1. Introduction
Urea is a widely used source of non-protein nitrogen (NPN) in ruminant nutrition, primarily due to its low cost per unit of nutrient. It represents a viable alternative to partially or completely replace true protein from commercial meals, which are expensive, and from oilseed cakes, which often contain antinutritional factors that may compromise nutrient digestibility [
1,
2].
However, the high solubility of urea makes it highly susceptible to rapid hydrolysis in the rumen, resulting in accelerated ammonia release [
3]. When supplied in excess, this ammonia may exceed the liver’s capacity for conversion back to urea, impairing recycling through saliva or urinary excretion [
4]. This overload increases blood ammonia concentration, potentially leading to ruminal alkalosis and acute toxicity. Therefore, the safe use of urea requires continuous adaptation of the ruminal microbiota to optimize its conversion into microbial protein and to reduce the risk of toxicity [
2].
Microbial protein synthesis in the rumen strongly depends on ammonia (NH
3-N) availability. Classical studies demonstrate that microbial synthesis efficiency is maximized when ruminal NH
3-N concentrations are approximately 5–11 mmol/L (≈7–15 mg·dL
−1) [
4,
5]; substantially lower concentrations can support only limited microbial growth, while rapid and excessive releases of N into the rumen increase the risk of ammonia release into portal and systemic blood [
5,
6]. In episodes of overload (e.g., acute urea ingestion), ruminal ammonia spikes from ≈9.7 to ≈32.0 mg/100 mL within minutes have been observed, with corresponding elevations in portal blood (up to ≈8.0 mg/100 mL) and subsequent arterial rise, illustrating that ruminal spikes can rapidly exceed hepatic detoxification capacity. Clinical signs of hyperammonemia typically appear around ≈1.0 mg/dL plasma ammonia, and associated mortality is generally reported when blood ammonia exceeds ≈2.0 mg/dL, emphasizing the narrow threshold between high N availability and systemic toxicity [
6]. Although the ruminant liver has the adaptive capacity to increase ureagenesis, and a significant portion of hepatic urea is recycled to the gastrointestinal tract (recent modeling indicates that a substantial fraction of urea-N may return to the lumen, model estimates even suggest that a large portion of hepatic N is recycled to the digestive tract), this recycling has limits. It does not eliminate the risk of acute ruminal peaks [
7]. Therefore, nutritional strategies that reduce rapid ruminal amino acid degradation (e.g., microencapsulated lysine) can improve N use efficiency and reduce nitrogen losses and hyperammonemia risk [
8].
In this context, strategies have been developed to control the rate of urea release in the rumen environment. By promoting a more gradual release of NPN, it becomes possible to synchronize nitrogen supply with the availability of fermentable energy, thereby enhancing microbial growth and microbial protein synthesis [
1,
2]. Technologies such as microencapsulation have been investigated for this purpose, using different materials and methods. Alternatives tested over the past few decades include starch-based systems [
1], formaldehyde treatments [
2], lipid protection [
3,
9], liquid urea with calcium chloride [
10], and the use of polymers [
11].
This type of approach is particularly relevant in arid and semi-arid regions worldwide, where forage scarcity or the predominance of low-quality feedstuffs, often with high fiber content and slow ruminal degradation, limits animal performance. In such environments, the lack of synchronization between nitrogen release and the availability of fermentable carbohydrates further restricts the utilization of free urea, compromising ruminal fermentation efficiency and microbial protein production [
12,
13].
Given this scenario, research efforts have intensified to develop and improve slow-release urea technologies aimed at enhancing the compatibility between nutrient degradation rates and maximizing the efficiency of non-protein nitrogen utilization [
14]. Therefore, this review summarizes advances in encapsulation techniques and evaluates their effects on nitrogen utilization and small ruminant performance.
2. Protein Metabolism in Ruminants
Protein is an essential macromolecule for the functioning and maintenance of animal organisms, playing roles in cellular structure, hormonal regulation, enzymatic activity, stimulus reception, and the storage of genetic information [
2,
14]. Protein metabolism in ruminants plays a central role in sustaining growth, reproduction, milk, and meat production. It is a complex process influenced by the interaction between dietary proteins and the rumen microbial ecosystem. Microorganisms in the rumen degrade dietary proteins into peptides, amino acids, and ammonia, which are later used for microbial protein synthesis [
11,
13]. This microbial protein is a major source of amino acids for the host animal once digested in the small intestine. The balance between rumen degradable and undegradable proteins strongly affects nutrient utilization efficiency. Thus, efficient protein metabolism reduces nitrogen losses, contributing to animal performance and environmental sustainability [
10].
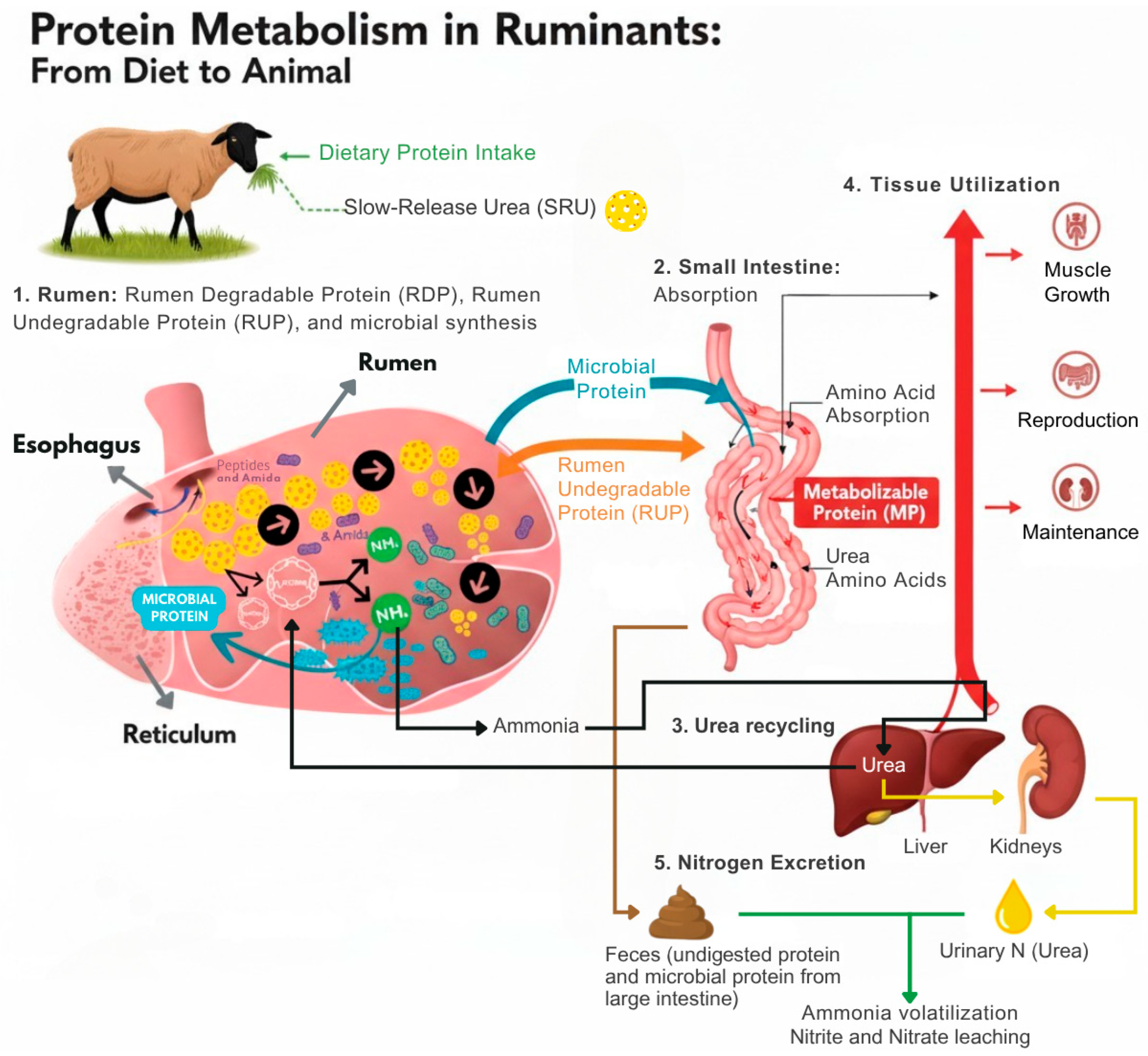
To facilitate the understanding of these processes, an infographic (
Figure 1) was prepared to schematically represent protein metabolism in sheep, highlighting the interaction between dietary proteins, ruminal microbial activity, nitrogen recycling through the urea cycle, and the contribution of slow-release urea (SRU) to synchronizing nitrogen availability with energy supply.
Understanding these mechanisms is essential for developing feeding strategies to optimize productivity in ruminant systems. In ruminants, dietary proteins are initially degraded in the rumen by microorganisms into peptides and amino acids, which are subsequently used for microbial protein synthesis [
9]. The extent and rate of this degradation vary widely depending on the type of feed: plant proteins, particularly those from legumes, tend to be more degradable than those of animal origin and usually have higher protein content compared to grasses [
13].
Given this dynamic, modern ruminant feeding systems adopt the concept of metabolizable protein (MP), defined as the total amount of amino acids truly absorbable in the small intestine, originating from both microbial protein and rumen undegraded dietary protein [
14,
15,
16,
17]. However, not all amino acids reaching the intestine are efficiently absorbed and utilized. The fraction of MP effectively incorporated into body tissues, milk synthesis, or metabolic maintenance is what determines the efficiency of metabolizable protein utilization [
18].
In this context, Galvani et al. [
19] suggested an average efficiency of 62% for MP utilization in sheep, a value considered safe to ensure adequate protein supply while preventing negative impacts on rumen fermentation. According to Van Soest [
20], diets with protein levels below 8% of dry matter (DM) may impair microbial growth and, consequently, reduce diet digestibility. Under conditions of low protein intake, physiological adaptations occur, such as increased renal urea reabsorption [
21] and enhanced ruminal urea transport [
22], allowing this recycled urea to be used as a nitrogen source by ruminal microorganisms.
On the other hand, excessive crude protein (CP) supplementation does not lead to greater productive gains and brings both economic and environmental drawbacks. Nitrogen excess requires additional energy for excretion and reduces CP utilization efficiency [
23]. Moreover, it increases N excretion, mainly as urinary urea, contributing to ammonia volatilization and nitrate leaching into groundwater [
24], thus raising the environmental impact of livestock systems.
Although ruminants can utilize NPN sources such as urea, the efficiency of this process remains limited. Nitrogen balance studies in dairy and beef systems have shown that only 27% and 14% of ingested N, respectively, were converted into milk or live weight gain [
25]. The remaining N is excreted via feces or urine, contributing to environmental pollution through soil and water contamination and atmospheric emissions of nitrogenous compounds [
26]. Importantly, urinary N is more susceptible to rapid losses via volatilization and leaching than fecal N, representing a mitigation opportunity through nutritional management [
27].
Fecal N excretion, largely derived from undigested microbial protein and proteins fermented in the large intestine, is directly influenced by diet quality. Diets with higher digestibility, in addition to supplying more fermentable energy to rumen microorganisms, tend to enhance microbial protein production. However, part of this protein may escape digestion in the small intestine and be excreted in feces [
28], particularly in diets with high CP content.
The efficiency of nitrogen metabolism in ruminants can also be assessed through nitrogen retention, defined as the difference between ingested N and total excretion. High retention rates are associated with adequate fermentable energy supply in the rumen, which favors microbial synthesis, reduces urinary urea excretion, and increases the proportion of N excreted in feces [
29]. Pfeffer et al. [
30] estimated that well-formulated diets should provide approximately 16 g of N/kg of dry matter, considering inevitable losses: 11 g in feces, 4 g as non-urea N in urine, and 1 g as urinary urea. This requirement can be met by either true protein sources or NPN, provided there is proper synchronization with the energy available to microorganisms.
3. Non-Protein Nitrogen (NPN)
The use of urea [(NH
2)
2CO] as a source of NPN has been widely adopted in ruminant nutrition, primarily because it represents an effective strategy to reduce production costs. Urea is rapidly hydrolyzed by ureolytic bacteria present in the rumen, releasing ammoniacal nitrogen (NH
3-N), which can be incorporated by ruminal microorganisms into microbial protein, essential for ruminant growth and maintenance [
31].
Among ruminal microorganisms, ureolytic bacteria play a key role in dietary urea hydrolysis since they produce the enzyme urease, which catalyzes the conversion of urea into ammonia (NH
3) and carbon dioxide [
32,
33]. However, the rate of urea hydrolysis often exceeds the capacity of ruminal bacteria to assimilate NH
3 into microbial protein, thereby reducing the efficiency of urea-N utilization by ruminants [
34]. Furthermore, the isolation and cultivation of these bacteria are complex, resulting in limited knowledge of the ureolytic ruminal microbiota [
35]. This knowledge gap hampers the development of targeted strategies to regulate the rate of urea hydrolysis [
36].
The NH
3-N peak in the rumen following urea supplementation usually occurs between 1 and 2 h after feeding. In contrast, when true protein sources are supplied, this peak is observed between 3 and 5 h after intake [
37,
38]. This difference reflects the rapid metabolism of urea in the ruminal environment. However, such rapid hydrolysis also raises concerns, such as the low incorporation rate of NH
3-N by the microbiota and the risk of ammonia intoxication when excessive amounts are provided [
20].
Urea remains an attractive alternative because of its low cost per nutrient unit and high protein equivalent, reaching up to 281% [
39]. This allows for its partial replacement of plant protein sources from meals and cakes. Nevertheless, its rapid degradation rate can lead to the sudden release of large amounts of NH
3-N, accumulating in the rumen. When absorption exceeds the liver’s capacity to convert ammonia into urea, it accumulates in the bloodstream, potentially leading to ruminal alkalosis, intoxication, and, in severe cases, animal death.
Ammonia production in ruminants is multifactorial. It can originate from dietary amino acids, glutamine catabolism in enterocytes, and peripheral tissues such as skeletal muscle. In mammals, at least 20 metabolic reactions produce ammonia, with glutaminase, glutamate dehydrogenase, and the purine nucleotide cycle being the most relevant [
40]. In the gastrointestinal tract (GIT), ammonia arises mainly through two mechanisms: microbial degradation of nitrogenous compounds in the lumen and microbial hydrolysis of urea diffusing across the intestinal wall from the bloodstream [
41].
In diets with very low protein levels, urea becomes the main NH
3 source in the rumen. Rumen undegradable protein and indigestible protein pass into the duodenum without influencing ruminal NH
3 production. Conversely, degradable NPN, such as urea, is rapidly converted into NH
3. Dietary nucleic acids are also degraded by bacterial peptidases and deaminases, generating ammonia [
42]. In animals with high protein intake, 10% to 15% of dietary protein may be deaminated to generate energy, leading to additional NH
3 production [
43].
Ammonia absorbed through the ruminal epithelium, small intestinal mucosa, and large intestine is transported via the portal vein to the liver, where it is converted into urea. This urea may then be recycled back to the rumen, intestine, or saliva; excreted by the kidneys; or secreted into milk or sweat [
44]. Hepatic metabolism, therefore, plays a central role in maintaining nitrogen homeostasis.
Urea intoxication in ruminants is an acute and often fatal syndrome that results from the excessive conversion of NPN into ammonia within the ruminal environment. The pathophysiological mechanism begins with the hydrolysis of urea by the enzyme urease, produced by ruminal microorganisms, leading to the release of ammonia and carbon dioxide. Under normal conditions, the ammonia generated is either incorporated by ruminal microbes for microbial protein synthesis or absorbed through the ruminal wall and converted to urea in the liver. However, when excessive amounts of urea are ingested, or when ammonia is released too rapidly, hepatic detoxification mechanisms become overwhelmed, resulting in the accumulation of ammonia in the bloodstream [
45].
Ammonia toxicity exerts its effects through multiple deleterious cellular mechanisms. Ammonia inhibits the citric acid cycle, induces lactic acidosis, decreases ATP production, and disrupts the Na
+/K
+-ATPase pump, severely impairing cellular energy metabolism. These effects are particularly pronounced in the central nervous system, where ammonia readily crosses the blood–brain barrier and directly interferes with neurotransmission. Critical blood ammonia thresholds for toxicity are well established in the literature. In cattle, clinical signs such as ataxia occur when blood ammonia-nitrogen exceeds 20 mg/L, with death usually occurring above 49 mg/L. In sheep, fatalities have been reported when blood ammonia-nitrogen surpasses 32 mg/L, indicating a slightly different sensitivity between species [
46]. Other studies report that death commonly occurs when blood ammonia reaches approximately 5 mg per 100 mL (equivalent to 50 mg/L) [
45].
The interval between urea ingestion and the onset of clinical signs varies markedly across species, generally ranging from 20 to 60 min in cattle and 30 to 90 min in sheep, and can be even longer in horses [
21]. This temporal variation reflects differences in urea hydrolysis rate, ammonia absorption, and detoxification capacity among species. In sheep, the progression of intoxication may be extremely rapid, with some animals found dead without overt clinical signs.
Early clinical signs of urea intoxication include muscle tremors, especially of the face and ears, abdominal pain, frothy salivation, polyuria, and bruxism. As intoxication progresses, animals may display restlessness, labored breathing, motor incoordination, bloat, and tetany. In severe cases, mydriasis, tetanic contractions, lateral recumbency, and convulsions develop, rapidly progressing to coma and death. In sheep specifically, experimental studies have documented distinctive clinical manifestations, including pronounced muscle fasciculations, tremors, teeth grinding, ataxia, lateral recumbency, bloat, regurgitation, hyperesthesia, mydriasis, and seizures. Anuria and absence of salivation have also been described as late signs [
47].
The practical aspects of urea intoxication in sheep involve both predisposing factors and preventive measures. Major risk factors include abrupt introduction of urea into the diet without proper adaptation, poor mixing of feed leading to localized areas of high urea concentration, administration of urea on an empty stomach when ruminal pH is elevated and microbial populations are reduced, and provision of urea without readily fermentable carbohydrates to balance ammonia release. Toxicity is further exacerbated under stress, dehydration, or conditions of low energy intake, which compromise the microbial capacity to assimilate ammonia.
From a diagnostic perspective, urea intoxication can be confirmed through clinical findings in association with specific laboratory alterations. Ruminal fluid pH becomes alkaline, typically ranging from 7.1 to 7.9 (normal 6.0–7.0), with elevated ruminal ammonia concentrations often exceeding 500–800 mg/L compared with normal values of 60–680 mg/L. The presence of white froth in the airways at necropsy and the characteristic odor of ammonia are supportive post-mortem findings. Blood sampling for ammonia determination must be performed immediately after collection and processed without delay, since values may artificially increase if samples are not properly preserved.
Treatment of urea intoxication is largely symptomatic and supportive, as no specific antidote exists. Therapeutic measures include the oral administration of weak acids such as vinegar (acetic acid) or diluted hydrochloric acid to neutralize ruminal alkalinity and reduce ammonia absorption, provision of easily fermentable carbohydrates to stimulate microbial growth and ammonia utilization, fluid therapy to correct dehydration and enhance renal excretion of nitrogenous compounds, and administration of sedatives or anticonvulsants when seizures occur. Prognosis depends critically on the rapidity of intervention, and animals in advanced stages of intoxication usually have a poor to grave prognosis.
Prevention of urea intoxication in sheep requires the implementation of strict nutritional management protocols. Urea introduction into the diet should be gradual, beginning with low doses and increasing progressively over 7 to 14 days to allow for ruminal microbiota adaptation. Uniform mixing of urea into feed is essential, and direct administration of powdered or granulated urea to animals must be avoided. The simultaneous provision of readily fermentable carbohydrates, such as molasses or grains, is crucial to synchronize ammonia release with energy availability for ruminal microbes. Additionally, urea should not exceed 1% of total dietary dry matter or 3% of concentrate, and animals must have continuous access to high-quality water.
To mitigate the risks associated with the rapid release of ammonia in the rumen, technological strategies such as urea microencapsulation have been explored. This technique aims to control the release of the compound, ensuring gradual and safer availability of ammonia, optimizing synchronization with fermentable carbohydrate supply [
20]. Controlled release improves nitrogen utilization efficiency and reduces toxicity risks. Freire et al. [
48] emphasized that the rate of ammonia release in the rumen is crucial both for microbial protein synthesis and for the adaptation of urea to the ruminal environment. Additionally, urea can improve the digestibility and utilization of low-quality roughages, such as hays and straws [
49], supporting animal performance even under nutritional restrictions.
A comprehensive understanding of the pathophysiological mechanisms of urea intoxication, coupled with knowledge of critical blood ammonia thresholds and practical aspects of prevention and management, is fundamental for the safe and effective use of urea as a source of non-protein nitrogen in sheep production systems. Proper implementation of these practices allows producers to benefit from the economic advantages of urea supplementation while minimizing the risks of intoxication, thereby contributing to the sustainability and efficiency of ruminant production.
Therefore, the use of urea as an NPN source remains a viable and economically attractive strategy, provided that practices are adopted to ensure metabolic safety and maximize microbial efficiency, particularly through technologies such as microencapsulation.
4. Microencapsulation Technique
Microencapsulation is a technology designed to package solids, liquids, or gases into small capsules, whose main function is to enable controlled release of their core content at defined rates, over time, and under specific conditions [
50]. In animal nutrition, this approach has gained increasing interest due to its potential to improve nutrient utilization efficiency, such as urea, while also favoring the modulation of ruminal microbiota and enhancing ruminant productive performance [
51,
52].
Historically, the microencapsulation technique was first described in the 1950s by Green and Schleicher, who patented capsules containing dyes initially developed for copying purposes [
51]. Since then, this technology has evolved, being applied to the encapsulation of liquids, solids, or gases within wall-forming materials, resulting in microcapsules with the capacity for controlled release of the core [
50]. This feature is particularly relevant in ruminant nutrition, as certain compounds, such as urea and methionine, undergo rapid transformations in the rumen, namely hydrolysis and deamination, respectively, thereby reducing their utilization efficiency [
53].
Although relatively recent in zootechnical applications, microencapsulation was already described in the 1970s as a process involving polymeric coatings applied to solid particles, liquid droplets, or gases, generating microcapsules capable of releasing their core under specific conditions of time and environment [
54]. This conceptual evolution reinforces the potential of the technique as a strategy to overcome ruminal constraints.
In ruminant nutrition, two main objectives justify the use of microencapsulation: (i) the controlled release of nitrogenous compounds, such as urea, in the rumen, and (ii) the protection of amino acids, fatty acids, and probiotic microorganisms against ruminal degradation [
55,
56,
57]. As highlighted by Comunian et al. [
58], several techniques have been investigated and employed for ingredient encapsulation, which can be grouped into three broad categories: physical (lyophilization, extrusion, spray-chilling, fluidized bed, co-crystallization, among others), chemical (polymerization, interfacial polymerization), and physicochemical (coacervation, solvent evaporation, liposomes, fusion-emulsion).
The choice of an appropriate technique depends on multiple factors, including the site and mode of application of the final product, the desired particle size, release mechanisms, and the physicochemical properties of both the core and the encapsulating material [
51]. Moreover, cost and safety of materials, particularly in the context of feed ingredients, are fundamental considerations [
58].
When lipid-based wall materials are used, the most common method is fusion-emulsification. This method consists of melting the lipids that compose the encapsulant, followed by core incorporation through dispersion or dissolution. To stabilize the emulsion, an emulsifier such as soy lecithin is added to the molten lipid [
59]. The emulsion is then dispersed into an aqueous phase containing an oil-in-water surfactant. Subsequently, the system is left at room temperature, allowing for lipid solidification and aqueous fraction evaporation [
60].
Another widely used method, especially for preserving heat-sensitive compounds, is lyophilization. According to Azeredo [
61], this technique involves freezing the material and subsequently removing water by sublimation under vacuum, resulting in a high-quality product with minimal structural changes and good stability. In microencapsulation, lyophilization can be applied to emulsions previously formed between the core and encapsulant, favoring content preservation and encapsulation efficiency.
Following encapsulation, one of the most relevant aspects for success in ruminant nutrition is understanding the mechanisms that regulate the release of the core in the ruminal environment. In this context, both the rate and mode of release are strongly influenced by the properties of the wall material, the nature of the core, and the ruminal physicochemical conditions.
In the rumen, the main identified release mechanisms are diffusion and biodegradation (or enzymatic erosion), both mediated by microorganisms and their digestive enzymes [
62,
63]. Diffusion occurs when the coating remains intact, allowing ruminal fluid to penetrate, dissolve the core, and subsequently release it through pores or channels within the encapsulant matrix [
64]. This process depends on factors such as solubility of the core, wall permeability, and microcapsule morphology [
65,
66]. Biodegradation, on the other hand, results from the action of ruminal enzymes, such as proteases and lipases, that hydrolyze wall constituents, particularly when composed of proteins or lipids, thereby exposing the core to the external environment and facilitating its release [
67].
Although some encapsulating materials, such as waxes or saturated fats, are considered relatively inert in the ruminal environment, evidence from electron micrographs suggests that the structure of these walls can be partially removed or disrupted by microbial action. Scanning electron microscopy (SEM) allows for the evaluation of surface morphology in both isolated phases and microencapsulated systems. For the preparation of micrographs, samples were fixed on aluminum stubs using double-sided adhesive tape and subsequently coated with gold. According to Medeiros et al. [
63], carnauba wax exhibits an irregular yet intact surface, sealed, non-porous, and marked by distinctive features. In contrast, urea displays a rough, porous surface with visible fissures [
52,
63]. When urea was encapsulated in carnauba wax, the microstructure retained the characteristic features of the wax, confirming its protective role. The observation that carnauba wax formed the only visible external phase supports the success of the microencapsulation process [
63]. The effectiveness of carnauba wax for material incorporation has also been reported in the literature, where it was employed to hydrophobize bentonite, vermiculite, and diatomite. Similarly, Carvalho Neto et al. [
53] demonstrated its potential as a microencapsulating agent for valine.
According to Carvalho et al. [
52], bee wax micrographs show a slightly irregular, intact, sealed, non-porous, and smooth surface, which contrasts with the more irregular morphology of carnauba wax [
63]. In the case of SRU encapsulated with beeswax, the surface appeared very similar to that of isolated bee wax, indicating that urea was located inside the microparticle, although minor surface exposure was detected. However, Carvalho et al. [
52] further observed that when sulfur was incorporated into the bee wax encapsulant, the microstructure became clearly rough and aggregated, suggesting a significant presence of exposed urea. This effect was likely associated with the sulfur source, which reduced the protective efficiency of the matrix and led to nitrogen exposure.
Medeiros et al. [
63] demonstrated from the scanning electron micrographs obtained before and after the in vitro ruminal digestion process of microcapsules formulated with carnauba wax as wall material, the capacity of ruminal microorganisms to promote physical and/or enzymatic modifications of microcapsules, potentially as an adaptive strategy to access metabolically valuable internal contents. Thus, interactions between capsules and ruminal microbiota appear more dynamic than previously assumed, with implications for release kinetics and compound bioavailability.
In practice, multiple mechanisms often act simultaneously or sequentially, and release may also be conditioned by specific triggers, such as pH, temperature, or the presence of particular enzymes, offering greater precision in nutrient delivery [
67,
68].
It is important to emphasize that the core purpose of microencapsulation is not only to protect the core but also to ensure its controlled release at the appropriate time and site. Without such control, the technique loses its functionality [
67]. When correctly applied, these systems provide a viable solution to challenges faced in ruminant nutrition, particularly in replacing soybean meal with alternative sources such as non-protein nitrogen compounds, protected amino acids, and functional lipids. Therefore, microencapsulation emerges as a promising technological tool to enhance feed efficiency and sustainability in animal production.
5. Methodology
This research followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework, structured as a systematic and descriptive review with a quantitative analytical approach. The review aimed to compile and critically assess the scientific evidence on slow- and controlled-release urea (SRU/CRU) technologies applied to sheep feeding systems, emphasizing their impact on nitrogen metabolism, feed efficiency, and animal performance.
5.1. Literature Search Strategy
A comprehensive and systematic search was conducted from September 2016 to April 2025 across three major academic databases: SciELO, ScienceDirect (Elsevier), and the National Library of Medicine (PubMed). The search strategy was developed using the Boolean method, combining controlled descriptors and free-text terms to maximize retrieval sensitivity and specificity.
The search string was formulated as follows: (“slow-release urea” OR “controlled-release urea”) AND (“sheep” OR “lamb”) AND (“feedlot” OR “intensive feeding”) AND (“nitrogen metabolism” OR “protein efficiency”).
After a pilot search, descriptions were selected to ensure adequate indexing under the Agricultural and Biological Sciences category. Only peer-reviewed articles published in English were considered. The reference management software Mendeley 1.19.3 (Elsevier®) was used to organize records, identify duplicates, and facilitate screening.
5.2. Eligibility Criteria
Inclusion and exclusion criteria were defined a priori to ensure methodological transparency and reproducibility. As inclusion criteria, studies were included if they: were original research articles published between 2016 and 2025; reported experimental data on the development, evaluation, or application of SRU or CRU in sheep; were conducted under feedlot or controlled feeding conditions using complete diets; and provided at least one quantitative response variable, such as nutrient intake, digestibility, ruminal or nitrogen metabolism, growth, or productive performance.
Priority was given to studies presenting novel encapsulation materials (e.g., lipid, wax, polymeric, or polysaccharide matrices), detailed characterizations of protection technologies, and trials assessing synchrony between nitrogen release and carbohydrate degradation.
In contrast, as an exclusion criterion, publications were excluded if they: were review papers, theses, dissertations, or book chapters; presented duplicated data across databases; lacked precise or reproducible methodological descriptions; reported results unrelated to SRU/CRU or nitrogen metabolism; or did not address the guiding research question of this review.
5.3. Study Selection Process
The selection process was conducted in three consecutive phases: Identification: Initial screening based on titles and keywords retrieved from the databases. Screening: Abstracts were evaluated to determine alignment with the review objectives. Eligibility and Inclusion: Full texts were read and assessed against all inclusion and exclusion criteria. From an initial pool of 246 studies (76 from SciELO, six from PubMed, and 164 from ScienceDirect), 23 manuscripts fulfilled all eligibility criteria and were retained for in-depth analysis.
A PRISMA [
69] flow diagram (
Figure 2) summarizes the article selection process, including the number of records identified, screened, excluded, and finally included in the synthesis.
5.4. Data Extraction and Synthesis
Two reviewers independently extracted and verified data from the selected studies to minimize bias. Extraction fields included: Author(s) and year of publication; Experimental design and animal category; Type of SRU/CRU and encapsulation material; Analytical and evaluation methods (chemical, metabolic, or performance-based); Main quantitative outcomes (intake, digestibility, ruminal parameters, nitrogen balance, growth rate, or carcass traits).
Information was organized into comparative tables and summary figures, focusing on the technological characteristics and biological responses of SRU systems. To enhance visual interpretation, results were synthesized into heatmaps and trend graphs highlighting relationships between the degradation rate of carbohydrate sources, nitrogen release kinetics, and ruminal synchronization efficiency.
5.5. Guiding Research Question
This review was structured around the question: “Which slow- or controlled-release urea technologies demonstrate effective applicability and productive advantages in sheep nutrition under feedlot or intensive feeding conditions?”.
5.6. Quality Assessment and Bias Control
Each included study was critically evaluated for methodological consistency, clarity of experimental design, replication, and statistical robustness. The risk of bias was assessed based on sample size, replication, data transparency, and the adequacy of control treatments. Studies with unclear reporting or methodological limitations were included only when their findings were essential to technological comparisons, with their limitations explicitly discussed in the results and discussion below.
6. New Systems of Slow-Release Urea in the Rumen
The use of protected urea is already a well-established practice in ruminant nutrition. However, recent advances in encapsulation processes have expanded the possibilities of this technology, enabling the development of more efficient and specific systems for controlled release. This has encouraged its adoption on a larger scale and stimulated the registration of new patents in the sector.
One of the main challenges in urea microencapsulation is the choice of the encapsulating material. Various industrial techniques have been employed to reduce the degradation rate of urea in the rumen. Among the pioneering strategies are “amireia” [
1], formaldehyde-treated urea [
2], lipid protection [
3], liquid urea associated with calcium chloride [
5], biuret [
9], and polymer encapsulation [
11]. To be effective, the encapsulating material must provide high protection and retention capacity, commercial availability, and safety for animals.
The wall material, or encapsulant, coats the core to form microparticles (
Figure 3). Compounds such as carbohydrates, mainly polymer [
37,
70,
71], lipids [
52,
53,
63,
72,
73], and cellulose [
49,
74] can be used, provided they are compatible with the core, stable at different pH values, capable of film formation, possess good emulsifying properties, and adequate porosity for the desired release. These characteristics must be evaluated in conjunction with the properties of the core and the environmental conditions at the release site.
To promote the slow release of urea in the rumen, the ideal encapsulant should be chemically inert, water-insoluble, and highly hydrophobic. Waxes such as carnauba and beeswax meet these criteria, offering good moisture resistance and a melting point close to 65 °C [
63,
74]. Carnauba wax, abundant in the Caatinga biome, characteristic of Brazil’s semiarid region, is a viable alternative because it is biodegradable, inert to ruminal microbiota, and hydrophobic [
63]. Composed of long-chain saturated lipids extracted from the palm
Copernicia cerifera, it is considered safe for animals [
75]. In addition, carnauba wax has environmental and economic importance for local communities in the Caatinga, being an important source of extractive income [
76].
Medeiros et al. [
63] evaluated carnauba wax as an encapsulating matrix in different core-to-encapsulant ratios using the lyophilization technique. All tested systems demonstrated satisfactory performance, reinforcing the potential of this wax in urea encapsulation. Beeswax also stands out as a lipidic encapsulant, with desirable characteristics such as hydrophobicity, thermal stability, and moisture resistance [
52,
74]. It also contains between 12 and 15% of free fatty acids, predominantly tetracosanoic acid, being inert to ruminal microorganisms [
77].
Different wall materials used for urea encapsulation exhibit distinct properties that directly influence microencapsulation efficiency, the protection of urea, and the release profile of nitrogen in the rumen (
Table 1).
Carnauba wax is initially available in flakes and is melted together with urea and lecithin to form an emulsion [
53,
63]. After drying and grinding, it results in a flour-like material. This matrix provides a continuous hydrophobic barrier without apparent porosity, offering efficient protection against the rapid solubilization of urea [
78,
79]. Its irregular but sealed surface suggests strong potential for encapsulation and controlled release.
Beeswax, typically sold in blocks, follows a process similar to that of carnauba wax, also yielding an emulsion and, after drying, a flour-like material. Its particles, however, display a smoother and less irregular surface compared to carnauba. While beeswax is capable of encapsulating urea, studies indicate a higher likelihood of superficial crystal exposure, particularly when combined with sulfur, which reduces its protective efficiency [
52,
80].
Vegetable fat is used to prepare emulsions with aqueous urea solutions and lecithin. After drying, it produces a homogeneous material with a pasty texture. This matrix is more flexible and less structured than waxes, which may compromise the physical resistance of the encapsulated product. On the other hand, its malleability allows for adjustments in the release rate through formulation strategies [
71].
Sodium alginate, in contrast to lipid matrices, forms solid beads through ionic gelation in calcium solutions. After drying, it produces circular and rigid capsules with high structural integrity. Being a hydrophilic system, it favors the production of uniform and resistant particles, but it may provide less protection against rapid diffusion of urea in aqueous environments, depending on the crosslinking density of the gel [
70].
In comparative terms, waxes (carnauba and beeswax) are hydrophobic and create effective physical barriers against the immediate solubilization of urea, but they differ in texture: carnauba produces a more sealed and irregular matrix, whereas beeswax forms a smoother surface with a greater chance of urea exposure [
81]. Vegetable fat offers flexibility and ease of processing but yields less robust structures, which may limit long-term protection [
71,
72]. Sodium alginate, due to its polysaccharide nature, forms well-defined solid capsules and represents a non-lipid alternative that combines simplicity of production with the potential for controlled release, although it is more susceptible to rapid hydration [
70].
However, not all encapsulating matrices are efficient in protecting urea. For example, Nayohan et al. [
82] attempted to encapsulate urea using chitosan, but this material proved ineffective. In vitro ruminal fermentation assays confirmed this inefficiency, as the degradation of urea in the rumen was not altered compared to the unprotected form, demonstrating that the choice of encapsulating material is crucial to ensuring the functionality of slow-release urea systems.
Overall, each encapsulant presents specific advantages and limitations: waxes stand out for their strong physical barrier, vegetable fat for its simplicity and adaptability, and sodium alginate for its structural robustness, albeit with a greater tendency toward quick hydration.
Table 1.
Comparison of different materials used as urea encapsulants.
Table 1.
Comparison of different materials used as urea encapsulants.
| Encapsulant | Form/Structure Obtained | Surface Characteristics | Advantages | Limitations |
|---|
| Carnauba wax [53,63,78,80,83] | Flakes → emulsion with urea and lecithin → dried and ground, flour-like material | Irregular but sealed surface, intact and non-porous | Hydrophobic barrier, good protection against rapid solubilization, controlled release, high structural integrity | More rigid processing; requires higher thermal energy during preparation |
Beeswax
[52,81,83] | Blocks → emulsion with urea and lecithin → dried and ground, flour-like material | Smooth, slightly irregular surface, sealed but may expose urea crystals | Hydrophobic, effective encapsulation, less irregular morphology | Higher risk of exposed urea; reduced efficiency when combined with sulfur |
Vegetable fat
[71,72] | Emulsion with aqueous urea solution + lecithin → dried homogeneous material, pasty texture | Less rigid, homogeneous structure, flexible consistency | Easy processing, flexible matrix, adjustable release rate | Lower physical resistance; less durable protection |
Sodium alginate
[70] | Beads formed by gelification in Ca2+ → circular and solid capsules after drying | Rigid, circular, uniform, hydrophilic structure | Simple production, resistant capsules, particle uniformity | Lower hydrophobic barrier, higher risk of rapid diffusion in aqueous medium |
Several experimental studies have been conducted to evaluate the performance of different encapsulating matrices and methodologies applied to the production of SRU, focusing on process yield, encapsulation efficiency, product stability, controlled nitrogen release, and the impact on animal metabolism.
In
Figure 4, the microencapsulation yield (MY) is presented, calculated as MY = (M
final/M
inicial) × 100, which compares the total mass of materials used in the encapsulation process with the mass obtained after processing. In
Figure 5, the microencapsulation efficiency (EM) is shown, determined as EM = (U
retained/U
inserted) × 100, which relates the amount of urea effectively retained in the final product to the amount initially used in the process. These parameters provide important insights for selecting the most effective combinations of core and wall materials, as well as for identifying operational and technological limitations, considering different formulations, urea proportions, drying methods, and the effects of sulfur addition.
Carvalho et al. [
52] used beeswax as an encapsulant, observing its effectiveness in reducing urea hygroscopicity, which favored homogenization with diet ingredients and prevented solidification during storage. The 33% urea proportion showed the best yield and urea content. However, sulfur addition impaired the quality of the formulation.
Another matrix evaluated was citrus pectin [
69], a natural polysaccharide successfully used with the external ionic extrusion/gelation technique. Pectin concentrations between 3 and 5% (
w/
v) and urea concentrations between 10 and 30% (
w/
w) were tested. Results indicated that pectin favored the slow release of non-protein nitrogen, optimizing its utilization and reducing nitrogen excretion.
When diets with high non-protein nitrogen levels, such as urea, are provided to ruminants, adequate sulfur supplementation is necessary to meet ruminal microbial requirements for sulfur amino acid synthesis. In this context, incorporating sulfur directly into encapsulated urea systems would logically optimize the simultaneous supply of these two essential nutrients for microbial metabolism. However, several studies have shown that sulfur addition during the encapsulation process can negatively affect the efficiency and quality of the formed microparticles.
Because it is a polar molecule, sulfur, especially in the form of magnesium sulfate, can alter the interaction between the core and encapsulating materials, affecting emulsion stability, microcapsule shape, and final encapsulation yield. Silva et al. [
70], for example, evaluated systems encapsulated with 3% (
w/
v) sodium alginate containing 20% (
w/
w) urea, using the external ionic extrusion/gelation technique and testing two drying methods (oven and freeze-drying). Results indicated that oven drying performed better; however, magnesium sulfate addition significantly compromised urea encapsulation efficiency. Similarly, Silva et al. [
83] compared carnauba and beeswax as encapsulating matrices using emulsification followed by lyophilization at a 2:1 (encapsulant:core) ratio. Again, sulfur addition reduced system efficiency, highlighting the need for caution in formulating encapsulated products with multiple components.
Medeiros [
84], evaluating stearic acid at a 2:1 ratio as wall material, also investigated the effect of sulfur addition to the urea core and compared two dehydration methods (oven drying and lyophilization). Results showed the same negative effect of sulfur on encapsulation. Still, under all tested conditions, stearic acid demonstrated high urea encapsulation efficiency, emphasizing the relevance of its physicochemical properties, such as high melting point (65 °C) and saturated nature, as determining factors for lipid application in protecting molecules in the ruminal environment. It is worth noting that stearic acid, in addition to being metabolically inert in the rumen, can be absorbed in the small intestine and used as an energy source by the animal.
Most SRU systems use about 33% urea in the formulation, considered the upper limit to maintain microparticle integrity and quality. Encapsulation yields (
Figure 4) are generally above 90%, with Silva et al. [
83] reporting nearly 100% when using carnauba wax. Conversely, systems with only 20% urea showed average yields of 87% [
63,
80], although SRU in pectin reached 97% [
69].
Values above 100%, such as those reported by Silva et al. [
83] with carnauba wax with and without sulfur, may be attributed to moisture retention during drying, due to wax hardness and the hygroscopicity of magnesium sulfate heptahydrate. Silva et al. [
70] also obtained yields exceeding 300% with sodium alginate, as urea concentration was calculated based on the hydrated solution, which concentrated after drying.
Particle size also influences urea degradation rate: larger particles degraded less and released more slowly [
63]. Microencapsulation efficiency was close to 100% for waxes and vegetable fat and exceeded 150% for pectin [
69] and sodium alginate [
70] systems.
The analyzed systems showed low water activity (~0.5 at 25 °C), an important parameter for the stability of powdered feeds, whose residual moisture must be below 4% [
85]. All formulations met this criterion. Results obtained with vegetable fat as encapsulant [
63] differed from those reported by Medeiros et al. [
63], Carvalho et al. [
52], and Netto et al. [
80], as increasing fat proportion did not improve yield, unlike other encapsulants. Lucena et al. [
71] highlighted a relevant finding regarding the ratio between wall material and core: higher wall content is not always associated with greater yields and efficiencies. This occurs because the interaction between urea, soy lecithin, and vegetable fat during emulsion formation can significantly influence this relationship, especially during dehydration. At this stage, vegetable fat may hinder water evaporation, compromising emulsion stability and, consequently, final yield and material stability.
7. Slow-Release Urea in Small Ruminant Feeding
The use of urea in ruminant nutrition is limited by the risk of ammonia intoxication, especially when there is a rapid release of NPN in the rumen, surpassing the microbial assimilation capacity. This abrupt release can cause an excessive increase in ruminal ammonia concentration, leading to systemic absorption and hepatic overload in the conversion to urea, with a consequent rise in serum urea levels and higher urinary excretion. As an alternative, slow-release urea has been proposed with the aim of synchronizing nitrogen release with carbohydrate fermentation in the rumen, favoring microbial growth and improving nitrogen use efficiency.
Based on bench-scale laboratory tests, it is possible to verify that the protection of urea through encapsulation with waxes and carbohydrates is effective, ensuring gradual release of NPN. However, it is crucial to understand how these systems behave in the actual ruminal environment, where variables such as passage rate, pH, and diet composition may directly influence the efficiency of encapsulated nitrogen utilization. Diets with high energy concentration and different forage-to-concentrate ratios, for instance, can affect the release pattern and utilization of protected urea.
Beyond the technical efficiency of encapsulation, it is essential to assess how these systems affect nutrient intake and digestibility, parameters directly related to diet acceptance and ingredient utilization. Some wall materials, such as carnauba wax, may present odors and specific sensory characteristics that influence palatability, potentially impacting voluntary intake. In this context, evaluating DM and CP intake allows for verification not only of diet acceptance but also of potential adjustments in the synchronization between NPN release and the availability of fermentable carbohydrates. Such synchronization is decisive to maximize NPN use efficiency by fibrolytic bacteria, whose activity is fundamental for fiber degradation and, consequently, energy utilization of the diet, reflecting in changes in the apparent digestibility of DM, CP, and NDF.
Table 2 presents the effects of SRU inclusion on DM, CP, and NDF intake and digestibility.
Table 3 compiles the data related to nitrogen metabolism, while
Figure 6 shows the animals’ average daily gain (ADG). A joint analysis of these results makes it possible to identify whether SRU contributes to a better balance between nutrient supply and utilization, optimizing ruminal fermentation and enhancing productive performance.
The inclusion of microencapsulated urea in sheep diets has promoted greater DM intake, especially with increasing levels of SRU [
52,
63,
80,
83], in addition to reducing urinary urea excretion, indicating better ruminal nitrogen utilization. However, Geron et al. [
38], using an established commercial SRU product, did not observe changes in intake.
The encapsulation process of urea can reduce or even eliminate the negative effect that free urea exerts on voluntary intake, even though wall materials may contain high ether extract (EE) levels. Lucena et al. [
71], by including up to 3% encapsulated urea using vegetable fat as wall material, raised the total dietary EE content to 6.25%, without reducing intake or altering ingestive behavior. Despite the high EE content, there was also no negative effect on fiber digestibility. Similar results were reported by Medeiros [
84] with the inclusion of 4.5% encapsulated urea with stearic acid.
Waxes also demonstrated positive effects on microbial activity on fiber [
52], possibly due to greater ruminal pH stability [
69,
71,
72]. This stability favors protozoal growth, whose contribution to fermentation and nutrition remains debated [
86]. Gradual ammonia release from SRU favors microbial nitrogen utilization and improves DM digestibility [
63,
69].
Regarding animal performance, responses to encapsulated urea vary according to wall material, dose, encapsulation technique, and animal category (
Figure 6). The best weight gain results were obtained with SRU encapsulated in carnauba wax [
78], reinforcing the superiority of this system over others. Systems with vegetable fat [
71] or combined waxes [
69] also proved viable, though with a more modest impact on performance.
SRU also contributes to the reduction in ruminal ammonia and serum urea peaks, promoting better nitrogen balance without compromising ruminal conditions [
70,
71]. The inclusion of SRU (2% of the diet) encapsulated with oven-dried sodium alginate proved efficient in nitrogen retention and reduction in urinary excretion [
70].
Netto et al. [
80] observed linear increases in DM, CP, and total digestible nutrient intake with SRU encapsulated in carnauba wax. Similarly, Silva et al. [
83] reported improved DM and NDF digestibility with sodium alginate-encapsulated SRU. Lucena et al. [
71] observed increased TDN intake and nitrogen retention, although Netto et al. [
80] did not identify changes in excretion despite higher nitrogen intake.
Urea encapsulation also showed positive effects on ruminal environment. SRU inclusion promotes greater ruminal pH stability, improving conditions for fiber fermentation and favoring cellulolytic microbial growth. These effects were more pronounced with sodium alginate and pectin encapsulation [
69,
70], which maintained pH within the optimal range for microbial enzymatic activity.
Ruminal ammonia nitrogen concentration was also positively influenced by SRU. Gradual release provides continuous ammonia supply, optimizing microbial assimilation and reducing losses through excessive absorption or urinary excretion [
52,
71]. These dynamic favors microbial protein synthesis, leading to greater dietary nitrogen use efficiency [
31,
63].
Lucena et al. [
71], although not observing significant effects of vegetable fat encapsulation on the ADG of feedlot lambs compared to free urea, reported that all treatments achieved expressive gains, with values above 200 g/day. A relevant aspect of this study is that SRU inclusion with vegetable fat allowed soybean meal to be reduced from 14% to 8% of the diet DM, without compromising animal performance. This represents a substantial reduction in the use of a costly and seasonally available ingredient, while demonstrating the efficiency of encapsulated NPN in partially replacing true protein sources without impairing ruminal fermentation or animal growth.
The effectiveness of SRU also depends on its interaction with forage sources and effective fiber levels. Its efficiency may be compromised by factors such as particle size, forage-to-concentrate ratio, and ruminal passage rate, which directly affect the retention time of encapsulated urea in the rumen and synchronization with fermentable energy availability.
In this context, carnauba wax-encapsulated SRU has shown contrasting results, possibly due to diet composition and physical structure. For example, Netto et al. [
80] reported a quadratic increase in ADG of feedlot lambs fed
Cynodon spp. hay, ranging from 90 to 148 g/day. Conversely, Batista et al. [
78], evaluating carnauba wax SRU in high-concentrate diets (60:40) with low physically effective forage, found no improvement in weight gain or carcass traits compared to free urea. Moreover, inclusions above 27.5 g/kg DM impaired performance. The authors attributed this to the small particle size, which accelerated passage rate, reducing ruminal exposure time of encapsulated urea and limiting nitrogen assimilation. These findings reinforce the importance of integrating diet formulation, ingredient structure, and synchronization of NPN release with carbohydrate availability.
Complementary in vitro evidence has been provided by Inácio et al. [
87], who evaluated SRU protected with carnauba wax associated with cassava starch and sugarcane molasses powder. The authors observed improved metabolic activity of ruminal microorganisms and linked these effects to changes in gas production dynamics, which were associated with the utilization of simple carbohydrates such as fructose (from molasses) and starch (from cassava), compared with fibrous carbohydrates (cellulose and hemicellulose) derived from forages. These results highlight the potential of combining SRU with readily fermentable carbohydrate sources to enhance microbial fermentation efficiency and modulate ruminal fermentation patterns.
Positive results were also obtained by Medeiros [
84], who, using corn silage, observed that stearic acid-encapsulated urea yielded ADG of 240 g/day in sheep, a 25% increase compared to commercial SRU. This superior performance may be linked to the physicochemical properties of stearic acid, such as its high melting point and saturated nature, which confer greater resistance to ruminal degradation and more controlled nitrogen release.
Besides ruminal metabolism and performance, it is also relevant to evaluate the impact of encapsulation systems on meat quality. Diet composition strongly influences lipid profile and nutritional value of meat, potentially yielding benefits for human health. Thus, it is crucial to assess whether encapsulating materials affect fatty acid deposition in body tissues, impacting not only productivity but also product quality and value.
Lucena et al. [
71] found that SRU protected with vegetable fat improved ruminal microbial efficiency. Although this fat, due to its high saturation degree, is considered inert in the rumen, it can be digested in the small intestine as a source of energy and fatty acids, potentially influencing meat lipid composition. According to Mazza et al. [
72], low-trans-fat SRU increased fatty acid and energy intake in lambs without altering carcass traits or physicochemical meat composition. However, including this encapsulate at 1.25% of diet DM increased trans-MUFA, n-6 PUFA, and CLA concentrations in meat compared to free urea. These findings suggest that vegetable fat-protected SRU may serve not only to optimize nitrogen utilization but also to improve meat lipid profile, potentially enhancing consumer health benefits.
Environmental parameters also benefit from SRU use. Reduced urinary urea excretion and greater nitrogen retention lower the risk of nitrous oxide emissions and soil/water contamination. Moreover, reduced ruminal ammonia peaks indirectly mitigate methane production by improving fiber fermentation and microbial efficiency [
80].
8. Conclusions
From a technological standpoint, advances in urea encapsulation have been driven by improved production techniques (extrusion, emulsification, freeze-drying), as well as careful selection of wall materials. Promising systems, such as those based on carnauba wax, sodium alginate, and pectin, offer excellent stability, core protection, and gradual NPN release, with potential for large-scale application.
It should be emphasized, however, that system efficiency also depends on diet composition, adaptation time, and feeding management. Diets with adequate fermentable carbohydrates and effective fiber are essential to ensure ammonia utilization and avoid ruminal disorders.
Based on available studies, it is concluded that slow-release encapsulated urea may represent a viable alternative to improve NPN use efficiency in small ruminants, and it has potential to improve nitrogen utilization and contribute to more efficient feeding strategies, though the results are variable. Developing systems with regional raw materials, such as carnauba wax, further adds social and environmental value to the technology, promoting sustainable resource use and strengthening local production chains.
Future research should focus on standardizing encapsulation efficiency evaluation methods, optimizing release according to ruminal passage rate, and assessing long-term effects on health, metabolism, and productivity. In addition, comparative economic studies between different slow-release technologies and traditional NPN sources may encourage broader adoption of this strategy in livestock production systems.
9. Limitations and Future Perspectives
Despite the promising advances in slow-release urea technologies for small ruminants, several limitations currently hinder their widespread adoption and optimal implementation. Importantly, the findings presented here should not be extrapolated to other species, such as goats or cattle, since the inclusion criteria restricted the review to sheep meat production.
One of the main technical challenges is the variability in coating materials. Waxes, lipids, and polysaccharides often show inconsistent release profiles under different ruminal conditions, making standardization across production systems difficult. Manufacturing complexity adds to this issue, as microencapsulation requires specialized equipment and expertise that are not always available to smaller feed producers, limiting scalability. In addition, the long-term stability of encapsulated products under varying temperature and humidity conditions during storage and transport remains poorly characterized, raising concerns about product efficacy over time.
Methodological gaps represent another critical limitation in the current state of slow-release urea research. The absence of universally accepted protocols for evaluating release kinetics and efficacy across different research groups has led to difficulties in comparing results and establishing clear performance benchmarks. Most existing research focuses on short-term trials, with insufficient data on long-term effects on animal health, reproduction, and overall performance, leaving important questions about chronic exposure and sustained benefits unanswered. The variable response factors observed in different studies, including inconsistent results due to differences in forage-to-concentrate ratios, ruminal passage rates, and individual animal variations, further complicate the development of universal application guidelines.
Economic and practical barriers also pose significant challenges to the broader implementation of slow-release urea technologies. Limited comprehensive economic evaluations comparing the costs of slow-release urea production and application versus the benefits obtained from conventional urea supplementation make it difficult for producers to make informed investment decisions. The scale-up of laboratory techniques to commercial production remains challenging, and producers are often reluctant to adopt new feed technologies without extensive field validation, creating market resistance.
Knowledge limitations persist in several key areas, including an incomplete understanding of the precise mechanisms governing nitrogen-energy synchronization in different ruminant species, which hampers the development of optimized formulations. Limited research on species-specific responses means that optimization for specific small ruminant breeds and production systems remains underdeveloped, while insufficient long-term studies on environmental benefits and potential ecological consequences leave important sustainability questions unanswered.
Looking toward the future, several promising research perspectives emerge that could address these limitations and unlock the full potential of slow-release urea technologies. Technological innovations hold particular promise, with the development of smart release systems that incorporate pH-responsive and time-controlled mechanisms capable of adapting to individual animal ruminal conditions representing a significant advancement opportunity. Nanotechnology applications, including the exploration of nanoencapsulation techniques, could provide improved precision in nitrogen release control, while research into biodegradable coating materials derived from agricultural by-products could offer environmentally sustainable and cost-effective alternatives to current synthetic options.
Methodological advancements will be crucial for the field’s progression, particularly through standardization initiatives that establish international protocols for testing and validating slow-release urea products. The development of in vivo monitoring technologies capable of real-time ruminal monitoring could enable precise optimization of nitrogen release patterns, while the creation of mathematical models to predict optimal formulations based on diet composition and animal characteristics could revolutionize formulation development processes.
Applied research priorities should focus on conducting extended trials lasting more than six months to evaluate chronic effects on animal health, reproduction, and productivity, providing the long-term data currently lacking in the literature. Comprehensive cost–benefit evaluations across different production systems and geographic regions are essential for demonstrating economic viability, while life-cycle analyses of slow-release urea technologies, including carbon footprint assessments and waste reduction potential evaluations, will be critical for establishing environmental credentials.
Integration and implementation strategies will require the development of precision nutrition approaches that combine slow-release urea with precision feeding systems for individualized supplementation strategies. Extension and education programs targeting veterinarians, nutritionists, and producers will be essential for ensuring optimal implementation, while collaboration with regulatory agencies to establish safety and efficacy standards for commercial products will facilitate market acceptance.
Emerging applications present exciting opportunities for the technology’s expansion beyond traditional supplementation roles. Investigation of slow-release urea’s potential in reducing greenhouse gas emissions from ruminant production could position it as a climate change mitigation tool, while exploration of incorporating urea derived from organic waste streams into slow-release formulations could contribute to circular economy initiatives. Research into simultaneous delivery of other nutrients, including vitamins, minerals, and probiotics through co-encapsulation technologies, could create value-added products that provide multiple benefits simultaneously.
To realize these future perspectives, research efforts should prioritize immediate goals including the establishment of standardized protocols for release kinetics evaluation, comprehensive economic feasibility studies, and the development of quality control standards for commercial products. Medium-term objectives should focus on completing long-term safety and efficacy trials in diverse production systems, optimizing formulations for specific small ruminant species and production stages, and validating environmental benefits through comprehensive life-cycle assessments. The long-term vision encompasses achieving commercial-scale production with cost-competitive pricing, integrating slow-release urea into precision livestock farming systems, and establishing global adoption frameworks with regulatory approval across major livestock-producing regions. Success in addressing these limitations and pursuing these future perspectives will ultimately determine whether slow-release urea technologies can fulfill their promise as a transformative approach to optimizing nitrogen utilization in small ruminant production systems.