; Ramin, P.; Udugama, I.; Young, B.R.; Gernaey, K.V.; Baroutian, S. Techno-economic and environmental impact assessment of biogas production and fertiliser recovery from pelagic Sargassum: A biorefinery concept for Barbados. Energy Conv. Manag. 2021, 245, 114605. [Google Scholar] [CrossRef]
- Henry, L.; McKenzie, B.; Goodridge, A.; Pivott, K.; Austin, J.; Lynch, K.; Spencer, S.; Cox, F.; Holder, N.; Murray, R.; et al. Experimental Evidence on the Use of Biomethane from Rum Distillery Waste and Sargassum Seaweed as an Alternative Fuel for Transportation in Barbados; Energy Division/Infrastructure and Energy Department: Washington, DC, USA, 2021. [Google Scholar]
- Morrison, M.; Gray, D. Anaerobic Digestion Economic Feasibility Study: Generating Energy from Waste, Sewage and Sargassum Seaweed in the OECS; The Caribbean Council: London, UK, 2017. [Google Scholar]
- Milledge, J.J.; Maneein, S.; Arribas López, E.; Bartlett, D. Sargassum inundations in Turks and Caicos: Methane potential and proximate, ultimate, lipid, amino acid, metal and metalloid analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef] [Green Version]
- BSI. Solid biofuels. Determination of moisture content. Oven dry method. Total moisture. Simplified method. In BS EN 14774-2:2009; BSI: London, UK, 2009. [Google Scholar]
- BSI. Solid biofuels-determination of ash content. In BS EN 14775:2009; BSI: London, UK, 2009. [Google Scholar]
- Milledge, J.; Nielsen, B.; Sadek, M.; Harvey, P. Effect of freshwater washing pretreatment on Sargassum muticum as a feedstock for biogas production. Energies 2018, 11, 1771. [Google Scholar] [CrossRef] [Green Version]
- IFRF—International Flame Research Foundation. Online Combustion Handbook. Method from Combustion File 24. 2004. Available online: https://ifrf.net/ (accessed on 10 December 2021).
- Heaven, S.; Milledge, J.; Zhang, Y. Comments on ‘Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable’. Biotechnol. Adv. 2011, 29, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367. [Google Scholar] [CrossRef]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Buswell, A.M.; Mueller, H.F. Mechanism of methane fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sustain. Energy Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Harvey, P.J.; Nielsen, B.V. Methane production from Sargassum muticum: Effects of seasonality and of freshwater washes. Energy Built Environ. 2021, 2, 235–242. [Google Scholar] [CrossRef]
- Barbot, Y.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.-P.; Zhou, X.-F.; Yang, X.-W.; Liu, Y. Chemical composition of seaweeds. In Seaweed Sustainability: Food and Non-Food Applications, 1st ed.; Tiwari, B., Troy, D., Eds.; Academic Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed; Pomin, V.H., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Hurd, C.L.; Harrison, P.; Bischof, K.; Lobban, C.S. Pollution. In Seaweed Ecology and Physiology, 2nd ed.; Hurd, C.L., Lobban, C.S., Bischof, K., Harrison, P.J., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 374–412. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. The effect of seasonal variation on biomethane production from seaweed and on application as a gaseous transport biofuel. Bioresour. Technol. 2016, 209, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, B.E.; West, L.E.; Sutton, T.T.; Hu, C. Ryther revisited: Nutrient excretions by fishes enhance productivity of pelagic Sargassum in the western North Atlantic Ocean. J. Exp. Mar. Biol. Ecol. 2014, 458, 46–56. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Jiang, X.M.; Han, X.X.; Ji, H.S. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Conv. Manag. 2013, 68, 273–280. [Google Scholar] [CrossRef]
- Oyesiku, O.O.; Egunyomi, A. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. Afr. J. Biotechnol. 2014, 13, 1188–1193. [Google Scholar] [CrossRef] [Green Version]
- McKennedy, J.; Sherlock, O. Anaerobic digestion of marine macroalgae: A review. Renew. Sustain. Energy Rev. 2015, 52, 1781–1790. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Anaerobic digestion and gasification of seaweed. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Spinger: Cham, Switzerland, 2018. [Google Scholar]
- D’Este, M.; Angelidaki, I.; Alvarado-Morales, M. Algal Biomass for Bioenergy and Bioproducts Production in Biorefinery Concepts.; Department of Environmental Engineering, Technical University of Denmark (DTU): Lyngby, Denmark, 2017. [Google Scholar]
- Chynoweth, D.P.; Ghosh, S.; Klass, D.L. Anaerobic digestion of kelp. In Biomass Conversion Processes for Energy and Fuels; Sofer, S.S., Zaborsky, O.R., Eds.; Springer: Boston, MA, USA, 1981. [Google Scholar]
- Chynoweth, D.P. Review of Biomethane from Marine Biomass; Department of Agricultural and Biological Engineering, University of Florida: Gainesville, FL, USA, 2002. Available online: https://arpa-e.energy.gov/sites/default/files/Review%20of%20Biomethane%20from%20Marine%20Biomass%202002.pdf (accessed on 10 December 2021).
- Angell, A.R.; Mata, L.; Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2015, 28, 511–524. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González López, C.V.; Garcia, M.D.C.; Fernandez, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Charton, M.; Pignolet, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Evaluation of the protein quality of Porphyridium cruentum. J. Appl. Phycol. 2013, 25, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Saldarriaga-Hernandez, S.; Melchor-Martínez, E.M.; Carrillo-Nieves, D.; Parra-Saldívar, R.; Iqbal, H.M.N. Seasonal characterization and quantification of biomolecules from sargassum collected from Mexican Caribbean coast—A preliminary study as a step forward to blue economy. J. Environ. Manag. 2021, 298, 113507. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Patel, D.P.; Lal, R.; Kumar, M.; Ramkrushna, G.I.; Layek, J.; Buragohain, J.; Ngachan, S.V.; Ghosh, P.K.; Choudhury, B.U.; et al. Impact of fodder grasses and organic amendments on productivity and soil and crop quality in a subtropical region of eastern Himalayas, India. Agric. Ecosyst. Environ. 2016, 216, 274–282. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Merrill, A.L.; Watts, B.K. Energy Values of Foods: Basis & Duration. Slight Revised February 1973; US Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]
- Milledge, J.J. The potential yield of microalgal oil. Biofuels Int. 2010, 4, 44–45. [Google Scholar]
- Vargas-Moreno, J.M.; Callejón-Ferre, A.J.; Pérez-Alonso, J.; Velázquez-Martí, B. A review of the mathematical models for predicting the heating value of biomass materials. Renew. Sustain. Energy Rev. 2012, 16, 3065–3083. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Ensilage and anaerobic digestion of Sargassum muticum. J. Appl. Phycol. 2016, 28, 3021–3030. [Google Scholar] [CrossRef]
- Milledge, J.J.; Staple, A.; Harvey, P. Slow pyrolysis as a method for the destruction of Japanese Wireweed, Sargassum muticum. Environ. Nat. Resour. Res. 2015, 5, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Van Ginneken, V.J.T.; Helsper, J.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef]
- Streefland, M. Algae and Aquatic Biomass for a Sustainable Production of 2nd Generation Biofuels-Deliverable 1.5-Report on Biofuel Production Processes from Micro, Macroalgae and Other Aquatic Biomass; AquaFUELs: Brussels, Belgium, 2010. [Google Scholar]
- Lenstra, W.J.; Hal, J.W.v.; Reith, J.H. Economic aspects of open ocean seaweed cultivation. In Proceedings of the Alg’n Chem 2011, Algae, New Resources for Industry, Montpellier, France, 10 November 2011. [Google Scholar]
- Milledge, J.J.; Smith, B.; Dyer, P.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, fatty acids, and fucoxanthin content from temperate and tropical Brown seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- World Weather Online. Cockburn Town Monthly Climate Averages, TC. Available online: https://www.worldweatheronline.com/cockburn-town-weather-averages/tc.aspx (accessed on 26 September 2021).
- Plouguerne, E.; Le Lann, K.; Connan, S.; Jechoux, G.; Deslandes, E.; Stiger-Pouvreau, V. Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat. Bot. 2006, 85, 337–344. [Google Scholar] [CrossRef]
- Lann, K.L.; Ferret, C.; VanMee, E.; Spagnol, C.; Lhuillery, M.; Payri, C.; Stiger-Pouvreau, V. Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: Spatial and specific variability. Phycol. Res. 2012, 60, 37–50. [Google Scholar] [CrossRef]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef]
- Gorham, J.; Lewey, S.A. Seasonal changes in the chemical composition of Sargassum muticum. Mar. Biol. 1984, 80, 103–107. [Google Scholar] [CrossRef]
- Connan, S.; Delisle, F.; Deslandes, E.; Gall, E.A. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot. Mar. 2006, 49, 39–46. [Google Scholar] [CrossRef]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Leon, E.S.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- ANSES. ANSES Makes Recommendations to Limit Cadmium Exposure from Consumption of Edible Seaweed. Available online: https://www.anses.fr/en/content/anses-makes-recommendations-limit-cadmium-exposure-consumption-edible-seaweed (accessed on 4 November 2021).
- Dodge, J.D. The Fine Structure of Algal Cells; Academic Press: London, UK, 1973. [Google Scholar] [CrossRef]
- Milinovic, J.; Vale, C.; Botelho, M.J.; Pereira, E.; Sardinha, J.; Murton, B.J.; Noronha, J.P. Selective incorporation of rare earth elements by seaweeds from Cape Mondego, western Portuguese coast. Sci. Total Environ. 2021, 795, 148860. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018-2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, K.; Konomi, A. Toxicity of so-called edible hijiki seaweed (Sargassum fusiforme) containing inorganic arsenic. Regul. Toxicol. Pharmacol. 2012, 63, 291–297. [Google Scholar] [CrossRef]
- Neff, J.M. Ecotoxicology of arsenic in the marine environment. Environ. Toxicol. Chem. 1997, 16, 917–927. [Google Scholar] [CrossRef]
- European Commission. Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs; European Commission: Brussels, Belgium, 2015; Available online: https://eur-lex.europa.eu/ (accessed on 10 November 2021).
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed—Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef] [Green Version]
- Taylor, V.F.; Li, Z.; Sayarath, V.; Palys, T.J.; Morse, K.R.; Scholz-Bright, R.A.; Karagas, M.R. Distinct arsenic metabolites following seaweed consumption in humans. Sci. Rep. 2017, 7, 3920. [Google Scholar] [CrossRef] [PubMed]
- Fourest, E.; Volesky, B. Alginate properties and heavy metal biosorption by marine algae. Appl. Biochem. Biotechnol. 1997, 67, 215–226. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrere, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Soto, M.; Vazquez, M.A.; de Vega, A.; Vilarino, J.M.; Fernandez, G.; de Vicente, M.E. Methane potential and anaerobic treatment feasibility of Sargassum muticum. Bioresour. Technol. 2015, 189, 53–61. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A brief review of anaerobic digestion of algae for bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef] [Green Version]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2018, 31, 779–786. [Google Scholar] [CrossRef]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.J.; Lewis, D.M.; Green, B. Anaerobic digestion of algae biomass: A review. Algal Res. Biomass Biofuels Bioprod. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol. 2008, 145, 375–395. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A review of seaweed pre-treatment methods for enhanced biofuel production by anaerobic digestion or fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.M.; Lumpkin, R.; Putman, N.F.; Smith, R.H.; Muller-Karger, F.E.; Rueda-Roa, D.T.; Hu, C.; Wang, M.; Brooks, M.T.; Gramer, L.J.; et al. The establishment of a pelagic Sargassum population in the tropical Atlantic: Biological consequences of a basin-scale long distance dispersal event. Prog. Oceanogr. 2020, 182, 102269. [Google Scholar] [CrossRef]
- UNEP. Sargassum White Paper-Sargassum Outbreak in the Caribbean: Challenges, Opportunities and Regional Situation; Scientific and Technical Advisory Committee (STAC) to the Protocol Concerning Specially Protected Areas and Wildlife (SPAW) in the Wider Caribbean Region: Panama City, Panama, 2018. [Google Scholar]
- Milledge, J.J.; Maneein, S. Chapter 6—Storage of seaweed for biofuel production: Ensilage. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 155–167. [Google Scholar] [CrossRef]


| Ash | %VS | C | H | N | O | Elemental Ratios | HHV | |||
|---|---|---|---|---|---|---|---|---|---|---|
| % Dry Weight (DW) | C:N | C:O | MJ kg−1 DW | MJ kg−1 VS | ||||||
| 20 September | ||||||||||
| Mixed sargassum | 41.75 | 58.25 | 27.68 | 2.72 | 1.64 | 26.21 | 16.88 | 1.06 | 9.7 | 16.7 |
| S. natans VIII | 41.63 | 58.37 | 23.55 | 2.08 | 1.93 | 30.81 | 12.2 | 0.76 | 7.2 | 12.3 |
| S. natans I | 41.08 | 58.92 | 23.96 | 1.88 | 1.96 | 31.12 | 12.22 | 0.77 | 7.1 | 12.1 |
| S. fluitans | 39.62 | 60.38 | 24.9 | 2.13 | 1.8 | 31.55 | 13.83 | 0.79 | 7.7 | 12.8 |
| 20 October | ||||||||||
| Mixed sargassum | 39.85 | 60.15 | 27.33 | 2.65 | 2.18 | 27.99 | 12.52 | 0.98 | 9.4 | 15.6 |
| S. natans VIII | 42.01 | 57.99 | 22.64 | 2.32 | 3.21 | 29.82 | 7.06 | 0.76 | 7.4 | 12.8 |
| S. natans I | 39.88 | 60.12 | 29.52 | 2.71 | 2.21 | 25.68 | 14.1 | 1.15 | 10.4 | 17.3 |
| S. fluitans | 41.05 | 58.95 | 29.06 | 3.2 | 2.22 | 24.46 | 13.06 | 1.19 | 10.9 | 18.5 |
| 20 November | ||||||||||
| Mixed sargassum | 40.95 | 59.05 | 26.76 | 2.9 | 2.22 | 27.17 | 11.3 | 0.98 | 9.5 | 16.1 |
| S. natans VIII | 24.61 | 75.39 | 23.95 | 2.53 | 2.37 | 46.55 | 9.92 | 0.51 | 6.3 | 8.4 |
| S. natans I | 41.36 | 58.64 | 25.4 | 2.19 | 1.8 | 29.25 | 14.11 | 0.87 | 8.1 | 13.8 |
| S. fluitans | 37.89 | 62.11 | 28.95 | 3.41 | 1.99 | 27.77 | 14.89 | 1.04 | 10.7 | 17.2 |
| 20 December | ||||||||||
| Mixed Ssargassum | 34.2 | 65.8 | 30.99 | 3.68 | 3.42 | 27.71 | 8.19 | 1.12 | 11.8 | 17.9 |
| S. natans VIII | 38.51 | 61.49 | 28.67 | 3.86 | 2.45 | 26.51 | 11.69 | 1.08 | 11.3 | 18.4 |
| S. natans I | 28.45 | 71.55 | 33.53 | 4.44 | 2.01 | 31.58 | 16.73 | 1.06 | 13 | 18.2 |
| S. fluitans | 44.34 | 55.66 | 25.67 | 2.87 | 2.52 | 24.6 | 10.19 | 1.04 | 9.4 | 16.9 |
| 21 January | ||||||||||
| Mixed sargassum | 37.96 | 62.04 | 31.62 | 3.79 | 1.32 | 25.31 | 23.95 | 1.25 | 12.2 | 19.7 |
| S. natans VIII | 32.44 | 67.56 | 28.61 | 3.12 | 2.74 | 33.08 | 10.44 | 0.86 | 9.9 | 14.7 |
| S. fluitans | 51.1 | 48.9 | 26.87 | 3.02 | 1.93 | 17.07 | 13.92 | 1.57 | 10.7 | 21.9 |
| 21 February | ||||||||||
| Mixed sargassum | 32.73 | 67.27 | 24.11 | 2.31 | 2.02 | 38.83 | 11.94 | 0.62 | 6.9 | 10.3 |
| S. natans VIII | 33.84 | 66.16 | 30.33 | 3.7 | 1.43 | 30.71 | 21.33 | 0.99 | 11.2 | 16.9 |
| S. natans I | 32.03 | 67.97 | 22.8 | 2.58 | 2.33 | 40.27 | 9.76 | 0.57 | 6.6 | 9.7 |
| S. fluitans | 34.44 | 65.56 | 28.7 | 3.42 | 1.5 | 31.94 | 19.11 | 0.9 | 10.2 | 15.6 |
| 21 March | ||||||||||
| Mixed sargassum | 40.03 | 59.97 | 27.18 | 3.92 | 2.45 | 26.42 | 11.1 | 1 | 10.8 | 18.0 |
| S. natans VIII | 39.33 | 60.67 | 26.32 | 2.66 | 2.59 | 29.1 | 10.16 | 0.9 | 9 | 14.8 |
| S. natans I | 34.02 | 65.98 | 27.91 | 2.97 | 2.22 | 32.87 | 12.57 | 0.85 | 9.4 | 14.2 |
| S. fluitans | 36.49 | 63.51 | 26.97 | 2.85 | 2.32 | 31.38 | 11.62 | 0.86 | 9.2 | 14.5 |
| 21 May | ||||||||||
| Mixed sargassum | 38.1 | 61.9 | 24.87 | 2.69 | 1.8 | 32.54 | 15.48 | 0.76 | 8.1 | 13.1 |
| S. natans VIII | 39.26 | 60.74 | 24.55 | 2.46 | 2.26 | 31.46 | 10.91 | 0.78 | 7.9 | 13.0 |
| S. natans I | 45.44 | 54.56 | 25.82 | 3.19 | 2.35 | 23.19 | 11.02 | 1.11 | 9.9 | 18.1 |
| S. fluitans | 45.95 | 54.05 | 23.59 | 2.39 | 2.02 | 26.06 | 11.65 | 0.91 | 8 | 14.8 |
| Ash | %VS | C | H | N | O | Elemental Ratios | HHV | |||
|---|---|---|---|---|---|---|---|---|---|---|
| % Dry Weight | C:N | C:O | MJ kg−1 DW | MJ kg−1 VS | ||||||
| Day 0 | 41.75 | 58.25 | 27.68 | 2.72 | 1.64 | 26.21 | 16.88 | 1.06 | 9.7 | 16.7 |
| Day 26 | 28.87 | 71.13 | 37.5 | 5.6 | 2.33 | 25.7 | 16.19 | 1.26 | 16.1 | 22.7 |
| Day 54 | 21.56 | 78.44 | 32.74 | 3.96 | 2.33 | 39.4 | 14.05 | 0.83 | 11.5 | 14.6 |
| Day 116 | 30.05 | 69.95 | 32.3 | 3.22 | 2.36 | 32.07 | 13.69 | 1.01 | 11.3 | 16.1 |
| Day 147 | 43.16 | 56.84 | 31.45 | 2.86 | 2.14 | 20.38 | 14.7 | 1.54 | 11.8 | 20.7 |
| Aluminium | Arsenic | Cadmium | Chromium | Lead | |
|---|---|---|---|---|---|
| Mixed sargassum | 15.78–50.11 | 63.14–175.88 | 0.11–0.22 | 0.0043–1.04 | ND * –0.52 |
| S. fluitans | 21.62–50.11 | 59.22–217.82 | ND *–0.23 | 0.004–5.15 | ND *–0.996 |
| S. natans I | 22.20–45.02 | 123.81–198.36 | ND *–0.392 | ND *–0.399 | ND *–0.499 |
| S. natans VIII | 26.72–124.13 | 82.44–197.95 | ND *–0.22 | 0.004–2.82 | ND *–0.66 |
| Arsenic | Aluminium | Cadmium | Chromium | Lead | |
| Basket Day 0 | 125.24 | 16.63 | 0.18 | 0.28 | 0.09 |
| Basket Day 26 | 99.18 | 23.29 | 0.27 | 0.29 | 0.37 |
| Basket Day 54 | 49.30 | 51.24 | 0.35 | 0.51 | 0.83 |
| Basket Day 116 | 55.01 | 71.31 | 0.33 | 0.69 | 1.59 |
| Basket Day 147 | 85.06 | 109.40 | 0.28 | 0.0098 | 1.69 |
| Theoretical CH4 | Methane Potential (MP) | BI | |
|---|---|---|---|
| mL CH4 g−1 VS | mL CH4 g−1 VS | ||
| Sep-20 | 451 | 54.66 | 12% |
| Oct-20 | 434 | 16.03 | 4% |
| Nov-20 | 429 | 33.90 | 8% |
| Dec-20 | 475 | 87.78 | 18% |
| Jan-21 | 517 | 47.13 | 9% |
| Feb-21 | 285 | 119.35 | 42% |
| Mar-21 | 432 | 81.13 | 19% |
| May-21 | 453 | 29.32 | 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, B.V.; Milledge, J.J.; Hertler, H.; Maneein, S.; Al Farid, M.M.; Bartlett, D. Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes. Phycology 2021, 1, 143-162. https://doi.org/10.3390/phycology1020011
Nielsen BV, Milledge JJ, Hertler H, Maneein S, Al Farid MM, Bartlett D. Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes. Phycology. 2021; 1(2):143-162. https://doi.org/10.3390/phycology1020011
Chicago/Turabian StyleNielsen, Birthe Vejby, John James Milledge, Heidi Hertler, Supattra Maneein, Md Mahmud Al Farid, and Debbie Bartlett. 2021. "Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes" Phycology 1, no. 2: 143-162. https://doi.org/10.3390/phycology1020011
APA StyleNielsen, B. V., Milledge, J. J., Hertler, H., Maneein, S., Al Farid, M. M., & Bartlett, D. (2021). Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes. Phycology, 1(2), 143-162. https://doi.org/10.3390/phycology1020011









