The Basics of Clinical Nutrition for Compromised Ruminants—A Narrative Review
Simple Summary
Abstract
1. Introduction
2. The Background of Ruminant Clinical Nutrition
3. Pathophysiology of Nutrient Utilization in Compromised Ruminants and Applicability to the Clinical Nutrition Intervention
3.1. Pathophysiology of Perturbed Utilization of Protein and Energy
3.2. Pathophysiology of Perturbed Utilization of Minerals, Vitamins and Water
3.3. Pathophysiology of Impaired Alimentary Function and Integrity
Pathophysiology of Forestomach Impaired Function and Integrity
3.4. Pre-Ruminant Stage
3.5. Summary of Pathophysiologic Alterations in Ruminants Important to Clinical Nutrition Intervention
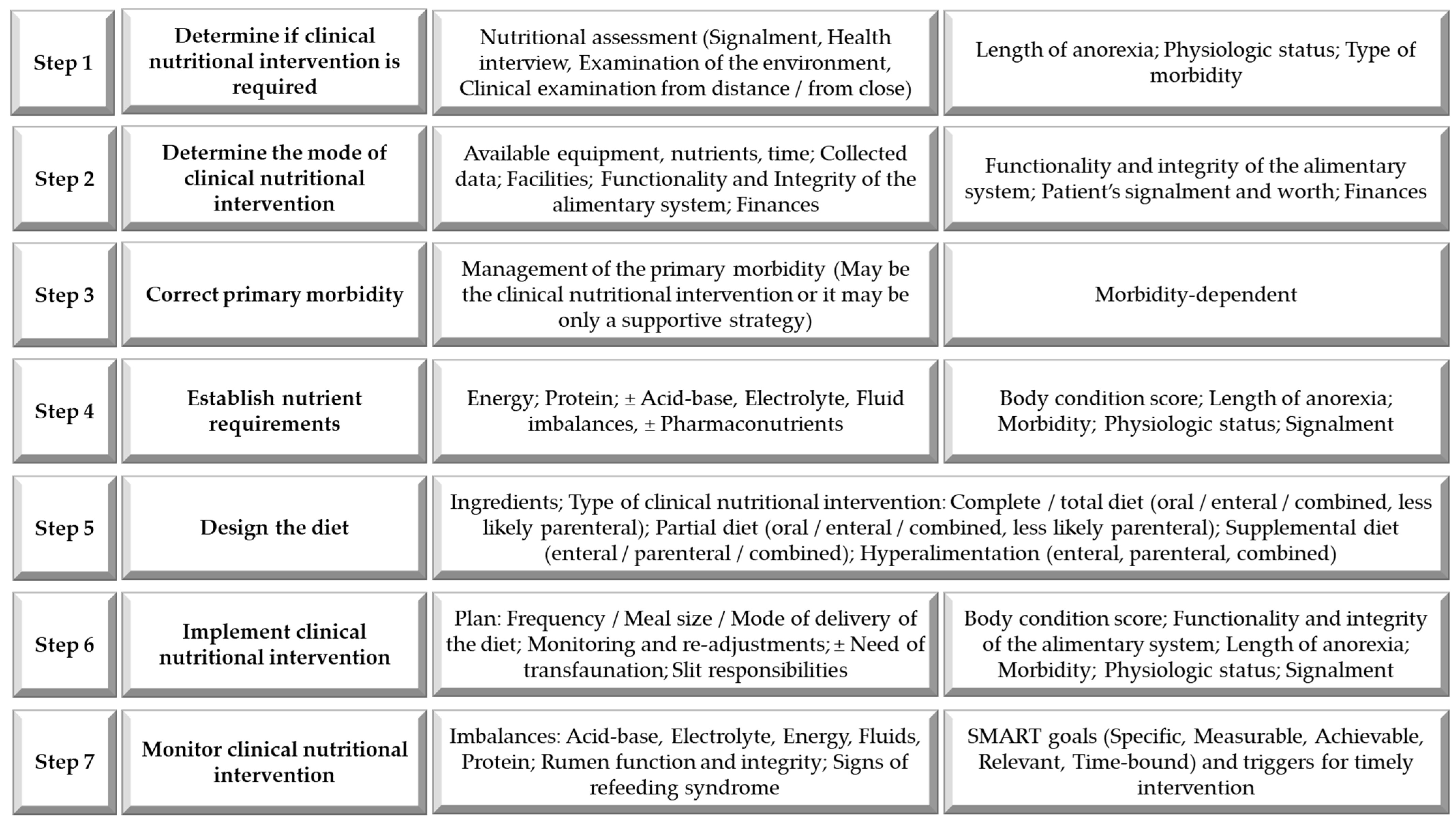
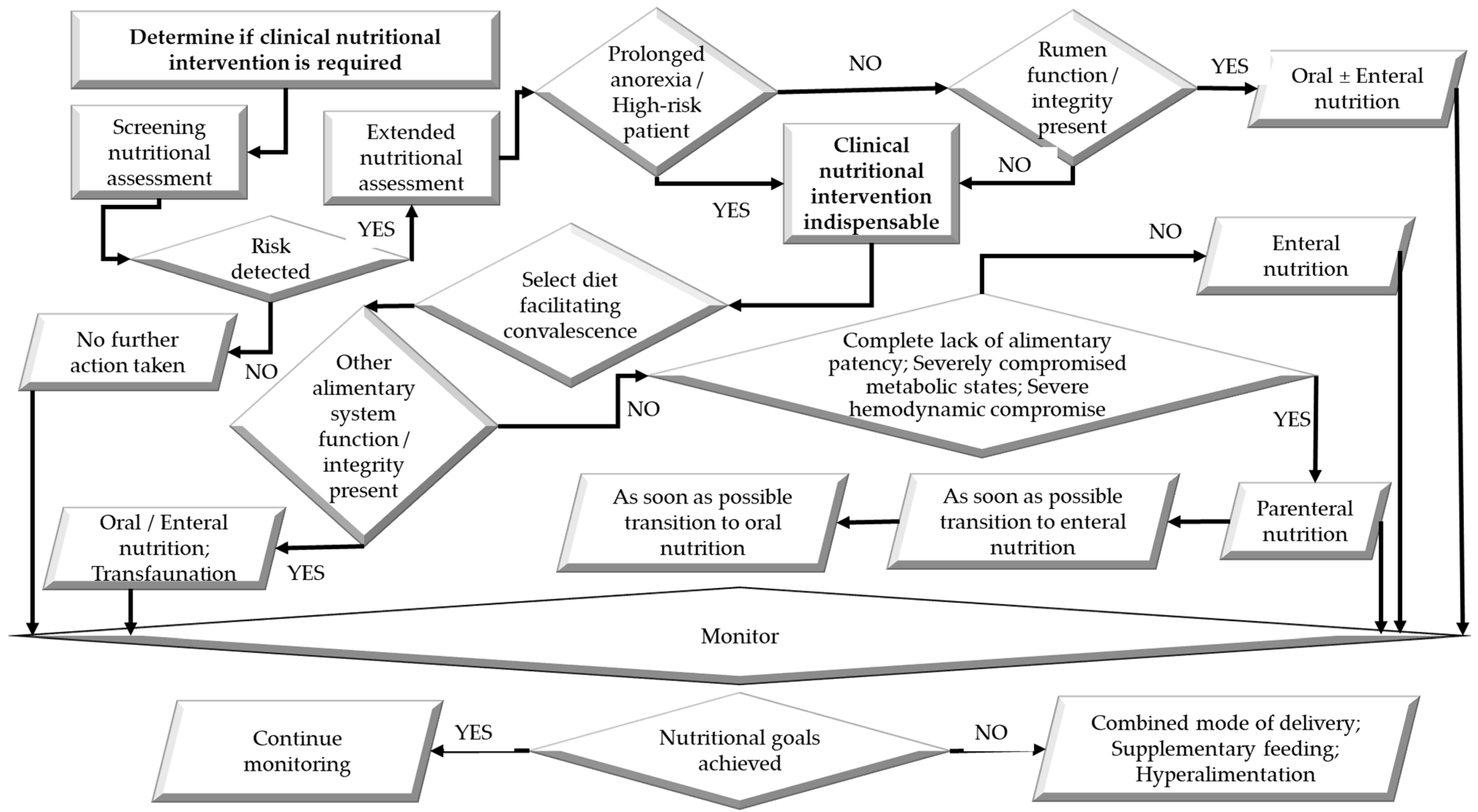
4. Establishing the Need for Clinical Nutrition Intervention in Compromised Ruminants
- Cachectic or debilitated ruminants (immediately);
- Growing ruminants (particularly during the exponential growth phase) when anorexic > 24–36 h;
- High-producing lactating cows expend significant amounts of energy to produce milk. A failure to consume sufficient food to meet the demands of milk production will result in a loss of substantial amounts of muscle and fat mass;
- Lactating females when anorexic for >12–24 h;
- Mature ruminants in average body condition scores (BCS; 3–4/5) being anorexic for a maximum of 2–3 days;
- Pregnant females in the last half of pregnancy, and particularly in the last trimester;
- Ruminants from extensively managed enterprises with irregular supervision should always be considered anorexic for a few days before detection;
- Ruminants with a high BCS (≥4/5), due to the risk of hepatic lipidosis;
- Ruminants with a low body condition score (BCS; ≤1.5/5) and prolonged malnutrition often have an unstable microbiota, underdeveloped rumen papillae, and increased requirements for specific vitamins, minerals, and amino acids;
- Ruminants that have undergone surgery experience high stress levels and may experience pain in the days following the procedure, which can affect their food intake;
- Ruminants with pre-existing metabolic derangements that would worsen with further anorexia;
- Severely immunocompromised ruminants;
- Specific morbidities and metabolic disorders, such as diarrhea.
5. Considerations in Designing a Clinical Diet for Compromised Ruminants
5.1. Nutritional Requirements
5.1.1. Energy Requirements
- 10% increase following elective surgery;
- 10–30% increase during heat stress/rumen failure;
- 10–30% increase in acute liver failure;
- 10–40% increase following parenteral loss of nutrients (e.g., nephropathy);
- 10–50% increase following enteral loss of nutrients (e.g., intestinal resection, severe parasitic gastroenteritis);
- 20–50% increase following bone fracture/major trauma;
- 30–50% increase following clostridial myositis/severe muscle trauma;
- 30–60% increase following major infection/sepsis/toxemia (approximately 10–13% increase for each 1 °C decrease/increase in body temperature);
- 40% increase following major peritonitis;
- 50–110% increase following major burns.
5.1.2. Protein Requirements
5.2. Meal Offering Frequency and Quantity
5.3. Medication–Nutrient Interaction
6. Options for the Delivery of the Diet During Clinical Nutrition Interventions to Compromised Ruminants
6.1. Oral Feeding Mode
Pre-Ruminant Stage
6.2. Enteral Feeding Mode
6.2.1. Transfaunation
6.2.2. Pre-Ruminant Stage
6.3. Parenteral Feeding Mode
Pre-Ruminant Stage
7. Pharmaconutrition for Compromised Ruminants
8. Future Directions
9. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
Glossary
| Acidosis | A condition characterized by excess acids in the body fluids |
| Anorexia | A complete absence of appetite (Synonym: aphagia) |
| Appetite | The desire to eat the offered diet |
| Big data | Aggregation and analysis of large/big data sets from multiple sources in management decisions in livestock enterprises (e.g., diet analysis, genomics, health records, production and product quality) |
| Bloat | Bloat is distention of a portion of the forestomaches (i.e., rumen) resulting from the accumulation of free gas or froth (Synonym: Tympany) |
| Clinical nutrition | The study and overall analysis of the interaction of nutrition and overall growth, health, and wellbeing of the (ruminant) body/individual |
| Colostrum | The first secretion from the mammary gland after calving (giving birth), rich in antibodies, growth-stimulating factors, other immune factors, and nutrients |
| Dysphagia | Difficulty swallowing; In broader term, it means difficulty in taking feed and/or liquids through the mouth, pharynx, and/or esophagus, so preventing entry into the stomach |
| Dysbiosis | An imbalance between the types of organism present in an animal’s microbiota, especially that of the alimentary system, thought to contribute to a range of ill health effects |
| Effective fiber (eNDF) | The fraction of fiber (NDF) that stimulates chewing activity, primarily related to the particle size (Synonym: physically effective fiber; peNDF) |
| Forage | The most important feed resource for ruminants globally. Representatives are grasses, forage crops, and legumes. May be fed as pastures or preserved forages (e.g., baleage, hay, or silage) |
| Hepatic lipidosis | A major metabolic disorder, most frequently in the very late pregnancy or the early lactation in female livestock as a result of overproduction of fatty acids and accumulation of lipids within the liver (synonym fatty liver disease) |
| Hyperalimentation | Administration of excess nutrients by enteral/parenteral route, particularly in patients unable to ingest enough diet orally |
| Immunocompetence | The ability of an individual’s immune system to work properly, allowing its body to mount an appropriate immune response as required |
| Immunostimulant | A substance of natural or pharmaceutical origin that stimulates the body immune system, usually in a non-specific manner, by activating or enhancing any of its components |
| Inappetence | A decreased appetite (Synonym: hypophagia) |
| Indigestion | A disruption of the ‘normal’ function of the reticulorumen (main portion of the forestomaches in ruminants) that may affect forestomach motility or microbial fermentation or both |
| Intelligent feeding system | A feeding system that incorporates an algorithm developed to monitor livestock ration intake, dependent on the data collected by precision technologies and/or human input |
| Morbidity | Any ill state in an individual; Proportion of the population affected by a particular condition/disorder/problem; State of being affected |
| Neonate | Newborn individual; In ruminants, typically first 3–4 weeks of life before any forestomach activity is present (pre-ruminant stage) |
| Nutrigenomics | The study of the complex interaction of genes and nutrients in livestock, to understand how various genes are expressed in response to specific nutrients |
| Obtundancy | A dulled or reduced levels of alertness or consciousness of an individual (common misnomer in veterinary medicine is depression, which is a symptom, not a sign) |
| Parenteral | Given/Occurring/Situated outside the intestines |
| Patency | The quality and state of a tubular organ/system being open and passage being uninterrupted |
| Prebiotic | A non-digestible food ingredient that promotes the growth of beneficial gut microbiota |
| Probiotic | Directly fed microbe which stimulates the growth of particular microbiota, especially those with beneficial properties (such as those of the gut microbiota) |
| Real-time monitoring | Monitoring involving automated systems, sensors, or wearable devices to continuously collect data on behavior, health, physiological parameters, and production |
| Refeeding syndrome | A potentially fatal shift in electrolytes and fluids that may occur in severely malnourished patients receiving artificial refeeding, whether oral, enteral, or parenteral |
| Reperfusion | The restoration of the blood flow to an organ or tissue after being significantly to completely blocked |
| Resting energy requirement (RER) | The energy requirement of a livestock individual at rest in a thermoneutral environment |
| Rumen | The first forestomach and the largest in mature ruminant. It is a muscular sac that contains large number of microbes involved in fermentation of the ingested diet. Fermented ingesta is passed into the reticulum. The fermentation of diet components unable to be digested by mammalian enzymes makes ruminants valuable in the eco system |
| Rumen acidosis | A metabolic disease that affects all ruminants. In cattle, both feedlot as well as dairy cattle. Rumen acidosis is usually associated with the ingestion of large amounts of highly fermentable, carbohydrate-rich feeds (e.g., cereal grains), which result in the excessive production and accumulation of acids in the rumen (pH of the rumen contents changes from mildly alkaline [around 7] to acidic [<5.6 down to <4.5]) |
| Rumenostomy | Surgical creation of temporary or permanent (insertion of rumen cannula) opening between the rumen and environment, including incising the skin, subcutaneous tissues, abdominal muscles, peritoneum, and the rumen wall |
| Siallorrhoea | Excessive flow of saliva (‘drooling’) |
| Splanchnic | Related to organ/s within the abdominal cavity |
| Stress | A Non-specific response of the body to any demand, usually from the environment where the livestock individual resides, management or nutrition |
| Transfaunation | Procedure consisting of removal of rumen fluid with healthy microbiota and good quality from one ruminant, and transfer of the removed fluid into the rumen of another ruminant individual |
References
- Galyean, M.L.; Perino, L.J.; Duff, G.C. Interaction of cattle health/immunity and nutrition. J. Anim. Sci. 1999, 77, 1120–1134. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7 (Suppl. S1), 112–122. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Dey, A. Nutrition in health and immune function of ruminants. Indian J. Anim. Sci. 2015, 85, 103–112. [Google Scholar] [CrossRef]
- Saha, S.K.; Pathak, N.N. Clinical and therapeutic nutrition. In Fundamentals of Animal Nutrition; Springer: Singapore, 2021; pp. 259–266. [Google Scholar]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.A.C.; Simões, J. Invited review: Ketosis diagnosis and monitoring in high-producing dairy cows. Dairy 2021, 2, 303–325. [Google Scholar] [CrossRef]
- Oetzel, G.R.; Berger, L.L. Protein-energy malnutrition in beef cows. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, New York, NY, USA, 19–22 November 1985; pp. 116–123. [Google Scholar] [CrossRef]
- Millen, D.D.; Pacheco, R.D.L.; da Silva Cabral, L.; Cursino, L.L.; Watanabe, D.H.M.; Rigueiro, A.L.N. Ruminal acidosis. In Rumenology; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 127–156. [Google Scholar]
- Goff, J.P. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 2006, 89, 1292–1301. [Google Scholar] [CrossRef]
- McGrath, J.; Duval, S.M.; Tamassia, L.F.M.; Kindermann, M.; Stemmler, R.T.; de Gouvea, V.N.; Acedo, T.S.; Immig, I.; Williams, S.N.; Celi, P. Nutritional strategies in ruminants: A lifetime approach. Res. Vet. Sci. 2018, 116, 28–39. [Google Scholar] [CrossRef]
- Sahoo, A. Clinical nutrition and therapeutic diets: New opportunities in farm animal practice. EC Vet. Sci. 2020, 5, 12–29. [Google Scholar]
- Gera, T. Efficacy and safety of therapeutic nutrition products for home based therapeutic nutrition for severe acute malnutrition: A systematic review. Indian Pediatr. 2010, 47, 709–718. [Google Scholar] [CrossRef]
- Jones, N.E.; Heyland, D.K. Pharmaconutrition: A new emerging paradigm. Curr. Opin. Gastroenterol. 2008, 24, 215–222. [Google Scholar] [CrossRef]
- Ramdani, D.; Yuniarti, E.; Jayanegara, A.; Chaudhry, A.S. Roles of essential oils, polyphenols, and saponins of medicinal plants as natural additives and anthelmintics in ruminant diets: A systematic review. Animals 2023, 13, 767. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, L.; Zhang, L.; Shi, Q.; Li, L. Branched-chain amino acids in liver diseases: Complexity and controversy. Nutrients 2024, 16, 1875. [Google Scholar] [CrossRef] [PubMed]
- Gallagher-Allred, C.R.; Voss, A.C.; Finn, S.C.; McCamish, M.A. Malnutrition and clinical outcomes: The case for medical nutrition therapy. J. Am. Diet. Assoc. 1996, 96, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Dworatzek, P.D.; Arcudi, K.; Gougeon, R.; Husein, N.; Sievenpiper, J.L.; Williams, S.L. Nutrition therapy. Can. J. Diabetes 2013, 37, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, A.S. Rumen management during aphagia: Review article. J. S. Afr. Vet. Assoc. 2008, 79, 106–112. [Google Scholar] [CrossRef]
- Galbat, S.; Keshta, H. Evaluation of rumen transfaunation after treatment of rumen acidosis in cows. Curr. Sci. Int. 2020, 9, 625–632. [Google Scholar] [CrossRef]
- DePeters, E.J.; George, L.W. Rumen transfaunation. Immunol. Lett. 2014, 162, 69–76. [Google Scholar] [CrossRef]
- Callan, R.J.; Applegate, T.J. Temporary rumenostomy for the treatment of forestomach diseases and enteral nutrition. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 525–537. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Bąkowski, M.; Kiczorowska, B. Probiotic microorganisms and herbs in ruminant nutrition as natural modulators of health and production efficiency—A review. Ann. Anim. Sci. 2021, 21, 3–28. [Google Scholar] [CrossRef]
- Reuben, R.C.; Elghandour, M.; Alqaisi, O.; Cone, J.W.; Marquez, O.; Salem, A.Z.M. Influence of microbial probiotics on ruminant health and nutrition: Sources, mode of action and implications. J. Sci. Food Agric. 2022, 102, 1319–1340. [Google Scholar] [CrossRef]
- Drackley, J.K.; Dann, H.M.; Douglas, N.; Guretzky, N.A.J.; Litherland, N.B.; Underwood, J.P.; Loor, J.J. Physiological and pathological adaptations in dairy cows that may increase susceptibility to periparturient diseases and disorders. Ital. J. Anim. Sci. 2016, 4, 323–344. [Google Scholar] [CrossRef]
- Naylor, J.M.; Ralston, S.L. Large Animal Clinical Nutrition; Mosby Year Book: St. Louis, MO, USA, 1991. [Google Scholar]
- Smith, B.P.; Van Metre, D.C.; Pusterla, N. Large Animal Internal Medicine, 6th ed.; Elsevier: St. Louis, MO, USA, 2020. [Google Scholar]
- Meganck, V.; Hoflack, G.; Opsomer, G. Advances in prevention and therapy of neonatal dairy calf diarrhoea: A systematical review with emphasis on colostrum management and fluid therapy. Acta Vet. Scand. 2014, 56, 75. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.W.; White, D.G.; Wagstaff, A.J.; Michell, A.R. Evaluation of a glutamine-containing oral rehydration solution for the treatment of calf diarrhoea using an Escherichia coli model. Vet. J. 1997, 153, 163–169. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, D.P. Nutritional consideration of the immune system. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Albuquerque, NM, USA, 16–19 September 1993; pp. 27–34. [Google Scholar]
- Watson, L.T. Nutritional management of the sick animal. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Indianapolis, IN, USA, 13–16 September 1990; pp. 104–106. [Google Scholar]
- Linneen, S. Beef nutrition 101: What a veterinarian needs to know to work with a nutritionist. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Long Beach, CA, USA, 22–24 September 2022; pp. 37–40. [Google Scholar]
- Goopy, J.P.; Korir, D.; Pelster, D.; Ali, A.I.M.; Wassie, S.E.; Schlecht, E.; Dickhoefer, U.; Merbold, L.; Butterbach-Bahl, K. Severe below-maintenance feed intake increases methane yield from enteric fermentation in cattle. Br. J. Nutr. 2020, 123, 1239–1246. [Google Scholar] [CrossRef]
- Hardy, J. Nutritional support and nursing care of the adult horse in intensive care. Clin. Tech. Equine Pract. 2003, 2, 193–198. [Google Scholar] [CrossRef]
- Petrovski, K.R.; Cusack, P.; Malmo, J.; Cockcroft, P. The value of ‘Cow Signs’ in the assessment of the quality of nutrition on dairy farms. Animals 2022, 12, 1352. [Google Scholar] [CrossRef]
- Hesta, M.; Shepherd, M. How to perform a nutritional assessment in a first-line/general practice. Vet. Clin. N. Am. Equine Pract. 2021, 37, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.Y.; Wang, Y.F.; Shen, Y.Z.; Zhou, G.; Wu, T.Y.; Zhang, X.; Wang, M.Z.; Loor, J.J.; Zhang, J. Effects of intravenous arginine infusion on inflammation and metabolic indices of dairy cows in early lactation. Animal 2020, 14, 346–352. [Google Scholar] [CrossRef]
- Carr, E.A. Enteral/Parenteral nutrition in foals and adult horses Practical guidelines for the practitioner. Vet. Clin. N. Am. Equine Pract. 2018, 34, 169–180. [Google Scholar] [CrossRef]
- Karinch, A.M.; Pan, M.; Lin, C.-M.; Strange, R.; Souba, W.W. Glutamine Metabolism in Sepsis and Infection. J. Nutr. 2001, 131, 2535S–2538S. [Google Scholar] [CrossRef]
- Dunkel, B.M.; Wilkins, P.A. Nutrition and the critically ill horse. Vet. Clin. N. Am. Equine Pract. 2004, 20, 107–126. [Google Scholar] [CrossRef]
- Ćwiek, A.; Borowiec, F. Effect of dietary fat source on nutrient digestibility and rumen fermentation in sheep. J. Anim. Feed Sci. 2005, 14 (Suppl. S1), 239–242. [Google Scholar] [CrossRef][Green Version]
- Mohamad Asrol, K.; Dek, P.; Suyub, I.; Sumita, S.; Jusoh, S. Production and optimisation of used cooking palm oil into protected fat calcium salts by fusion method using response surface methodology (RSM). Int. Food Res. J. 2023, 30, 696–708. [Google Scholar] [CrossRef]
- Warner, C.M.; Hahm, S.-W.; Archibeque, S.L.; Wagner, J.J.; Engle, T.E.; Roman-Muniz, I.N.; Woerner, D.; Sponsler, M.; Han, H. A comparison of supplemental calcium soap of palm fatty acids versus tallow in a corn-based finishing diet for feedlot steers. J. Anim. Sci. Technol. 2015, 57, 25. [Google Scholar] [CrossRef]
- Pan, X.; Nan, X.; Yang, L.; Jiang, L.; Xiong, B. Thiamine status, metabolism and application in dairy cows: A review. Br. J. Nutr. 2018, 120, 491–499. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F. Biological properties of vitamins of the B-complex, part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmy, K.; Virmani, M.; Malik, R.; Rajalakshmi, K.; Kasthuri, S. The role of B Vitamins in livestock nutrition. J. Vet. Med. Res. 2018, 5, 1162. [Google Scholar]
- Singh, A.; Kerketta, S.; Kumari, P.; Mahesh, M.; Rajak, S.; Kumar, R. Recent developments in B-Vitamin nutrition of dairy cattle. In Feed Additives and Supplements for Ruminants; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 399–421. [Google Scholar]
- Fitzgerald, T.; Norton, B.; Elliott, R.; Podlich, H.; Svendsen, O. The influence of long-term supplementation with biotin on the prevention of lameness in pasture fed dairy cows. J. Dairy Sci. 2000, 83, 338–344. [Google Scholar] [CrossRef]
- Sharma, V.; Rodionov, D.A.; Leyn, S.A.; Tran, D.; Iablokov, S.N.; Ding, H.; Peterson, D.A.; Osterman, A.L.; Peterson, S.N. B-vitamin sharing promotes stability of gut microbial communities. Front. Microbiol. 2019, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Graulet, B.; Matte, J.J.; Desrochers, A.; Doepel, L.; Palin, M.F.; Girard, C.L. Effects of dietary supplements of Folic Acid and Vitamin B12 on metabolism of dairy cows in early lactation. J. Dairy Sci. 2007, 90, 3442–3455. [Google Scholar] [CrossRef]
- Li, H.; Liu, Q.; Wang, C.; Yang, Z.; Guo, G.; Huo, W.; Pei, C.; Zhang, Y.; Zhang, S.; Wang, H. Effects of dietary supplements of rumen-protected folic acid on lactation performance, energy balance, blood parameters and reproductive performance in dairy cows. Anim. Feed Sci. Technol. 2016, 213, 55–63. [Google Scholar] [CrossRef]
- Ceja, G.; Boerman, J.P.; Neves, R.C.; Jorgensen, M.W.; Johnson, J.S. L-glutamine supplementation reduces gastrointestinal permeability and biomarkers of physiological stress in preweaning Holstein heifer calves. J. Dairy Sci. 2023, 106, 9663–9676. [Google Scholar] [CrossRef] [PubMed]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.; Moatshe, G.; LaPrade, R.F. Efficacy of vitamin C supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: A systematic review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H.; Stowe, H.D. Fat-soluble vitamin nutrition for dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 1991, 7, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.; Weiss, W. Invited Review: Mineral and vitamin nutrition in ruminants. Prof. Anim. Sci. 2014, 30, 180–191. [Google Scholar] [CrossRef]
- McGill, J.L.; Kelly, S.M.; Guerra-Maupome, M.; Winkley, E.; Henningson, J.; Narasimhan, B.; Sacco, R.E. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci. Rep. 2019, 9, 15157. [Google Scholar] [CrossRef]
- Weiss, W. Requirements of fat-soluble vitamins for dairy cows: A review. J. Dairy Sci. 1998, 81, 2493–2501. [Google Scholar] [CrossRef]
- Bourne, N.; Laven, R.; Wathes, D.; Martinez, T.; McGowan, M. A meta-analysis of the effects of Vitamin E supplementation on the incidence of retained foetal membranes in dairy cows. Theriogenology 2007, 67, 494–501. [Google Scholar] [CrossRef]
- Tang, S.; Ruan, Z.; Ma, A.; Wang, D.; Kou, J. Effect of vitamin K on wound healing: A systematic review and meta-analysis based on preclinical studies. Front. Pharmacol. 2022, 13, 1063349. [Google Scholar] [CrossRef] [PubMed]
- Riner, K.; Boos, A.; Hässig, M.; Liesegang, A. Vitamin D receptor distribution in intestines of domesticated sheep Ovis ammon f. aries. J. Morphol. 2008, 269, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Vigh, A.; Criste, A.D.; Corcionivoschi, N.; Gerard, C. Rumen solubility of copper, manganese and zinc and the potential link between the source and rumen function: A systematic review. Agriculture 2023, 13, 2198. [Google Scholar] [CrossRef]
- Brooks, H.W. Oral fluid therapy-supporting the gut mucosa. Cattle Pract. 1998, 6, 325–327. [Google Scholar]
- Constable, P. Fluid and electrolyte therapy in ruminants. Vet. Clin. Food Anim. Pract. 2003, 19, 557–597. [Google Scholar] [CrossRef]
- Pugh, D.G.; Baird, A.N. Sheep and Goat Medicine, 2nd ed.; Elsevier/Saunders: Maryland Heights, MO, USA, 2012. [Google Scholar]
- Bravo, D.M.; Wall, E.H. The rumen and beyond: Nutritional physiology of the modern dairy cow. J. Dairy Sci. 2016, 99, 4939–4940. [Google Scholar] [CrossRef]
- Garcia Diaz, T.; Ferriani Branco, A.; Jacovaci, F.A.; Cabreira Jobim, C.; Pratti Daniel, J.L.; Iank Bueno, A.V.; Gonçalves Ribeiro, M. Use of live yeast and mannan-oligosaccharides in grain-based diets for cattle: Ruminal parameters, nutrient digestibility, and inflammatory response. PLoS ONE 2018, 13, e0207127. [Google Scholar] [CrossRef]
- Tao, S.; Duanmu, Y.; Dong, H.; Ni, Y.; Chen, J.; Shen, X.; Zhao, R. High concentrate diet induced mucosal injuries by enhancing epithelial apoptosis and inflammatory response in the hindgut of goats. PLoS ONE 2014, 9, e111596. [Google Scholar] [CrossRef]
- Schcolnik, E. Basics of dairy diet design: Feeds and how they are included in diets. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, St. Louis, MO, USA, 12–13 February 2021; pp. 70–73. [Google Scholar]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]
- van Vuuren, A.M.; Calsamiglia, S.; Udén, P. Rumen health: A 360° analysis. Anim. Feed Sci. Technol. 2012, 172, 1–3. [Google Scholar] [CrossRef]
- Lean, I.J.; Golder, H.M.; Hall, M.B. Feeding, evaluating, and controlling rumen function. Vet. Clin. Food Anim. Pract. 2014, 30, 539–575. [Google Scholar] [CrossRef]
- Troutt, H.F. Pathology associated with rations. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Fort Worth, TX, USA, 2–5 December 1973; pp. 68–73. [Google Scholar]
- Zou, H.; Hu, R.; Wang, Z.A.-O.; Shah, A.A.-O.; Zeng, S.; Peng, Q.A.-O.; Xue, B.; Wang, L.A.-O.; Zhang, X.A.-O.; Wang, X.; et al. Effects of nutritional deprivation and re-alimentation on the feed efficiency, blood biochemistry, and rumen microflora in yaks (Bos grunniens). Animals 2019, 9, 607. [Google Scholar] [CrossRef]
- Clauss, M.; Hummel, J. Physiological adaptations of ruminants and their potential relevance for production systems. Rev. Bras. Zootec. 2017, 46, 606–613. [Google Scholar] [CrossRef]
- Hallowell, G.; Remnant, J. Fluid therapy in calves. Clin. Pract. 2016, 38, 439–449. [Google Scholar] [CrossRef]
- Hernández, J.; Benedito, J.L.; Abuelo, A.; Castillo, C. Ruminal acidosis in feedlot: From aetiology to prevention. Sci. World J. 2014, 702572, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.C.; Chigerwe, M. Diagnosis and treatment of infectious enteritis in neonatal and juvenile ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 101–117. [Google Scholar] [CrossRef]
- Lorenz, I. d-Lactic acidosis in calves. Vet. J. 2009, 179, 197–203. [Google Scholar] [CrossRef]
- Buechner-Maxwell, V.A. Nutritional support for neonatal foals. Vet. Clin. N. Am. Equine Pract. 2005, 21, 487–510. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut microbiome colonization and development in neonatal ruminants: Strategies, prospects, and opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Lucas, A.; Bloom, S.R.; Aynsley-Green, A. Gut hormones and ‘minimal enteral feeding’. Acta Paediatr. Scand. 1986, 75, 719–723. [Google Scholar] [CrossRef]
- Cerdo, T.; Garcia-Santos, J.A.; Rodriguez-Pohnlein, A.; Garcia-Ricobaraza, M.; Nieto-Ruiz, A.; Bermúdez, M.G.; Campoy, C. Impact of total parenteral nutrition on gut microbiota in pediatric population suffering intestinal disorders. Nutrients 2022, 14, 4691. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef]
- Harris, P.; Nelson, S. Approach to clinical nutrition. UK-Vet Equine 2022, 6, 50–55. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Amagwula, I.O. Nutritional diseases and nutrient toxicities: A systematic review of the diets and nutrition for prevention and treatment. Int. J. Adv. Acad. Res. 2020, 6, 1–46. [Google Scholar] [CrossRef]
- Pirie, R.S.; Jago, R.C. Nutritional support for the dysphagic adult horse. Equine Vet. Educ. 2015, 27, 430–441. [Google Scholar] [CrossRef]
- Tackett, V.L.; Bertrand, J.A.; Jenkins, T.C.; Pardue, F.E.; Grimes, L.W. Interaction of dietary fat and acid detergent fiber diets of lactating dairy cows. J. Dairy Sci. 1996, 79, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Szumacher-Strabel, M. Microbial protein net synthesis in sheep fed meadow hay supplemented with different source and level of fat. J. Anim. Feed Sci. 1998, 7, 385–394. [Google Scholar] [CrossRef][Green Version]
- Onetti, S.G.; Shaver, R.D.; McGuire, M.A.; Palmquist, D.L.; Grummer, R.R. Effect of supplemental tallow on performance of dairy cows fed diets with different corn silage:alfalfa silage ratios. J. Dairy Sci. 2002, 85, 632–641. [Google Scholar] [CrossRef]
- Clark, A.; Imran, J.; Madni, T.; Wolf, S.E. Nutrition and metabolism in burn patients. Burn. Trauma 2017, 5, 12. [Google Scholar] [CrossRef]
- Yadav, B.; Singh, G.; Verma, A.K.; Dutta, N.; Sejian, V. Impact of heat stress on rumen functions. Vet. World 2013, 6, 992–996. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Hirsch, K.R.; Saylor, H.E.; Gould, L.M.; Blue, M.N.M. Nutritional considerations and strategies to facilitate injury recovery and rehabilitation. J. Athl. Train. 2020, 55, 918–930. [Google Scholar] [CrossRef]
- Hynd, P.I. Animal Nutrition: From Theory to Practice; CSIRO Publishing: Clayton, VIC, Australia, 2019. [Google Scholar]
- Gouvêa, V.N.; Cooke, R.F.; Marques, R.S. Impacts of stress-induced inflammation on feed intake of beef cattle. Front. Anim. Sci. 2022, 3, 10. [Google Scholar] [CrossRef]
- Campbell, R.M.; Cuthbertson, D.P. Effect of environmental temperature on the metabolic response to injury. Exp. Physiol. 1967, 52, 114–129. [Google Scholar] [CrossRef]
- Luethy, D.; Stefanovski, D.; Sweeney, R.W. Refeeding syndrome in small ruminants receiving parenteral nutrition. J. Vet. Intern. Med. 2020, 34, 1674–1679. [Google Scholar] [CrossRef]
- Santos, C.A.; Boullata, J.I. An approach to evaluating drug-nutrient interactions. Pharmacotherapy 2005, 25, 1789–1800. [Google Scholar] [CrossRef]
- Kakazu, E.; Kanno, N.; Ueno, Y.; Shimosegawa, T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J. Immunol. 2007, 179, 7137–7146. [Google Scholar] [CrossRef]
- Spears, J.W. Ionophores and nutrient digestion and absorption in ruminants. J. Nutr. 1990, 120, 632–638. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Rodríguez, C. Bioavailability of in-feed tetracyclines is influenced to a greater extent by crude protein rather than calcium. Anim. Feed Sci. Technol. 2014, 198, 323–332. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Ellis, K.J. Issues and challenges in developing ruminal drug delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 1415–1436. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.; Angel, T.; Rogers, I. Practical use of non-steroidal anti-inflammatory drugs in farm practice. Livestock 2020, 25, 202–209. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota-host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Qiu, Q.A.-O.; Gao, C.; Su, H.A.-O.; Cao, B. Rumen fermentation characteristics require more time to stabilize when diet shifts. Animals 2021, 11, 2192. [Google Scholar] [CrossRef]
- Steiner, S.; Neidl, A.; Linhart, N.; Tichy, A.; Gasteiner, J.; Gallob, K.; Baumgartner, W.; Wittek, T. Randomised prospective study compares efficacy of five different stomach tubes for rumen fluid sampling in dairy cows. Vet. Rec. 2015, 176, 50. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Davis, T.A. Proteins and amino acids in enteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Divers, T.J.; Sweeney, R.W.; Galligan, D. Parenteral nutrition in cattle. Bov. Pract. 1987, 22, 56–57. [Google Scholar] [CrossRef]
- Doley, J. Enteral nutrition overview. Nutrients 2022, 14, 2180. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.M.; Zello, G.A.; Abeysekara, S. Advances in oral and intravenous fluid therapy of calves with gastrointestinal disease. In Proceedings of the World Buiatrics Congress, Nice, France, 15–19 October 2006. [Google Scholar]
- Naylor, J.M. Effects of electrolyte solutions for oral administration on clotting of milk. J. Am. Vet. Med. Assoc. 1992, 201, 1026–1029. [Google Scholar] [CrossRef]
- Bachmann, L.; Homeier, T.; Arlt, S.; Brueckner, M.; Rawel, H.; Deiner, C.; Hartmann, H. Influence of different oral rehydration solutions on abomasal conditions and the acid-base status of suckling calves. J. Dairy Sci. 2009, 92, 1649–1659. [Google Scholar] [CrossRef]
- Constable, P.D.; Trefz, F.M.; Sen, I.; Berchtold, J.; Nouri, M.; Smith, G.; Grünberg, W. Intravenous and oral fluid therapy in neonatal calves with diarrhea or sepsis and in adult cattle. Front. Vet. Sci. 2021, 7, 603358. [Google Scholar] [CrossRef]
- Alves, S.R.; Avanza, M.F.B.; Silva, M.O.d.; Ermita, P.A.N.; Gomes, L.L.; Monteiro, L.C.; Santos, P.V.d.M.; Costa, C.M.; Ferreira, G.M.M.; Mattos, F.S.; et al. Two enteral solutions with different chloride concentrations administered by naso-ruminal route for fluid therapy in adult cattle. Ciência Rural 2019, 49, 6. [Google Scholar] [CrossRef]
- Rabaza, A.; Banchero, G.; Cajarville, C.; Zunino, P.; Britos, A.; Repetto, J.L.; Fraga, M. Effects of feed withdrawal duration on animal behaviour, rumen microbiota and blood chemistry in feedlot cattle: Implications for rumen acidosis. Animal 2020, 14, 66–77. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Kim, Y.H.; Kanazawa, T.; Ikuta, K.; Sato, S. Effects of short-term fasting on ruminal pH and volatile fatty acids in cattle fed high-roughage versus high-concentrate diets. J. Vet. Med. Sci. 2020, 82, 1415–1420. [Google Scholar] [CrossRef]
- Rager, K.D.; George, L.W.; House, J.K.; DePeters, E.J. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J. Am. Vet. Med. Assoc. 2004, 225, 915–920. [Google Scholar] [CrossRef]
- Steiner, S.; Linhart, N.; Neidl, A.; Baumgartner, W.; Tichy, A.; Wittek, T. Evaluation of the therapeutic efficacy of rumen transfaunation. J. Anim. Physiol. Anim. Nutr. 2020, 104, 56–63. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Divers, T.J. The use of parenteral nutrition in calves. Vet. Clin. N. Am. Food Anim. Pract. 1990, 6, 125–131. [Google Scholar] [CrossRef]
- Jones, M.; Navarre, C. Fluid therapy in small ruminants and camelids. Vet. Clin. N. Am.—Food Anim. Pract. 2014, 30, 441–453. [Google Scholar] [CrossRef]
- Pugh, D.G. Sheep, Goat, and Cervid Medicine, 3rd ed.; Elsevier: Edinburgh, UK, 2021. [Google Scholar]
- Grove-White, D. Practical intravenous fluid therapy in the diarrhoeic calf. In Practice 2007, 29, 404–408. [Google Scholar] [CrossRef]
- Dore, V.; Foster, D.M.; Ru, H.; Smith, G.W. Comparison of oral, intravenous, and subcutaneous fluid therapy for resuscitation of calves with diarrhea. J. Dairy Sci. 2019, 102, 11337–11348. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, F.; Kong, F.; Cao, Z.; Wang, W.; Yang, H.; Wang, Y.; Bi, Y.; Li, S. Effect of supplementing different levels of L-glutamine on Holstein calves during weaning. Antioxidants 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Joshi, M. Clinical nutrition for udder health management in dairy animals. Intas Polivet 2020, 21, 348–355. [Google Scholar]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Shaheen, H.M.; Abdel-Latif, M.A.; Noreldin, A.E.; Khafaga, A.F. The applications of Origanum vulgare and its derivatives in human, ruminant and fish nutrition—A review. Ann. Anim. Sci. 2020, 20, 389–407. [Google Scholar] [CrossRef]
- Mirzaei-Aghsaghali, A.; Syadati, S.A.; Fathi, H.; Rasouli, S.; Sadaghian, M.; Tarahomi, M. Garlic in ruminant feeding. Asian J. Biol. Sci. 2012, 5, 328–340. [Google Scholar] [CrossRef]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed tannins as antioxidants in ruminants-effectiveness and action mechanisms to improve animal antioxidant status and oxidative stability of products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef]
- Charmpilas, N.; Ruckenstuhl, C.; Sica, V.; Büttner, S.; Habernig, L.; Dichtinger, S.; Madeo, F.; Tavernarakis, N.; Bravo-San Pedro, J.M.; Kroemer, G. Acyl-CoA-binding protein (ACBP): A phylogenetically conserved appetite stimulator. Cell Death Dis. 2020, 11, 7. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- National Research Council; Committee on Nutrient Requirements of Small Ruminants. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Research Council: Washington, DC, USA, 2007. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Brooks, H.W.; Hall, G.A.; Wagstaff, A.J.; Michell, A.R. Detrimental effects on villus form during conventional oral rehydration therapy for diarrhoea in calves; alleviation by a nutrient oral rehydration solution containing glutamine. Vet. J. 1998, 155, 263–274. [Google Scholar] [CrossRef]
- De-Souza, D.A.; Greene, L.J. Intestinal permeability and systemic infections in critically ill patients: Effect of glutamine. Crit. Care Med. 2005, 33, 1125–1135. [Google Scholar] [CrossRef]
- Yue, H.Y.; Wang, Y.; Zeng, J.; Jiang, H.; Li, W. Enteral glutamine supplements for patients with severe burns: A systematic review and meta-analysis. Chin. J. Traumatol. Engl. Ed. 2024, 27, 359–367. [Google Scholar] [CrossRef]
- Amsathkumar, L.; Jadhav, S.E.; Pattanaik, A.K.; Dutta, N. Nutrient utilization and performance of endotoxin exposed kids supplemented with phytogenic feed additive. Anim. Nutr. Feed Technol. 2019, 19, 371–383. [Google Scholar] [CrossRef]
- Renaud, D.L.; Kelton, D.F.; Weese, J.S.; Noble, C.; Duffield, T.F. Evaluation of a multispecies probiotic as a supportive treatment for diarrhea in dairy calves: A randomized clinical trial. J. Dairy Sci. 2019, 102, 4498–4505. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Molatová, Z.; Marounek, M. Effects of caprylic acid and triacylglycerols of both caprylic and capric acid in rabbits experimentally infected with enteropathogenic Escherichia coli O103. Vet. Microbiol. 2008, 126, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Chirase, N.K.; Hutcheson, D.P.; Thompson, G.B. Feed intake, rectal temperature, and serum mineral concentrations of feedlot cattle fed zinc oxide or zinc methionine and challenged with infectious bovine rhinotracheitis virus. J. Anim. Sci. 1991, 69, 4137–4145. [Google Scholar] [CrossRef]
- Moonsie-Shageer, S.; Mowat, D.N. Effect of level of supplemental chromium on performance, serum constituents, and immune status of stressed feeder calves. J. Anim. Sci. 1993, 71, 232–238. [Google Scholar] [CrossRef]
- Wright, A.J.; Mowat, D.N.; Mallard, B.A. Supplemental chromium and bovine respiratory disease vaccines for stressed feeder calves. Can. J. Anim. Sci. 1994, 74, 287–295. [Google Scholar] [CrossRef]
- Khorrami, B.; Vakili, A.R.; Mesgaran, M.D.; Klevenhusen, F. Thyme and cinnamon essential oils: Potential alternatives for monensin as a rumen modifier in beef production systems. Anim. Feed Sci. Technol. 2015, 200, 8–16. [Google Scholar] [CrossRef]
- Carr, E.A.; Holcombe, S.J. Nutrition of critically ill horses. Vet. Clin. N. Am. Equine Pract. 2009, 25, 93–108. [Google Scholar] [CrossRef]
- Lo, E.K.K.; Felicianna; Xu, J.H.; Zhan, Q.; Zeng, Z.; El-Nezami, H. The emerging role of branched-chain amino acids in liver diseases. Biomedicines 2022, 10, 1444. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. World J. Gastroenterol. 2013, 19, 7620–7629. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, W.; Socha, P.; Lebensztejn, D.; Wierzbicka, A.; Mazur, A.; Neuhoff-Murawska, J.; Matusik, P. Omega-3 fatty acids for treatment of non-alcoholic fatty liver disease: Design and rationale of randomized controlled trial. BMC Pediatr. 2013, 13, 85. [Google Scholar] [CrossRef]
- Spooner, M.H.; Jump, D.B. Nonalcoholic fatty liver disease and omega-3 fatty acids: Mechanisms and clinical use. Annu. Rev. Nutr. 2023, 43, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Pomar, C.; Remus, A. Fundamentals, limitations and pitfalls on the development and application of precision nutrition techniques for precision livestock farming. Animal 2023, 17, 100763. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J. Nutrigenomics in livestock—Recent advances. J. Appl. Genet. 2020, 61, 93–103. [Google Scholar] [CrossRef]
- Nunes, A.T.; Faleiros, C.A.; Poleti, M.D.; Novais, F.J.; López-Hernández, Y.; Mandal, R.; Wishart, D.S.; Fukumasu, H. Unraveling ruminant feed efficiency through metabolomics: A systematic review. Metabolites 2024, 14, 675. [Google Scholar] [CrossRef] [PubMed]
| Alteration in | Common Causes | |
|---|---|---|
| Syndromes | Diagnoses | |
| Appetite | Alimentary syndromes Generalized malaise Heat stress Neurologic syndromes Severe fever/inflammation/pain/sepsis/toxemia Insufficient Diet delivery/preparation/Nutrient supply Offensive diet smell/taste Poor diet quality/spoilage Sudden diet change/Unfamiliar diet | Actinobacillosis Actinomycosis Any generalized morbidity Cheilitis Foreign body Glossitis Mandibular Fracture Sinusitis Stomatitis |
| Prehension | Congenital abnormalities Cranial nerve dysfunction Lameness Localized pain Oral trauma Stomatitis Tongue disorders Offensive diet smell/taste Poor diet quality Presence of corrosive compounds | Actinobacillosis Cheilitis Foreign body Glossitis Mandibular Fracture Sinusitis Stomatitis |
| Mastication | Congenital abnormalities Cranial nerve dysfunction Dental problems Localized pain Oral trauma Pharyngeal abscessation/cellulitis/trauma Quidding Stomatitis Tongue disorders Offensive diet smell/taste Poor diet quality Presence of corrosive compounds | Actinobacillosis Actinomycosis Balling gun injury Botulism Foreign body Lead/Salt/Sulfur/Water toxicosis Listeriosis Mandibular fracture Tetanus |
| Swallowing | Congenital abnormalities Cranial nerve dysfunction Dysphagia Neoplasia Odynophagia Oral trauma Esophageal abscessation/cellulitis/diverticulum/neoplasia/trauma Pharyngeal abscessation/cellulitis/trauma Stomatitis Tongue disorders Presence of corrosive compounds | Balling gun injury Botulism Choke Foreign body Goiter Hypocalcemia Lead/Sulfur toxicosis Megaesophagus Rabies |
| Rumen fermentation | Alimentary syndromes Poor diet quality/spoilage Sudden diet change | Imbalanced diet/Indigestion Rumen acidosis (less likely alkalosis) |
| Eructation | Alimentary syndromes Congenital abnormalities Neurologic syndromes | Bloat Choke Foreign body |
| Rumination | Alimentary syndromes Congenital abnormalities Neurologic syndromes | Rumen acidosis (less likely alkalosis)/impaction Rumenitis Traumatic reticulo-peritonitis Vagus indigestion |
| Chime passage | Alimentary syndromes Congenital abnormalities Neoplasia Neurologic syndromes | Foreign body Herniation Omasal impaction Obstructive disorders Use of laxatives Vagus indigestion |
| Digestion | Alimentary syndromes Congenital abnormalities Maldigestion syndrome (rare in ruminants) | Abomasal displacement/impaction/ulceration Abomasitis Cholestasis Enteritis |
| Absorption | Alimentary syndromes Iatrogenic interventions Diarrhea Malabsorption syndrome | Parasitic gastro-enteritis Use of binders in diet |
| Utilization | Alimentary/Hepatic/Urinary loss Alimentary/Endocrine syndromes Cachectic state Chronic morbidity Heat stress Neoplasia Parasitism Severe morbidity (fever/inflammation/pain/sepsis/toxemia) Toxicities resulting in the prevention of nutrient transport and metabolism Malnutrition | Amyloidosis Cholestasis Congestive heart failure Endocrinopathy Enteritis/Enteropathy Hepatitis/Hepatopathy Nephritis/Nephropathy |
| Parameter | Oral | Enteral 1 | Parenteral |
|---|---|---|---|
| Indication/s | Alimentary function is grossly maintained Alimentary health is minimally affected Alimentary patency maintained Some appetite present | Alimentary health is minimally affected Caudal alimentary function is grossly maintained Caudal alimentary patency is maintained Complete absence of dietary intake Inability to obtain adequate nutrition by the oral route | Alimentary health is grossly affected Complete lack of alimentary function/patency Dehydration > 8% Failure to obtain adequate nutrition by other modes Severely compromised metabolic states (e.g., hepatic lipidosis, pregnancy toxemia) Severe hemodynamic compromise |
| Absolute contraindication | Completely interrupted patency of the alimentary system caudal to the forestomaches Congenital abnormalities Hemodynamic instability (e.g., shock) Lack of swallowing reflex Non-functional alimentary system | Completely interrupted patency of the alimentary system caudal to the forestomaches Congenital abnormalities Hemodynamic instability Non-functional alimentary system | |
| Advantages | Allows for the provision of roughage 2 Cheapest Improved protein utilization through microbial digestion Lowest labor requirements Least stressful for the ruminant individual Maintenance of the functionality of the entire alimentary system Maintenance of the gut-associated lymphoid tissue (GALT) Maintenance of the integrity of the entire alimentary system Maintenance of the rumen microbes Maximized enteral resistance to pathogens Minimal gut permeability Physiologic function | Allows for provision of roughage 2 Attenuation of hypermetabolic response to injury 3 Cheap Improved protein utilization through microbial digestion Lower labor requirements than parenteral feeding Rumenostomy after the intervention is less stressful Maintenance of functionality of a major portion of the alimentary system Maintenance of the GALT Maintenance of the integrity of a major portion of the alimentary system Maintenance of the rumen microbes Maximized enteral resistance to pathogens Minimal gut permeability Minimal risk of fluid overload More physiologic compared to parenteral | Providing essential nutrients when other modes are not possible Rapid rehydration and correction of acid–base, electrolyte, fluid, and nutrient imbalances |
| Disadvantages | Intake must be carefully monitored, as the diet may not be eaten, or selection of feedstuffs may occur | Administration of a liquid diet generally results in a shorter transit rate Higher demand for nutrients for the enterocytes Requirements for specialized equipment Supplementation of effective fiber | Altered function of the alimentary system (e.g., enzymatic dysfunction, rumen inactivity/loss of microbes) Altered integrity of the alimentary system (e.g., increased gut permeability, intestinal atrophy) Does not allow for the provision of roughage Expensive High risk of fluid overload Major labor requirements (e.g., requirement of regular monitoring and checks for side effects) Minimized enteral resistance to pathogens Requirements for specialized equipment |
| Risk of side effects | Minimal | Medium | The highest |
| Common side effects | Aspiration pneumonia Refeeding syndrome Worsening of diarrhea | Refeeding syndrome Worsening of diarrhea | Imbalanced nutrient supplementation (e.g., amino acids, electrolytes, fluid) High risk of infection (e.g., catheter/giving set contamination; fluids being excellent media for microbial growth) High risk of liver disorders Refeeding syndrome Thrombophlebitis |
| What can be fed | Usual diet, usually finally chopped/maximized palatability 4/moistened | Usual diet, usually finally chopped/moistened Blended diet Ground/Pulverized diet | Simple nutrients (e.g., amino acids, minerals, mono- to di-saccharides, peptides, and vitamins) |
| Commonly used methods | Bunk feeding/Pasture grazing | Oro-ruminal tubing 5 Permanent/Temporary rumenostomy 6 | Intravenous route |
| Alteration in | Method of Delivery of Clinical Nutrition | |
|---|---|---|
| Type in Order of Preference | Notes | |
| Appetite * | Maximize palatability 2 Use of pharmaceutical appetite stimulators 1 | Mainly generalized morbidity |
| Prehension * | Moistened diet 2 Finely chopped and moistened diet 2 Ruminal feeding Pellets | Mainly localized morbidity (e.g., Dental morbidity, Dysfunction of some cranial nerves, Stomatitis, Trauma) but also some generalized morbidity (e.g., Botulism, Tetanus) |
| Mastication * | Moistened diet 2 Finely chopped and moistened diet 2 Ruminal feeding | Mainly localized morbidity (e.g., Actinobacillosis, Dental morbidity, Dysfunction of some cranial nerves, Osteomyelitis, Stomatitis, Trauma) but also some generalized morbidity (e.g., Botulism, Tetanus) |
| Swallowing * | Moistened diet 2 Finely chopped and moistened diet 2 Ruminal feeding | For esophageal ± pharyngeal alterations, feeding from an elevated surface (30–50 cm above the ground) |
| Rumen fermentation | Decrease/Increase particle size (as required) 2 Use of ruminal buffers 1 Transfaunation | Water contents may need modification, particularly after prolonged anorexia |
| Eructation | Use of condensed tannins 2 Use of methane abatement products 1 Use of plant secondary compounds 1,2 Transfaunation | |
| Rumination | Increase particle size 2 | |
| Chyme passage | Increase the inclusion of highly fibrous components in the diet | |
| Digestion | Feed manufacturing, involving water and heat High-quality forage Grain processing, involving chemical, physical, or thermal methods | Mainly a syndrome of maldigestion |
| Absorption | Oral rehydration compounds containing glutamine, glycine, or glucose | Mainly a syndrome of malabsorption Severe internal parasitism |
| Elimination | Water intake Physical activity Improving rumen function Emesis Use of pharmaceuticals (e.g., laxatives) 1 | Mainly syndromes of constipation and diarrhea, but also the hindgut fermentation |
| Utilization | Amino acids Enzymes Glucose Minerals Vitamins | Mainly generalized morbidity |
| Compound | Type of Ruminant | |||
|---|---|---|---|---|
| Calves 1 | Calves 2 | Small Ruminants 1 | Small Ruminants 2 | |
| Vehicle | Balanced electrolyte solution or Sterile water | Balanced electrolyte solution or Sterile water | Balanced electrolyte solution | Balanced electrolyte solution |
| Dextrose 50% | 20% | 16.6% | 10% | 10% |
| Amino acids 8.5% | 3.4% | 2.8% | 20% | 20% |
| Lipids | 2% | 3.3% | 10% | |
| B-complex | Separate line | Separate line | 4 mL/L | |
| Potassium chloride (as indicated) | Separate line | Separate line | 20–40 mEq/L | |
| Calcium gluconate 23% (as indicated) | Separate line | Separate line | 4–10 mL/L | |
| Reference | [26,110] | [26,110] | [120] | [65] |
| Antioxidants 1 | Appetite Stimulators 2 | Gut Integrity Protectors 3 | Immunostimulants | Liver Protectants | Rumen Modifiers | Wound Healing Stimulants |
|---|---|---|---|---|---|---|
| Amino acids Arginine, Glutamine [38,87,109,124] Branched chain amino acids (e.g., isoleucine, leucin, and valine) [99] Sulfur-containing amino acids (e.g., lysine, methionine) Minerals Copper, Selenium, Zinc [10,41,125] Plants or Plant-based products Condensed tannins, Garlic (Allium sativum), Oregano (Origanum vulgare) [126,127,128] Vitamins Beta-carotene, Vitamins C and E [10,41,125] | Acetyl-Coenzyme-A- binding protein [129] Benzodiazepines (although more known in monogastric animals) with a note that all have more or less sedative activity as well Beta-agonists [130,131,132] Vitamins of the B-complex [32] Various hormones, medications, plant products, proteins, and vitamins [130,131,132] | Orally administered glutamine [7,29,30,39,41,53,63,87,124,133,134,135] Plant-based products [136] Prebiotics [67] Probiotics [67,80,137] Vitamin A [125] Zinc [125] Yeasts [137] | Amino acids, including arginine, glutamine and leucine [30,38,41,53,63,87,107,124,133] Fatty acids, including omega-3 fatty acids [11,41,138] Minerals, including calcium, chromium, copper, iodine, iron, selenium, and zinc [31,32,125,139,140,141] Plants or plant-based products including ashwagandha/winter cherry (Withania somnifera), black-berry nightshade (Solanum nigrum), cinnamon (Cinnamomum verum), echinacea (Echinacea purpurea), fenugreek (Trigonella foenum-graecum), fire-flame bush (Woodfordia fruticosa), garlic (Allium sativum), guduchi/heart-leaved moonseed (Tinospora cordifolia), oregano (Origanum vulgare), shatawari (Asparagus reacemosus), tulsi/holy basil (Ocimum sanctum), turmeric (Curcuma longa), and thyme (Thymus vulgaris) [125,126,127,136,142] Some probiotics [24,32] Vitamins including beta-carotenes, Vitamin A, B-complex, C, and E [26,31,32,125] | Branched chain amino acids [39,99,143,144,145,146] Fatt acids [147,148] Glutamine [30] Sulfur-containing amino acids [68,144,146] | A large group of products have been tested to improve rumen health (e.g., antimicrobials, mannan-oligosaccharides, rumen buffers, and yeasts). Many of these compounds improve rumen health by maintaining optimal rumen pH [2,3,67]. Others are used to decrease the inflammatory response of the rumen [67] and/or improve nutrient utilization [2,3,67] and/or prevent bacterial or toxin translocation from the rumen into the general circulation [67]. | Arginine [53] Glutamine [134,135] Vitamin A [125] Vitamin K [60] Zinc [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira Rodrigues de Almeida, S.; Caetano, M.; Kirkwood, R.N.; Petrovski, K.R. The Basics of Clinical Nutrition for Compromised Ruminants—A Narrative Review. Ruminants 2025, 5, 51. https://doi.org/10.3390/ruminants5040051
Teixeira Rodrigues de Almeida S, Caetano M, Kirkwood RN, Petrovski KR. The Basics of Clinical Nutrition for Compromised Ruminants—A Narrative Review. Ruminants. 2025; 5(4):51. https://doi.org/10.3390/ruminants5040051
Chicago/Turabian StyleTeixeira Rodrigues de Almeida, Saulo, Mariana Caetano, Roy Neville Kirkwood, and Kiro Risto Petrovski. 2025. "The Basics of Clinical Nutrition for Compromised Ruminants—A Narrative Review" Ruminants 5, no. 4: 51. https://doi.org/10.3390/ruminants5040051
APA StyleTeixeira Rodrigues de Almeida, S., Caetano, M., Kirkwood, R. N., & Petrovski, K. R. (2025). The Basics of Clinical Nutrition for Compromised Ruminants—A Narrative Review. Ruminants, 5(4), 51. https://doi.org/10.3390/ruminants5040051







