Biochemical and Elemental Composition of Pelagic Sargassum Biomass Harvested across the Caribbean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Samples
2.2. Ash and Moisture Analysis
2.3. Elemental Composition Analysis by Inductively Coupled Plasma Mass Spectrometry (ICP–MS)
2.4. Amino Acid Analysis
2.5. Biogenic Amines Analysis
2.6. Vitamin Analysis
2.7. Fatty-Acid Analysis
2.8. Monosaccharide Analysis of the Non-Cellulosic Fraction
3. Results and Discussion
3.1. Ash and Moisture
3.2. Elemental Composition
3.3. Vitamins
3.4. Fatty Acids
3.5. Amino Acids and Biogenic Amines
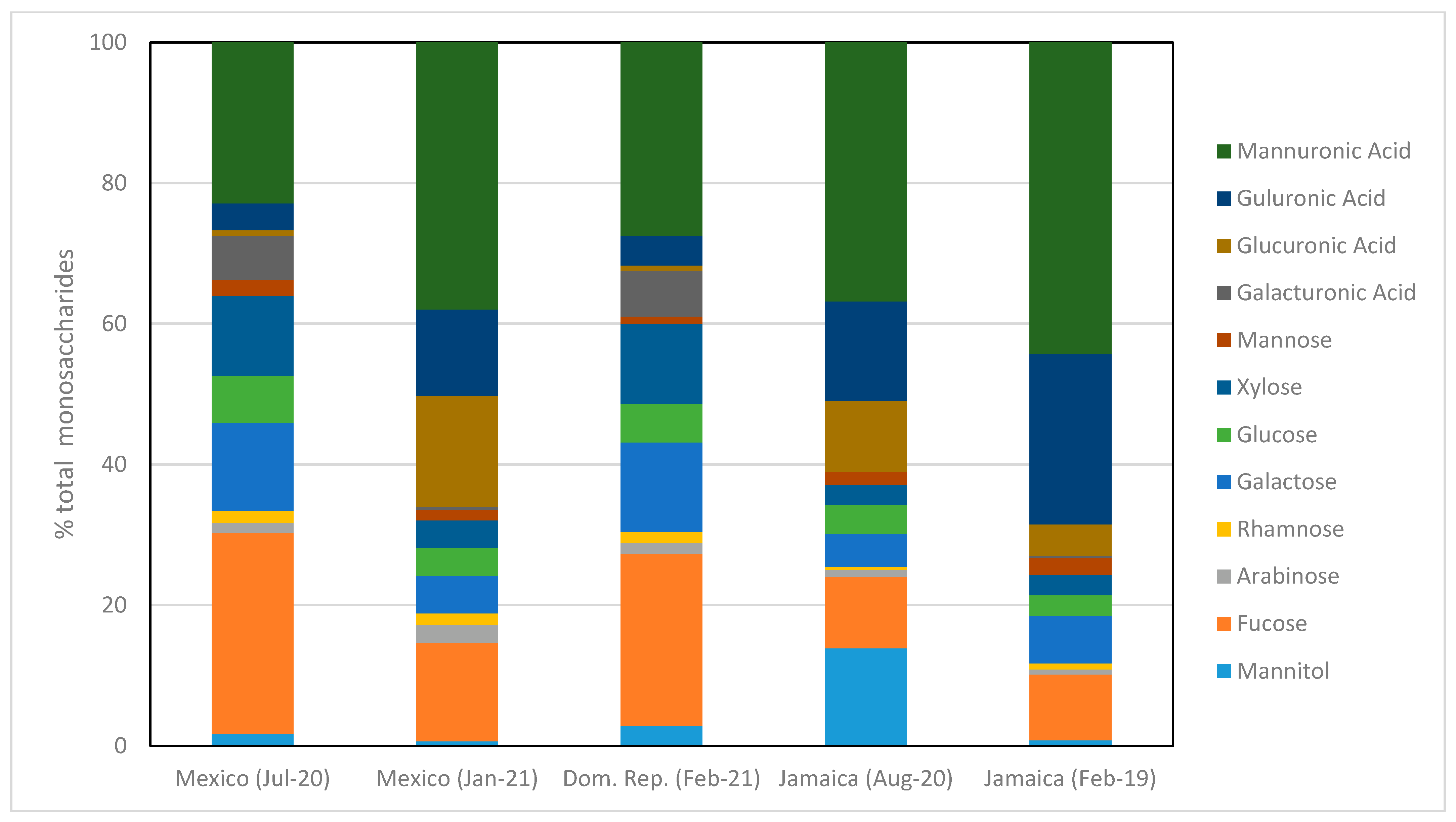
3.6. Monosaccharide Composition of the Non-Cellulosic Fraction
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oxenford, H.A.; Cox, S.-A.; van Tussenbroek, B.I.; Desrochers, A. Challenges of turning the Sargassum crisis into gold: Current constraints and implications for the Caribbean. Phycology 2021, 1, 27–48. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.-A.; Oxenford, H.A.; van Tussenbroek, B. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers. Report Prepared for the Climate Change Adaptation in the Eastern Caribbean Fisheries Sector (CC4FISH) Project of the Food and Agriculture Organization (FAO) and the Global Environment Facility (GEF); Centre for Resource Management and Environmental Studies (CERMES), University of the West Indies, Cave Hill Campus: Bridgetown, Barbados, 2020. [Google Scholar]
- Bartlett, D.; Elmer, F. The Impact of Sargassum Inundations on the Turks and Caicos Islands. Phycology 2021, 1, 83–104. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.; et al. Massive influx of pelagic Sargassum spp. on the coasts of the Mexican Caribbean 2014–2020: Challenges and opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Milledge, J.J.; Maneein, S.; López, E.A.; Bartlett, D. Sargassum Inundations in Turks and Caicos: Methane potential and proximate, ultimate, lipid, amino acid, metal and metalloid analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef] [Green Version]
- Gray, L.A.; Bisonó León, A.G.; Rojas, F.E.; Veroneau, S.S.; Slocum, A.H. Caribbean-Wide, Negative Emissions Solution to Sargassum spp. Low-Cost Collection Device and Sustainable Disposal Method. Phycology 2021, 1, 49–75. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.V.; Milledge, J.J.; Hertler, H.; Maneein, S.; Al Farid, M.M.; Bartlett, D. Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes. Phycology 2021, 1, 143–162. [Google Scholar] [CrossRef]
- Machado, C.B.; Maddix, G.M.; Francis, P.; Thomas, S.-L.; Burton, J.-A.; Langer, S.; Larson, T.R.; Marsh, R.; Webber, M.; Tonon, T. Pelagic Sargassum events in Jamaica: Provenance, morphotype abundance, and influence of sample processing on biochemical composition of the biomass. Sci. Total Environ. 2022, 817, 152761. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Dash, J.; Tompkins, E.L.; Tonon, T. A systematic review of floating and beach landing records of Sargassum beyond the Sargasso Sea. Environ. Res. Commun. 2020, 2, 122001. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef] [PubMed]
- Kaap, R.W., Jr. Arsenic. Encycl. Toxicol. 2014, 3, 308–312. [Google Scholar]
- Pereira, L.A. Review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Coimbra, Portugal, 2011; pp. 15–47. [Google Scholar]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible Seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Pilsbury, A.; Kumar, V.; Herrera-Rodriguez, L.; Suarez, J.V.; Allen, M.J. Phytohormone analysis of British and Mexican seaweeds for use as potential plant growth stimulants. manuscript in preparation.
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]

| Mexico (July 2020) | Mexico (January 2021) | Dom. Republic (February 2021) | Jamaica (August 2020) | Turks & Caicos (June 2019) | Jamaica (February 2019) | |
|---|---|---|---|---|---|---|
| Ash | 58.60 ± 0.43 | 30.07 ± 1.31 | 16.63 ± 0.09 | 36.97 ± 0.12 | 46.94 ± 1.31 | 34.12 ± 3.46 |
| Moisture | 11.83 ± 0.40 | 13.60 ± 0.14 | 18.80 ± 0.01 | 8.50 ± 0.08 | 81.98 ± 0.89 * | 8.19 ± 0.71 |
| Mexico (July 2020) | Mexico (January 2021) | Dom. Republic (February 2021) | Jamaica (August 2020) | Turks & Caicos (June 2019) | |
|---|---|---|---|---|---|
| Na | 32,368.08 ± 2390.29 | 36,705.04 ± 745.92 | 7382.63 ± 60.28 | 30,803.70 ± 2714.54 | NA |
| Mg | 7424.02 ± 560.86 | 8574.62 ± 162.14 | 6553.47 ± 42.82 | 8306.53 ± 225.96 | 12,053.19 |
| Al | 10.75 ± 5.90 | 19.14 ± 5.56 | 28.45 ± 1.26 | 62.82 ± 9.45 | 37.50 |
| K | 34,177.79 ± 2687.81 | 48,213.60 ± 887.80 | 18,939.46 ± 138.93 | 56,013.25 ± 4395.27 | 69,359.39 |
| Ca | 24,198.68 ± 2062.31 | 36,894.929 ± 1672.95 | 41,717.14 ± 618.85 | 34,476.17 ± 3984.21 | 70,305.77 |
| V | 1.42 ± 0.18 | 5.38 ± 1.03 | 2.18 ± 0.09 | 1.57 ± 0.20 | NA |
| Cr | 0.69 ± 0.35 | 0.73 ± 0.15 | 1.99 ± 0.36 | 1.47 ± 0.93 | <0.3 |
| Mn | 11.03 ± 0.84 | 13.60 ± 0.30 | 14.49 ± 0.17 | 22.30 ± 0.72 | 30.15 |
| Fe | 45.30 ± 11.39 | 47.90 ± 8.67 | 58.79 ± 3.80 | 53.35 ± 11.63 | 3811.37 |
| Co | 0.54 ± 0.02 | 0.70 ± 0.02 | 0.47 ± 0.02 | 0.46 ± 0.07 | NA |
| Ni | 4.59 ± 0.84 | 4.72 ± 0.15 | 4.40 ± 0.62 | 3.75 ± 0.34 | NA |
| Cu | 2.38 ± 0.16 | 2.25 ± 0.15 | 3.53 ± 0.02 | 2.11 ± 0.11 | 2.51 |
| Zn | 4.84 ± 2.05 | 11.49 ± 2.39 | 13.73 ± 2.99 | 3.87 ± 1.52 | 5.81 |
| As | 55.91 ± 4.53 | 53.89 ± 1.30 | 21.42 ± 0.93 | 86.84 ± 5.11 | 123.69 |
| Cd | 0.40 ± 0.086 | 0.77 ± 0.20 | 0.35 ± 0.01 | 0.39 ± 0.02 | 0.13 |
| Ba | 22.56 ± 2.24 | 19.63 ± 0.06 | 26.93 ± 0.82 | 15.17 ± 1.77 | NA |
| Pb | 0.50 ± 0.50 | 3.12 ± 1.76 | 0.45 ± 0.19 | 0.85 ± 0.05 | 0.26 |
| U | 0.35 ± 0.03 | 0.54 ± 0.01 | 0.59 ± 0.02 | 0.47 ± 0.01 | NA |
| TOTAL | 98,329.83 ± 7686.39 | 130,572.04 ± 3448.87 | 74,770.46 ± 550.06 | 129,855.076 ± 4623.48 | NA |
| Mexico (July 2020) | Mexico (January 2021) | Dom. Republic (February 2021) | Jamaica (August 2020) | |
|---|---|---|---|---|
| Vitamin A (trans-retinol, IU/g) | <1.00 | <1.00 | <1.00 | <1.00 |
| Vitamin B1 (thiamine HCl, mg/kg) | 0.30 ± 0.15 | 0.19 ± 0.07 | 0.11 ± 0.04 | 0.07 ± 0.01 |
| Vitamin B2 (riboflavin, mg/kg) | 0.29 ± 0.15 | 1.77 ± 0.04 | 0.41 ± 0.03 | 0.29 ± 0.03 |
| Vitamin B3 (mg/kg) | 1.39 ± 0.05 | 4.21 ± 0.43 | 2.24 ± 0.28 | 2.16 ± 0.66 |
| Vitamin B6 (pyridoxine, mg/kg) | <0.50 | <0.50 | <0.50 | <0.50 |
| Vitamin B9 (free folic acid, mg/kg) | 0.31 ± 0.03 | 0.25 ± 0.02 | <0.12 | <0.12 |
| Vitamin B12 (cyanocobalamin, µg/100 g) | 4.97 ± 0.17 | 4.86 ± 0.38 | 5.74 ± 0.33 | 2.28 ± 0.15 |
| Vitamin C (ascorbic acid, mg/kg) | <1.00 | <1.00 | <1.00 | <1.00 |
| Vitamin E (as DL tocopherol acetate, IU/kg) | 3.70 ± 0.91 | 7.23 ± 0.26 | 5.97 ± 0.37 | 6.70 ± 0.16 |
| Mexico (July 2020) | Mexico (January 2021) | Dom. Republic (February 2021) | Jamaica (August 2020) | Turks & Caicos (June 2019) | |
|---|---|---|---|---|---|
| Caprylic Acid—C08:0 | <0.05 | 0.07 ± 0 | <0.05 | <0.05 | <0.05 |
| Capric Acid—C10:0 | <0.05 | 0.10 ± 0.01 | 0.05 ± 0.01 | <0.05 | <0.05 |
| Undecylic Acid—C11:0 | <0.05 | 0.19 ± 0.01 | <0.05 | 0.25 ± 0.01 | <0.05 |
| Lauric Acid—C12:0 | 0.24 ± 0.01 | 1.32 ± 0.02 | 0.20 ± 0.01 | 0.08 ± 0.01 | 0.14 |
| Tridecylic Acid—C13:0 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.34 ± 0.02 | 0.05 ± 0.01 | <0.05 |
| Myristic Acid—C14:0 | 2.58 ± 0.01 | 2.58 ± 0.03 | 1.60 ± 0.04 | 3.83 ± 0.01 | 2.01 |
| Myristoleic Acid—C14:1 | 0.22 ± 0.01 | 0.27 ± 0.01 | 0.56 ± 0.03 | 0.33 ± 0.01 | 0.43 |
| Pentadecanoic Acid—C15:0 | 0.54 ± 0.01 | 0.51 ± 0.01 | 0.48 ± 0.02 | 0.65 ± 0.01 | 0.46 |
| Pentadecenoic Acid—C15:1 | <0.05 | 0.28 ± 0.05 | 0.05 ± 0.01 | <0.05 | 0.39 |
| Palmitic Acid—C16:0 | 31.90 ± 0.44 | 22.13 ± 0.33 | 25.57 ± 0.84 | 34.19 ± 0.094 | 26.68 |
| Palmitoleic Acid—C16:1 | 6.41 ± 0.06 | 7.87 ± 0.10 | 6.26 ± 0.01 | 7.52 ± 0.01 | 4.03 |
| Heptadecanoic Acid—C17:0 | 0.70 ± 0.02 | 0.92 ± 0.04 | 0.28 ± 0.03 | 0.77 ± 0.04 | 1.17 |
| Heptadecenoic Acid—C17:1 | 0.49 ± 0.01 | 0.14 ± 0.01 | 0.27 ± 0.02 | 0.37 ± 0.01 | <0.05 |
| Stearic Acid—C18:0 | 3.12 ± 0.04 | 3.45 ± 0.06 | 2.50 ± 0.01 | 2.75 ± 0.01 | 4.73 |
| Oleic Acid—C18:1 | 13.06 ± 0.09 | 14.80 ± 0.13 | 20.84 ± 0.39 | 13.09 ± 0.02 | 12.71 |
| Linoleic Acid—C18:2 | 5.00 ± 0.05 | 7.52 ± 0.12 | 12.72 ± 0.30 | 5.89 ± 0.01 | 5.32 |
| Linolenic Acid—C18:3 | 1.89 ± 0.04 | 3.12 ± 0.05 | 3.75 ± 0.07 | 4.15 ± 0.01 | 4.4 |
| Stearidonic Acid—C18:4 | 0.20 ± 0.01 | 0.39 ± 0.16 | 0.21 ± 0.12 | 0.47 ± 0.01 | 0.07 |
| Arachidic Acid—C20:0 | 0.52 ± 0.02 | 0.75 ± 0.06 | 0.64 ± 0.01 | 0.59 ± 0.02 | 0.47 |
| Gadoleic Acid—C20:1 | 0.64 ± 0.67 | 0.27 ± 0.15 | 0.54 ± 0.23 | 0.19 ± 0.01 | 0.18 |
| Arachidonic Acid—C20:4 | 4.71 ± 0.08 | 0.57 ± 0.01 | 0.43 ± 0.01 | 0.45 ± 0.01 | 7.79 |
| Eicosapentaenoic Acid—C20:5 | 0.38 ± 0.01 | 0.98 ± 0.01 | 0.43 ± 0.02 | 0.79 ± 0.01 | 3.75 |
| Behenic Acid—C22:0 | 1.09 ± 0.04 | 0.94 ± 0.01 | 0.85 ± 0.02 | 0.68 ± 0.01 | 0.63 |
| Erucic Acid—C22:1 | 0.18 ± 0.02 | 0.49 ± 0.06 | 0.25 ± 0.01 | 0.31 ± 0.03 | 1.59 |
| Adrenic Acid—C22:4 | 0.10 ± 0.01 | 0.66 ± 0.01 | 0.19 ± 0.01 | 0.15 ± 0.01 | 1.17 |
| Docosapentaenoic Acid—C22:5 | <0.05 | <0.05 | 0.16 ± 0.02 | 0.113 ± 0.03 | 0.36 |
| Docosahexaenoic Acid—C22:6 | 0.29 ± 0.01 | 0.89 ± 0.01 | 0.19 ± 0.01 | 0.24 ± 0.01 | 6.44 |
| Lignoceric Acid—C24:0 | 0.56 ± 0.01 | 0.52 ± 0.01 | 0.58 ± 0.02 | 0.41 ± 0.01 | 0.42 |
| Saturated Fatty Acids | 41.41 ± 0.40 | 33.54 ± 0.51 | 33.09 ± 0.93 | 44.14 ± 0.14 | 36.71 |
| Monounsaturated Fatty Acids | 20.84 ± 0.86 | 24.13 ± 0.36 | 28.78 ± 0.49 | 21.84 ± 0.03 | 19.33 |
| Polyunsaturated Fatty Acids | 12.57 ± 0.06 | 14.12 ± 0.24 | 18.07 ± 0.43 | 12.25 ± 0.02 | 29.3 |
| Unidentified Fatty Acids | 25.17 ± 0.86 | 28.20 ± 0.63 | 20.07 ± 0.17 | 21.77 ± 0.15 | 14.66 |
| Mexico (July 2020) | Mexico (January 2021) | Dom. Republic (February 2021) | Jamaica (August 2020) | Turks & Caicos (June 2019) | |
|---|---|---|---|---|---|
| Alanine | 0.32 ± 0.02 | 0.32 ± 0.01 | 0.37 ± 0.01 | 0.30 ± 0.01 | 0.34 |
| Arginine | 0.26 ± 0.01 | 0.28 ± 0.01 | 0.37 ± 0.01 | 0.20 ± 0.01 | 0.18 |
| Aspartic acid | 0.56 ± 0.03 | 0.6 ± 0.01 | 0.70 ± 0.01 | 0.52 ± 0.01 | 0.47 |
| Cystine | <0.10 | 0.11 ± 0.01 | 0.13 ± 0.01 | <0.10 | 0.09 |
| Glutamic acid | 0.64 ± 0.01 | 0.71 ± 0.01 | 0.76 ± 0.01 | 0.81 ± 0.01 | 0.85 |
| Glycine | 0.35 ± 0.01 | 0.38 ± 0.01 | 0.45 ± 0.01 | 0.35 ± 0.01 | 0.32 |
| Histidine | <0.10 | <0.10 | <0.10 | <0.10 | 0.06 |
| Isoleucine | 0.23 ± 0.02 | 0.22 ± 0.01 | 0.27 ± 0.01 | 0.17 ± 0.01 | 0.16 |
| Leucine | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.43 ± 0.01 | 0.25 ± 0.01 | 0.27 |
| Lysine | 0.22 ± 0.01 | 0.25 ± 0 | 0.30 ± 0.01 | 0.20 ± 0.01 | 0.24 |
| Methionine | <0.10 | 0.11 ± 0.01 | 0.14 ± 0.01 | <0.10 | 0.10 |
| Phenylalanine | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.30 ± 0.01 | 0.19 ± 0.01 | 0.18 |
| Proline | 0.16 ± 0.03 | 0.27 ± 0.04 | 0.34 ± 0.01 | 0.20 ± 0.01 | 0.18 |
| Serine | 0.22 ± 0.01 | 0.27 ± 0.01 | 0.32 ± 0.01 | 0.22 ± 0.01 | 0.22 |
| Threonine | 0.23 ± 0.01 | 0.26 ± 0.01 | 0.32 ± 0.01 | 0.21 ± 0.01 | 0.19 |
| Tryptophan | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | <0.05 | 0.04 |
| Tyrosine | <0.10 | <0.10 | 0.19 ± 0.01 | <0.10 | 0.01 |
| Valine | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.38 ± 0.01 | 0.25 ± 0.01 | 0.24 |
| Mexico (July 2020) | Mexico (January 2021) | Dom. Republic (February 2021) | Jamaica (August 2020) | Jamaica (February 2019) | |
|---|---|---|---|---|---|
| Alginate (% biomass DW) | 1.36 ± 0.30 | 6.62 ± 0.87 | 2.46 ± 0.13 | 6.98 ± 0.65 | 13.50 ± 4.61 |
| Alginate (% total monosaccharides) | 26.72 ± 3.73 | 50.23 ± 2.36 | 31.72 ± 2.56 | 50.97 ± 2.36 | 68.51 ± 4.29 |
| M/G ratio | 5.98 ± 0.51 | 3.10 ± 0.11 | 6.47 ± 0.22 | 2.61 ± 0.05 | 1.83 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonon, T.; Machado, C.B.; Webber, M.; Webber, D.; Smith, J.; Pilsbury, A.; Cicéron, F.; Herrera-Rodriguez, L.; Jimenez, E.M.; Suarez, J.V.; et al. Biochemical and Elemental Composition of Pelagic Sargassum Biomass Harvested across the Caribbean. Phycology 2022, 2, 204-215. https://doi.org/10.3390/phycology2010011
Tonon T, Machado CB, Webber M, Webber D, Smith J, Pilsbury A, Cicéron F, Herrera-Rodriguez L, Jimenez EM, Suarez JV, et al. Biochemical and Elemental Composition of Pelagic Sargassum Biomass Harvested across the Caribbean. Phycology. 2022; 2(1):204-215. https://doi.org/10.3390/phycology2010011
Chicago/Turabian StyleTonon, Thierry, Carla Botelho Machado, Mona Webber, Deanna Webber, James Smith, Amy Pilsbury, Félix Cicéron, Leopoldo Herrera-Rodriguez, Eduardo Mora Jimenez, Julio V. Suarez, and et al. 2022. "Biochemical and Elemental Composition of Pelagic Sargassum Biomass Harvested across the Caribbean" Phycology 2, no. 1: 204-215. https://doi.org/10.3390/phycology2010011
APA StyleTonon, T., Machado, C. B., Webber, M., Webber, D., Smith, J., Pilsbury, A., Cicéron, F., Herrera-Rodriguez, L., Jimenez, E. M., Suarez, J. V., Ahearn, M., Gonzalez, F., & Allen, M. J. (2022). Biochemical and Elemental Composition of Pelagic Sargassum Biomass Harvested across the Caribbean. Phycology, 2(1), 204-215. https://doi.org/10.3390/phycology2010011






