Simple Summary
Mycotoxins are fungal toxins that can cause health problems in cattle, which can lead to losses in productive performance. Considering the importance of beef cattle production in Brazil’s economy, we analyzed 152 total mixed ration samples from beef cattle feedlots in Brazil to evaluate mycotoxin occurrences. Our evaluation found that every sample analyzed was contaminated to some extent, and in most cases, with more than one mycotoxin present. Our findings can help to monitor cattle exposure and contribute to future studies regarding mycotoxin effects in beef cattle.
Abstract
Contamination by multi-mycotoxins in cattle feed can lead to increased susceptibility to diseases and loss of performance. The present study aimed to investigate the occurrence of multiple mycotoxins present in the diet of beef cattle feedlots in Brazil. Chromatographic analyses were performed on 152 TMR samples from seven states, representing the diet provided to 1,246,522 animals. Contamination by mycotoxins was found in 100% of the TMR samples analyzed, with the most frequent being fumonisins, present in 100% of the samples, followed by zearalenone, which contaminated 79.6% of the samples, and subsequently by aflatoxins, deoxynivalenol, and T-2, while HT-2 was not detected in any of the samples. Furthermore, 2.6% of samples showed co-occurrence of five different types of mycotoxins, 23.7% presented four mycotoxins, 41.4% three mycotoxins, 22.4% two mycotoxins, and 9.9% of the samples showed contamination by only one mycotoxin. The significant prevalence of mycotoxins of the Fusarium and Aspergillus genera in the samples of the present study indicates a notable degree of pre- and post-harvest contamination in these beef cattle diets. Further studies are needed to define methods for monitoring cattle exposure to clarify its effects, even at low levels, and reduce the impacts on beef cattle production in Brazil.
1. Introduction
Multi-mycotoxin contamination in animal feed is a global problem that affects several sectors, from animal production to the food industry. Their presence in food affects the safety and quality of food, resulting in significant economic losses [1].
Mycotoxins are defined as fungal metabolites that can cause pathogenic changes in humans and animals and, consequently, mycotoxicosis, a toxicity syndrome as the consequence of the absorption of mycotoxins [2]. Mycotoxins can be produced before and after harvest, during storage and processing, and are produced by a series of consecutive reactions catalyzed by enzymes [3]. It is suggested that mycotoxins are formed when there is an accumulation of primary metabolic precursors and, to avoid this accumulation, fungi divert the excess of these precursors to the elaboration of secondary metabolites, which keep the primary ones operating [4].
Toxinogenic fungi belong mainly to the Aspergillus, Fusarium, Penicillium, and Alternaria genera. Currently, more than 500 mycotoxins have been identified, among which the most relevant to animal and human health are aflatoxins, fumonisins, deoxynivalenol, zearalenone, and T-2 and HT-2 toxins [5]. Brazil’s largely hot and humid tropical climate provides favorable conditions for the growth of certain toxinogenic fungi [6].
One must also consider the ability of the same species of fungus to produce different types of mycotoxins, thus multiple contamination can occur in the same feed ingredient. Alternatively, different types of mycotoxins can be found in the same feed composed of different contaminated ingredients. When they are present simultaneously, their interactions can have additive, antagonistic, or synergistic effects [7].
Cattle are generally exposed to mycotoxins through the consumption of contaminated feed and silage. In cattle, chronic ingestion of mycotoxins causes a decrease in dry matter intake by the animals, reducing nutrient absorption and impairing the metabolism, alterations in the endocrine system and suppression of the immune system, leading to increased susceptibility to disease and loss of reproductive performance and, in the case of dairy cattle, a decrease in milk production and quality [8].
There are an estimated 1 billion head of cattle worldwide, with the United States, Brazil, and the European Union producing 48% of the world’s beef. Beef production generally involves the simultaneous use of intensive production, in the form of feedlots, and extensive production, where herds are allowed to graze freely on large pastures. In the future, the growing demand for beef will likely involve a process of sustainable intensification, due to the limited number of pastures and climate change processes. The shift from extensive to intensive production will require adjustments in animal nutrition practices [9].
This is of great importance for the food security role of Brazil, which is the world’s largest meat supplier, with a total slaughter of 41.96 million head of cattle in 2023, of which 16.6% were finished in confinement. Beef exports resulted in a revenue of USD 339.7 billion [10].
Thus, the present study aimed to investigate the levels of multi-mycotoxins (aflatoxins, fumonisins, deoxynivalenol, zearalenone, T-2 toxin, and HT-2) present in the diet of confined beef cattle in Brazil, evaluating the occurrence of these mycotoxins throughout the years 2023 and 2024.
2. Materials and Methods
2.1. Property Selection
Feedlots from the seven largest beef cattle producing states in Brazil: Goiás, Mato Grosso, Mato Grosso do Sul, Tocantins, Rio Grande do Sul, Minas Gerais, and São Paulo were selected to represent 4 regions: Midwest, North, Southeast, and South [11]. Combined, these feedlots have a static capacity of almost 500 thousand head of cattle, being able to finish almost 1.5 million head/year in a confinement system. Regarding the animals, 62% of the farms confined exclusively male animals, 18% female, and 20% both male and female. The Nelore breed, pure or crossed with other breeds, was utilized on 92% of the properties. Additionally, other breeds, including Angus, Aberdeen Angus, Santa Gertrudes, Hereford, Caracu, and Simmental, were also utilized in purebred or crossbreed animals.
2.2. Experimental Design
A total of 152 samples were collected from a variety of feedlots, including at least two samples from each property evaluated in the study. These samples were obtained from the total diet, which is defined as TMR (total mixed ration, e.g., corn silage and commercial concentrate feed) intended for the general feeding of cattle on the selected properties. Information was also collected on the formulation/composition of the total diet and the use or not of anti-mycotoxin additives in the formulation.
2.3. Sampling
The following procedure was adopted for TMR sampling: a sample weighing 2.5 kg was formed from several primary subsamples randomly collected from the trough of an entire lot of animals. The collection occurred prior to the distribution before any animal came into contact with the diet. All samples were carefully obtained to cover all areas of the lot and to be representative. These samples were properly identified, labeled, packaged in polyethylene bags, and kept refrigerated until sent to the Mycotoxin Control and Decontamination Laboratory of the School of Animal Science and Food Engineering of the University of São Paulo (LCDM/ZEA/FZEA-USP) located in Pirassununga, SP, for chromatographic analysis of the total diet. Upon arrival at the laboratory, the samples were homogenized and dried to obtain dry matter, after which they were crushed to standardize the granulometry. From this already homogeneous material, a subsample was taken for the analytical process.
The animals were fed on the chosen farms under normal practical conditions. The contamination of the food was not intentional or controlled; therefore, the initial concentration or even the presence of mycotoxins in the samples was not known until the results of the analyses.
2.4. Reagents
The analytical standards of Biopure® mycotoxins (Biopure®, Getzersdorf, Austria) employed in this study were as follows: aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), fumonisin B1 (FB1), fumonisin B2 (FB2), deoxynivalenol (DON), zearalenone (ZEA), T-2 toxin, HT-2 toxin, Mix 11 (13C Aflatoxins), U-[13C18] Zearalenone, and Mix10 (13C Fusarium Toxins).
The solvents used were methanol, acetonitrile, and HPLC grade isopropanol and Milli-Q® ultrapure water (Merck Millipore®, Darmstadt, Germany). Acetic acid, formic acid, and ammonium acetate were used as mobile phase additives for Liquid Chromatography coupled to Mass Spectrometry (LC-MS/MS) analyses.
2.5. Total Diet Extraction Procedure
The TMR samples were ground to acquire the same particle size and finally homogenized for the extraction and determination of AFB1, AFB2, AFG1, AFG2, FB1, FB2, DON, ZEA, T-2, and HT-2. Using the methodology described by Sulyok, Krska, and Schuhmacher [12] for sample extraction, multi-mycotoxin analyses were performed using the “dilute-and-shoot” methodology, with some modifications. The method consists of a single extraction step, where an acidified mixture of acetonitrile and water is used, and the solution obtained from this extract is directly injected into the mass spectrometer.
Using an analytical balance (Radwag® AS 220/C/2, Toruńska, Warszawa, Poland), a 2 g aliquot of the previously homogenized sample was weighed into a 15 mL Falcon tube. After weighing, 4 mL of the extraction solvent acetonitrile/water/acetic acid (80:20:0.1, v/v/v) were added, and the tube was vigorously shaken for 1 min using a vortex mixer (Scilogex MX-S®, Cromwell, Rocky Hill, CT, USA), followed by shaking on a shaker (Tecnal®, Piracicaba, São Paulo, Brazil) for 60 min. After the process was completed, the vortex shaking was repeated (1 min) and then 30 min of shaking on a shaker. After this step, the sample was then centrifuged (4000 rpm) for 5 min. After centrifugation, the supernatant was filtered through disposable PTFE syringe filters with a 0.22 mm membrane (Millex, Millipore Corp®, Darmstadt, Germany). The final extract was transferred to glass inserts properly inserted into vials, followed by shaking, for subsequent analysis by LC-MS/MS [13].
2.6. Instrumentation
Mass spectrometry was performed using an Acquity UPLC I class chromatographic system (Waters®, Milford, MA, USA) with a binary pump system and an automatic injector interfaced with a Xevo TQ S® mass spectrometer. The system also has a Z-orthogonal electrospray ionization source (Waters®). The equipment is located at the Laboratory of Food Microbiology and Mycotoxicology (LMMA) of FZEA/USP. The data were processed using Masslynx 4.1® software and the TargetLynx® 4.1 platform (Waters®).
For the chromatographic separation of mycotoxins, a C18 BEH® column measuring 2.1 × 50 mm, 1.7 mm (Waters®) was used, which was maintained at a temperature of 40 °C during the analyses. The experimental parameters used in the analyses were as follows: capillary voltage for a positive mode of 3.00 V, and for a negative mode of 2.00 V; source temperature of 150 °C; desolvation temperature of 500 °C; cone gas flow of 150 L/h; desolvation gas flow of 700 L/h; collision gas flow of 0.15 mL−1; nebulizer gas pressure of 7.00 bar. The injection volume of standards and samples is 5 mL.
Mass spectrometry parameters were manually optimized for individual mycotoxins in each matrix type. Table S1 (Supplementary Material) presents the MRM transitions (major precursor ion > fragment ion) used for mycotoxins in feed samples.
2.7. Results Analysis
Identifying the levels of detectable mycotoxins in the animals’ diet, the position and dispersion measures (mean, variance, and standard deviation) of the results obtained in the analyses were determined. In addition, the mycotoxin levels were also compared using the PROC-CORR method of the SAS Studio 3.82 software (SAS Institute, New York, NY, USA, 2023) in order to statistically evaluate the correlation between the mycotoxins found in the diet. In all statistical treatments, α = 0.05 was adopted as the rejection level.
After determining the average daily dry matter intake (DMI) of the animals, the average probable daily intake (PDI) of the different mycotoxins was calculated by multiplying the DMI by the average contamination and dividing it by the average body weight (BW) of a beef cattle, adopted in this study as 550 kg [14].
3. Results
A total of 152 beef cattle feedlot TMR samples were analyzed over a one-year period (August 2023–August 2024). The analyzed samples are representative of the diet supplied to 1,246,522 animals. Of the total, 299,764 (24%) belonged to the state of Mato Grosso (MT), 264,432 (21.2%) to Minas Gerais (MG), 199,000 (16%) were from Goiás (GO), 150,800 (12.1%) from Rio Grande do Sul (RS), 149,526 (12%) from Mato Grosso do Sul (MS), 122,000 (9.8%) were from Tocantins (TO), and 61,000 (4.9%) from São Paulo (SP). Of the 152 samples evaluated, only 18 (12%) responded that they used some anti-mycotoxin additive in the animals’ diet, while the remaining 134 samples (89%) did not use any. Of those, 12 (66.6%) presented aflatoxin contamination (13.48 μg/kg), 5 (27.7%) had some level of deoxynivalenol (134.74 μg/kg), 18 (100%) fumonisins (2366.9 μg/kg), and 14 (77.7%) had zearalenone (19.33 μg/kg). In addition, the DMI found was 11.11 kg.
Mycotoxin contamination was found in 100% of the analyzed TMR samples, with the most frequent being fumonisins (B1 and B2), present in 100% of the samples, followed by zearalenone, which contaminated 79.6% of the samples. In addition, 62.5% of the samples contained aflatoxins (B1 + B2 + G1 + G2), 35.5% were contaminated with deoxynivalenol, and 8.6% had T-2, while HT-2 was not detected in any of the samples (Table 1).

Table 1.
Occurrence of mycotoxins in TMR samples from beef feedlot cattle in Brazil.
The occurrence of zearalenone was high in all states, with the highest prevalence in the state of Tocantins, where it was present in all TMR samples. Followed by Goiás, where it was the second most prevalent mycotoxin, together with aflatoxins, present in 95.2% of the samples analyzed. Aflatoxins were also present in 83.3% of the samples from Mato Grosso do Sul. Rio Grande do Sul was the third state with the highest occurrence of zearalenone, being present in 94.1% of the samples.
The highest mean levels of fumonisin contamination were found in the samples from Minas Gerais, with an average of 2634.91 µg/kg/DM; however, the highest level of fumonisin contamination in a sample was found in Mato Grosso, which presented 15,861.05 µg/kg/DM. T-2 toxin had the lowest occurrence in all states, among the samples above the LOD, and was not found in the states of Tocantins and São Paulo. In addition, aflatoxins were also not found in the samples from Tocantins (Table 2).

Table 2.
Occurrence of mycotoxins in TMR samples from beef feedlot cattle in Brazil by state.
Samples above the limit of detection of the analytical methods exhibited variable levels of contamination (Figure 1).
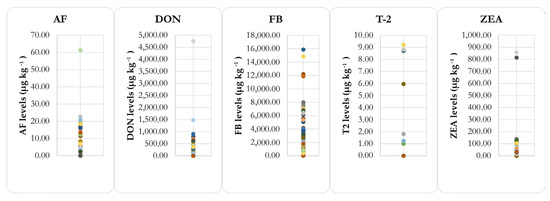
Figure 1.
Levels (μg/kg) of mycotoxins found in TMR samples from beef cattle feedlots in Brazil.
Total mixed ration (TMR) samples consisted of a variety of ingredients and concentrations. Among the samples, 107 (70.4%) contained corn silage, 27 (17.8%) contained some sorghum derivative (e.g., sorghum silage, ground sorghum, dry or wet grain), 35 (23.0%) contained grass silage, 5 (3.3%) wheat silage, 118 (77.6%) contained corn derivatives (e.g., ground corn, rehydrated corn, flaked corn or grains), 98 (64.5%) cotton derivatives (e.g., cottonseed or cottonseed cake), 91 samples (59.9%) of soybean derivatives (e.g., hulls, bran or grains), 85 (55.9%) of distillers dried grains (DDG), 16 (10.5%) of distillers wet grains (WDG), 3 (2.0%) had barley, 12 (7.9%) ground oats, 28 (5.9%) (18.4%) sugarcane bagasse, and 169 (12.5%) contained citrus pulp in different concentrations (Table 3). The occurrence of mycotoxins was correlated with the presence of certain ingredients in the feed (Figure 2).

Table 3.
Heat map of the occurrence of mycotoxins in TMR samples containing certain ingredients above the limit of detection. Red indicates higher and green indicates lower occurrence.
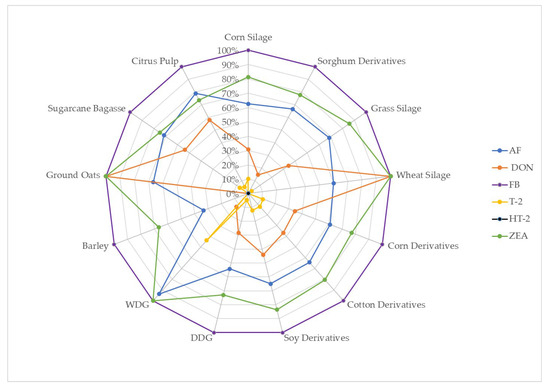
Figure 2.
Percentage of samples demonstrating mycotoxin concentrations above the detection limit, by ingredients present in TMR samples.
Furthermore, 4 (2.6%) samples showed co-occurrence of five different types of mycotoxins, 36 (23.7%) presented four mycotoxins, 63 (41.4%) showed three mycotoxins, 34 (22.4%) showed two mycotoxins, and 15 (9.9%) of the samples showed contamination by only one mycotoxin. The co-occurrence of each two combinations of mycotoxins (Table 4) and their correlation (Table 5) were also calculated.

Table 4.
Co-occurrence of mycotoxins in TMR samples from the diet of feedlot cattle in Brazil.

Table 5.
Heat map of correlation coefficients between mycotoxins detected in TMR samples from the diet of beef cattle feedlots in Brazil. * Indicates significant correlation coefficients (p < 0.05). Green indicates high and red indicates low correlation.
4. Discussion
It is well established that fungi can contaminate grains and silages in the pre- and post-harvest phase. The growth conditions of fungal species differ significantly in the field compared to the post-harvest phase, where changes in temperature, moisture, water activity, and relative humidity occur [15]. Fusarium fungi are generally considered field contaminants, as they do not tolerate anaerobic and low pH environments, conditions that are found in silage [16]. Fusarium is normally found in corn, with the species F. verticillioides being the most regularly found in corn production in Brazil [17].
There are several factors that will influence the contamination of crops by toxigenic fungi, such as the date of sowing and harvesting, planting density, crop rotation, cultivation techniques, and the previously mentioned environmental factors. Contamination by mycotoxins will also vary depending on the variety of corn plants since they have different levels of susceptibility or resistance to certain species and strains of fungi [18].
A survey with 36 feedlot cattle nutritionists in Brazil revealed that corn was the most commonly used grain in feedlot operations, with 97.2% of respondents indicating that it was the primary grain source. Sorghum was the secondary source, used by 95.6% of respondents. Co-products, such as cottonseed, were indicated by 52.8% of respondents as the primary co-product used in the animals’ diet. The following were citrus pulp (30.6%), distillers dried grains (DDG, 8.3%), soybean hulls (2.8%), distiller’s wet grains (WDG, 2.8%), and cottonseed cake (2.8%) [19].
These findings are in line with those of our research, which identified corn silage and its derivatives as the most prevalent ingredients in the analyzed samples, present in 70.4% and 77.6% of the samples, respectively. Although the secondary source of grains was soybean and its derivatives, present in 59.9% of the samples, we also identified that the main co-product used in the analyzed diet was cotton derivatives, present in 64.5%. The frequency with which we found grains such as corn may be indicative of the high prevalence of mycotoxins produced by Fusarium, such as fumonisins, observed in this study.
There are few studies that have evaluated the occurrence of mycotoxins in the total diet of cattle in Brazil, and in confinement they are even scarcer. Custódio et al. [20] analyzed 41 TMR samples from Brazilian cattle feedlots and also found a high prevalence of fumonisins, present in 93.3% of the samples, and with all samples showing some level of mycotoxin contamination. It is known that fumonisins can cause kidney and liver damage in cattle, with the presence of increased serum levels of specific diagnostic enzymes for these organs, in addition to high levels of cholesterol and bilirubin [2]. A study conducted on sheep also identified liver and kidney lesions in animals that received daily administration of 45,500 µg and 22,200 µg of fumonisins in aqueous solution, some of which subsequently presented diarrhea and died [21].
The results described by Oliveira et al. [22] also corroborate this hypothesis, where 100% of the 148 corn samples analyzed, from southern states of Brazil (Rio Grande do Sul, Paraná and Santa Catarina), were contaminated with fumonisins (62.4–66,274 µg/kg), in which zearalenone was the second most present mycotoxin.
In the present study, zearalenone (ZEA) was also the second most present mycotoxin. Like fumonisins, it is also produced by fungi of the Fusarium genera, and, in addition to corn, it is frequently present in wheat, oats and barley [23], grains present in lower frequency in the analyzed samples. The proportion of wheat, oats, and barley in the samples analyzed was lower, comprising, respectively, 3.3%, 7.9%, and 2.0%. This may explain its second position.
Because it has a molecular structure analogous to that of steroids, it exerts agonist effects on bovine estrogen receptors [23]. However, an experiment conducted by Pião et al. [24] provided zearalenone-contaminated feed to twenty healthy Nelore heifers (Bos taurus indicus) using contaminated concentrate, equivalent to 300 ppb ZEA/heifer/day, and found no significant differences in dry matter intake and average daily weight gain. However, considering that the present study detected higher contamination levels, with total values of 368.85 µg/ZEA/animal/day and PDI of 0.67 µg/kg, it is not feasible to rule out production losses resulting from zearalenone contamination.
Among all the samples analyzed, the maximum level of DON found was 4752.3 µg/kg. Brazil does not have current legislation regulating contamination limits for DON and other mycotoxins in beef cattle, only Ordinance No. 07 of 11/09/1988 of the Ministry of Agriculture, which determined the maximum level of aflatoxins (50 µg/kg) in animal feed components, currently revoked by Normative Instruction No. 30 of 08/05/2009 of the Ministry of Agriculture, Livestock and Food Supply [25]. However, the United States sets limits of 10 mg/kg of DON for the diet of finishing beef cattle [26]. Therefore, none of the samples exceeded the recommended levels.
Although cattle are considered more resistant to DON than other mammalian species, they may also experience a decrease in dry matter intake, as evidenced by Trenholm et al. [27], after contaminating non-lactating dairy cows with 70 µg/kg/bw of DON.
We analyzed only five (3.3%) samples that contained wheat silage in their composition, but all of them showed some level of DON contamination. We also found that 31% of the TMR samples containing corn silage were contaminated with DON, with the number rising to 35% in samples containing other corn co-products. This contradicts a study on the prevalence of DON in food products, which found that 73% of wheat and 92% of corn in the United States were contaminated with DON [28]. However, the climatic differences between the two countries and the small sample size would justify these differences.
In ensiled corn, most fungal growth is limited by low oxygen levels and the production of organic acids by lactic acid bacteria. However, some genera, such as Aspergillus, can survive the low pH microaerophilic environment of silage, probably explaining the high reported frequency of toxigenic fungi isolated from ensiled corn [29].
Aflatoxins (AFB1, AFB2, AFG1 and AFG2) are produced primarily by the fungi Aspergillus flavus and A. parasiticus, which are typically found in warm climates. Several nutritional factors have also been identified as affecting aflatoxin production. These include the presence of various carbohydrate and nitrogen sources, phosphates, lipoperoxides, and trace metals in the growth medium [30].
The effects caused in beef cattle have been reported since the 1960s; Garrett, Heitman, and Booth [31] observed that animals that ingested feed contaminated with aflatoxins showed lower weight gain and increased liver and kidney weights, indicating damage to these organs. Similarly, studies carried out with dairy cattle indicated that acute poisoning with aflatoxins at varying levels (20 μg–13 mg) caused a reduction in dry matter intake, a drop in milk production, and a decrease in reproductive efficiency [32,33,34]. A more recent study, conducted in Pakistan, reported contamination levels of 100 μg/kg/DM in the diet of dairy cows, which resulted in reduced milk production and abortions [35].
The samples from Goiás presented the highest occurrence of aflatoxins, with 95.2% of the analyzed samples contaminated, with an average level of 5.53 μg/kg. On the other hand, Minas Gerais presented a lower occurrence of contamination (50%), but a higher average level of aflatoxin contamination (10.87 μg/kg) and the highest value found (61.30 μg/kg). In Minas Gerais, the property responsible for the highest aflatoxin value found (61.30 μg/kg) was one of two properties which reported the usage of anti-mycotoxin additives in the diet, with the other one presenting no aflatoxin contamination. In Goiás, only six properties (28.6%) reported to use anti-mycotoxin additives, presenting an average contamination of 11.46 μg/kg.
While no proper evaluation of the storage condition of the different farms was conducted, these data demonstrate that the properties located in these states have problems in feed storage, since aflatoxins are considered storage mycotoxins.
A Brazilian study [36] which evaluated the occurrence of mycotoxins in distillery grains in the state of Bahia, identified a contamination by aflatoxins in 33.75% of 80 samples analyzed, with levels between 1 and 3 μg/kg. This may be an indication that the contamination by aflatoxins found by us (62.5%, with levels between 1.1 and 61.3 μg/kg), is due to contamination of other components of the total diet. Despite this, we observed that the occurrence of mycotoxins found in samples containing WDGs was higher compared to samples containing DDGs, including the highest occurrence of T-2 (44%), which can be explained by the higher water content present in the first group.
T-2 toxin was the least present mycotoxin, being identified in only 8.6% of all samples. Of these, 17.4% came from feedlots in Mato Grosso, with an average of 4.71 μg/kg and values ranging from 1.0 to 9.2 μg/kg). The low occurrence may be associated with its producing fungus, F. sporotrichioides, whose optimum growth temperature is relatively lower, between 22.5 and 27.5 °C [37]. The low incidence of T-2 and the absence of HT-2 in our study can be explained by considering the tropical and subtropical climate of Brazil.
A 10-year global study to monitor the occurrence of mycotoxins in animal feed revealed that T-2 was detected in 21.5% of samples from South America, with a higher average concentration of 31 μg/kg. The higher occurrence of T-2 can be attributed to the inclusion of other countries with colder climates, such as Argentina, Chile, and Uruguay. However, fumonisins were the most frequently detected mycotoxins, present in 75.3% of samples at an average concentration of 1390 μg/kg, zearalenone was also the second most frequently detected mycotoxin, present in 46.9% of samples [38]. These results are consistent with those of our study.
Intoxication levels between 0.5 and 204 mg/kg have been related to the appearance of submandibular edema and necrosis of the digestive mucosa in cattle [39]. Another study carried out with calves that received 0.16 mg/kg/BW of T-2, also identified ulcers in the abomasum, with some animals dying after ingesting 0.60 mg/kg/BW for 20 consecutive days [40].
When considering the legislation adopted in the European Union (EU) [41,42], where the most prevalent mycotoxins are regulated in some raw materials and compound feed, in order to analyze the average levels found in each state, we can conclude that just three samples exceeded the maximum EU guidance levels (AFB1: 0.02 mg/kg, DON: 5 mg/kg, ZEA: 3 mg/kg, FBs: 50 mg/kg) for total feed for beef cattle. Two were from Minas Gerais (61.3 μg/kg, 20.8 μg/kg) and the other was from Goiás (22.8 μg/kg).
However, the legislation only regulates exposure to a single mycotoxin, failing to consider the potential co-occurrence of multiple toxins. TMRs are composed of several ingredients, which may be contaminated by different agents. Furthermore, the same fungus has the capacity to produce more than one type of mycotoxin, and the contamination of a single ingredient is sufficient for the presence of multiple mycotoxins, which may have additive effects [7].
The few studies evaluating the effects of multi-mycotoxins in cattle suggest that a reduced concentration of each mycotoxin is necessary for them to produce clinical and subclinical effects [43]. Abeni et al. [44] observed that heifers fed feed contaminated with levels of 19.9 μg of AFB1 and 23,200 μg of FB showed decreased intake and delayed development when compared to the control group and the group contaminated only with significant levels of AFB1. The authors attributed the delay in puberty to changes in nutritional status and the presence of lasting liver damage.
In a study conducted by Kiyothong et al. [45], simultaneous contamination with 38 μg/kg AFB1, 270 μg/kg T-2, 720 μg/kg DON, 701 μg/kg FB1, 541 μg/kg ZEA, and 501 μg/kg OTA mg/kg was able to reduce DM intake, crude protein, and neutral detergent fiber digestibility and impact milk protein production of cows, compared to cows supplemented with anti-mycotoxin additives. The presence of multi-mycotoxins also caused a reduction in the ruminal microbial population and immunosuppression, evidenced by the reduction in serum IgA levels.
As demonstrated in the present study, a high prevalence of mycotoxins was identified, among the 152 samples analyzed, 90.81% contained at least two mycotoxins. It was observed that 23.7% and 41.4% of the samples contained four and three of the six mycotoxins analyzed, respectively. The most frequent concomitant mycotoxins were fumonisins and zearalenone (79.6%), followed by fumonisins and aflatoxins (62.5%) and aflatoxins and zearalenone (53.9%). The highest co-occurrence observed was between fumonisins and zearalenone, since both are produced by the same fungus, Fusarium, which indicates a possible high rate of contamination in the pre-harvest phase. However, the co-occurrence of aflatoxins with other mycotoxins of the genus Fusarium suggests management failures at different production stages, indicating that the feed may be contaminated both in the fields and during storage.
According to Biscoto et al. [46], high frequencies of co-occurrences were verified in cattle feed in Brazil, the most frequent combinations were deoxynivalenol and zearalenone (45.2%), aflatoxins and deoxynivalenol (42.1%), and aflatoxins and zearalenone (41.5%). Although the co-occurrence of deoxynivalenol was predominant, the concomitance of planting and storage mycotoxins strengthens the initial hypothesis.
There are a number of factors that may explain the obtained results. During the pre-harvest period, the climatic factors of the region, such as temperature and humidity, the susceptibility of cultivars and the presence of insects, are some of the factors that influence grain contamination by mycotoxins. The fungicides to be used must have high lethality against Fusarium spp., since they may stimulate the production of mycotoxins if they do not. In addition, the planting and harvesting dates may also influence the final levels of contamination; late harvesting generally implies greater contamination [47].
It is also important to note that, even though TMR samples were collected in certain states, it is not synonymous with the provenance of the commodities that compose the TMRs. Brazil’s large areas of arable land make it possible for the trade of goods in the national market, making it difficult to determine a state responsible for the found field contamination [48]. This may explain differences found in neighboring states, such as São Paulo and Minas Gerais.
Furthermore, inadequate storage, accompanied by high temperatures and high moisture content in the grains, also favors the production of mycotoxins and results in reduced grain quality. Aspergillus and Penicillium are the fungi most commonly associated with the post-harvest period; however, in conditions of high humidity, fungi of the genus Fusarium spp. demonstrate the ability to continue producing mycotoxins even after harvest [49,50].
However, even the most efficient agricultural management is not capable of completely eradicating mycotoxin contamination [51], which results in the implementation of strategies to mitigate production impacts, such as the inclusion of anti-mycotoxin agents in the animals’ diet. Only 18 (12%) of the 152 samples analyzed use some type of anti-mycotoxin additive. These samples demonstrated mean levels of AFBs (13.48 μg/kg) and FBs (2366.9 μg/kg) above the found national means and may explain its use by these properties.
Aluminosilicate minerals represent the most widely used and studied class of mycotoxin adsorbents in the mycotoxin decontamination process. Although many minerals demonstrate the ability to strongly adsorb polar toxins, such as AFB1 and FB1, they are ineffective in adsorbing other mycotoxins, such as DON and ZEA [51]. Alternatively, a study evaluated the efficiency of additives composed of a mixture of inorganic components, biological components, enzymes, and phycophytic compounds in cow diets, observing a reduction in all mycotoxins evaluated at levels below the detection limit in blood, urine, and milk, except for AFM1 in milk and urine [52].
Given the proven efficiency of using certain additives, one hypothesis for the non-use of anti-mycotoxin additives by producers may be associated with a lack of awareness, since, when observing the levels as within the recommended levels, producers do not consider the effects of possible interactions. We have not gathered any data on animal health impacts after mycotoxin exposure. Further studies are needed to elucidate the effects of exposure to different mycotoxins in different levels of contamination and the effectiveness of anti-mycotoxin additives in order to draw more conclusions.
In addition to the existence of other classes of mycotoxins, such as emerging ones, those that are not routinely determined nor legislatively regulated, and although data are limited, exposure to these mycotoxins has been associated with reproductive disorders [53]. In addition to the above, it is essential to consider masked mycotoxins, which may escape analysis due to altered physicochemical properties of their molecules. These issues result in underestimation of the total mycotoxin content of the sample [54].
Other points to be considered are the variety of ingredients present in different concentrations and the non-homogeneous distribution of mycotoxins in TMRs, factors that make it difficult to obtain a representative sample and accurately identify the problematic ingredients. Within the same species, variations in the composition of the diet are observed to meet specific nutritional needs [55].
5. Conclusions
In conclusion, a significant prevalence of mycotoxins of the Fusarium and Aspergillus genera were identified in the samples of the present study, indicating a notable degree of pre- and post-harvest contamination in these diets of beef cattle. Although ruminants are commonly considered more resistant to the action of mycotoxins, the particularities of this supposed resistance have not been fully elucidated and do not take into account chronic exposure and the occurrence of more than one mycotoxin. The presence of multiple mycotoxins in the diet of confined cattle can affect the health of the animals and, consequently, the productive performance of the activity, which constitutes one of the pillars of Brazil’s economy. Therefore, it is crucial not to disregard this concern.
The composition of TMRs is complex, comprising a variety of ingredients in different concentrations. Therefore, confirming that a single ingredient is contaminated by mycotoxins is a challenging process, requiring further studies to define methods for monitoring cattle exposure, in order to clarify the effects of this exposure, even at low levels, and reduce the impacts on beef cattle production in Brazil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ruminants5020012/s1, Table S1: MS/MS parameters for TMR mycotoxin determination.
Author Contributions
Conceptualization, C.H.C.; methodology, R.D.P., T.A.e.S. and A.M.B.; software, R.D.P. and A.M.B.; validation, C.H.C.; formal analysis, R.D.P., T.A.e.S. and A.M.B.; investigation, R.D.P., T.A.e.S., A.M.B., C.S.C. and V.V.d.C.; resources, C.H.C.; data curation, C.H.C.; writing—original draft preparation, R.D.P., T.A.e.S., A.M.B., C.S.C. and V.V.d.C.; writing—review and editing, C.H.C., C.S.C. and V.V.d.C.; visualization, R.D.P. and C.H.C.; supervision, C.H.C.; project administration, C.H.C.; funding acquisition, C.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed, in part, by the São Paulo Research Foundation (FAPESP), Brazil. Process Number #2023/16007-6 and #2022/07439-7; National Council for Scientific and Technological Development (CNPq)—Grant #304262/2021-8, and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the São Paulo Research Foundation (FAPESP), the National Council for Scientific and Technological Development (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for partly funding this study.
Conflicts of Interest
Authors Cristina Simões Cortinhas and Victor Valério de Carvalho were employed by the company dsm-firmenich. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Iqbal, S.Z. Mycotoxins in Food, Recent Development in Food Analysis and Future Challenges; a Review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Rosim, R.E.; de Oliveira, C.A.F.; Corassin, C.H. The in Vitro Ability of Different Saccharomyces Cerevisiae—Based Products to Bind Aflatoxin B1. Food Control 2015, 47, 298–300. [Google Scholar] [CrossRef]
- Bovo, F.; Corassin, C.H.; Rosim, R.E.; de Oliveira, C.A.F. Efficiency of Lactic Acid Bacteria Strains for Decontamination of Aflatoxin M1 in Phosphate Buffer Saline Solution and in Skimmed Milk. Food Bioprocess Technol. 2013, 6, 2230–2234. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. The Role of Mycotoxins in the Health and Performance of Dairy Cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef]
- Hajslova, J.; Zachariasova, M.; Cajka, T. Analysis of Multiple Mycotoxins in Food. Methods Mol. Biol. 2011, 747, 233–258. [Google Scholar] [PubMed]
- Paterson, R.R.M.; Lima, N.; Taniwaki, M.H. Coffee, Mycotoxins and Climate Change. Food Res. Int. 2014, 61, 1–15. [Google Scholar] [CrossRef]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.; Fremy, J.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H.; et al. Review of Mycotoxin—Detoxifying Agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety. EFSA Support. Publ. 2009, 6, 22E. [Google Scholar] [CrossRef]
- Alonso, V.A.; Pereyra, C.M.; Keller, L.A.M.; Dalcero, A.M.; Rosa, C.A.R.; Chiacchiera, S.M.; Cavaglieri, L.R. Fungi and Mycotoxins in Silage: An Overview. J. Appl. Microbiol. 2013, 115, 637–643. [Google Scholar] [CrossRef]
- McAllister, T.A.; Stanford, K.; Chaves, A.V.; Evans, P.R.; Eustaquio de Souza Figueiredo, E.; Ribeiro, G. Nutrition, Feeding and Management of Beef Cattle in Intensive and Extensive Production Systems. Anim. Agric. 2020, 1, 75–98. [Google Scholar]
- ABIEC Beef Report 2024—Perfil da Pecuária no Brasil. Available online: https://www.abiec.com.br/publicacoes/beef-report-2024-perfil-da-pecuaria-no-brasil/ (accessed on 14 January 2025).
- McManus, C.; Barcellos, J.O.J.; Formenton, B.K.; Hermuche, P.M.; De Carvalho, O.A.; Guimarães, R.; Gianezini, M.; Dias, E.A.; Do Nascimento Lampert, V.; Zago, D.; et al. Dynamics of Cattle Production in Brazil. PLoS ONE 2016, 11, e0147138. [Google Scholar] [CrossRef]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A Liquid Chromatography/Tandem Mass Spectrometric Multi-Mycotoxin Method for the Quantification of 87 Analytes and Its Application to Semi-Quantitative Screening of Moldy Food Samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-Occurrence of Mycotoxins in Maize Food and Maize-Based Feed from Small-Scale Farms in Brazil: A Pilot Study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Condotta, I.C.F.S.; Musgrave, J.A.; Brown-Brandl, T.M.; Mulliniks, J.T. Estimating Body Weight and Body Condition Score of Mature Beef Cows Using Depth Images. Transl. Anim. Sci. 2023, 7, txad085. [Google Scholar] [CrossRef]
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of Milling Procedures on Mycotoxin Distribution in Wheat Fractions: A Review. LWT Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage Review: Mycotoxins in Silage: Occurrence, Effects, Prevention, and Mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.F.; Reis, E.M.; Casa, R.T. Quantificação Da Transmissão de Fusarium Moniliforme de Sementes Para Plântulas de Milho. Fitopatol. Bras. 2004, 29, 456–458. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize Food Chain and Mycotoxins: A Review on Occurrence Studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar]
- Silvestre, A.M.; Millen, D.D. The 2019 Brazilian Survey on Nutritional Practices Provided by Feedlot Cattle Consulting Nutritionists. Rev. Bras. Zootec. 2021, 50, e20200189. [Google Scholar] [CrossRef]
- Custódio, L.; Prados, L.F.; Yiannikouris, A.; Holder, V.; Pettigrew, J.; Kuritza, L.; de Resende, F.D.; Siqueira, G.R. Mycotoxin Contamination of Diets for Beef Cattle Finishing in Feedlot. Rev. Bras. Zootec. 2019, 48, e20190079. [Google Scholar] [CrossRef]
- Edrington, T.S.; Kamps-Holtzapple, C.A.; Harvey, R.B.; Kubena, L.F.; Elissalde, M.H.; Rottinghaus, G.E. Acute Hepatic and Renal Toxicity in Lambs Dosed with Fumonisin-Containing Culture Material. J. Anim. Sci. 1995, 73, 508–515. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural Mycotoxin Contamination of Maize (Zea mays L.) in the South Region of Brazil. Food Control 2017, 73, 127–132. [Google Scholar] [CrossRef]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and Reproductive Function in Farm Animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Pião, D.d.O.; Mello, M.R.B.d.; Barbero, M.M.D.; Silva, A.L.d.; Moura, A.M.; Barbero, R.P. Does the Mycotoxin Ingestion by Beef Heifers on Feedlot Change the Productive Parameters? Biosci. J. 2023, 39, e39048. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastec. Instrução Normativa No 30, de 05 de Agosto de 2009; Diário Oficial da União: Brasília, DF, Brasil, 2009.
- FDA. US Food and Drug Administration Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed; FDA: Rockville, MD, USA, 2018. [Google Scholar]
- Trenholm, H.L.; Thompson, B.K.; Martin, K.E.; Greenhalgh, R.; McAllister, A.J. Ingestion of Vomitoxin (Deoxynivalenol)-Contaminated Wheat by Nonlactating Dairy Cows. J. Dairy Sci. 1985, 68, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of Deoxynivalenol and Its Acetylated and Modified Forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Mansfield, M.A.; Kuldau, G.A. Microbiological and Molecular Determination of Mycobiota in Fresh and Ensiled Maize Silage. Mycologia 2007, 99, 269–278. [Google Scholar] [CrossRef]
- Gonçalves, L.; Dalla Rosa, A.; Gonzales, S.L.; Feltes, M.M.C.; Badiale-Furlong, E.; Dors, G.C. Incidence of Aflatoxin M1 in Fresh Milk from Small Farms. Food Sci. Technol. 2017, 37, 11–15. [Google Scholar] [CrossRef]
- Garrett, W.N.; Heitman, H.; Booth, A.N. Aflatoxin Toxicity in Beef Cattle. Exp. Biol. Med. 1968, 127, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.; Anderson, P. Recent Aflatoxin Feeding Experiments in Cattle. Vet. Rec. 1982, 110, 60. [Google Scholar] [CrossRef]
- Jones, M.; Ewart, J. Effects on Milk Production Associated with Consumption of Decorticated Extracted Groundnut Meal Contaminated with Aflatoxin. Vet. Rec. 1979, 105, 492–493. [Google Scholar] [CrossRef]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Responses of Dairy Cows to Dietary Aflatoxin: Feed Intake and Yield, Toxin Content, and Quality of Milk of Cows Treated with Pure and Impure Aflatoxin. J. Dairy Sci. 1982, 65, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Mahmood, M.; Sulyok, M.; Rafique, K.; Khan, M.R.; Zebeli, Q.; Krska, R.; Metzler-Zebeli, B. Outbreak of Aflatoxicosis in a Dairy Herd Induced Depletion in Milk Yield and High Abortion Rate in Pakistan. Toxicon 2024, 246, 107799. [Google Scholar] [CrossRef]
- Simas, M.M.S.; Botura, M.B.; Correa, B.; Sabino, M.; Mallmann, C.A.; Bitencourt, T.C.B.S.C.; Batatinha, M.J.M. Determination of Fungal Microbiota and Mycotoxins in Brewers Grain Used in Dairy Cattle Feeding in the State of Bahia, Brazil. Food Control 2007, 18, 404–408. [Google Scholar] [CrossRef]
- Bacon, C.W.; Nelson, P.E. Fumonisin Production in Corn by Toxigenic Strains of Fusarium Moniliforme and Fusarium Proliferatum. J. Food Prot. 1994, 57, 514–521. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Gabal, M.A.; Awad, Y.L.; Morcos, M.B.; Barakat, A.M.; Malik, G. Fusariotoxicoses of Farm Animals and Mycotoxic Leucoencephalomalacia of the Equine Associated with the Finding of Trichothecenes in Feedstuffs. Vet. Hum. Toxicol. 1986, 28, 207–212. [Google Scholar]
- Pier, A.C.; Cysewski, S.J.; Richard, J.L.; Baetz, A.L.; Mitchell, L. Experimental Mycotoxicoses in Calves with Aflatoxin, Ochratoxin, Rubratoxin, and T-2 Toxin. Proc. Annu. Meet. U S Anim. Health Assoc. 1976, 80, 130–148. [Google Scholar]
- European Union. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; European Comission: Brussels, Belgium, 2019; pp. 1–10. [Google Scholar]
- European Union. Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding (2006/576/EC); European Comission: Brussels, Belgium, 2016; pp. 1–7. [Google Scholar]
- Borowsky, A.M.; Rosim, R.E.; Tonin, F.G.; de Oliveira, C.A.F.; Corassin, C.H. Co-Occurrence of Mycotoxins in the Diet and in the Milk of Dairy Cows from the Southeast Region of Brazil. Toxins 2024, 16, 492. [Google Scholar] [CrossRef]
- Abeni, F.; Migliorati, L.; Terzano, G.M.; Capelletti, M.; Gallo, A.; Masoero, F.; Pirlo, G. Effects of Two Different Blends of Naturally Mycotoxin-Contaminated Maize Meal on Growth and Metabolic Profile in Replacement Heifers. Animal 2014, 8, 1667–1676. [Google Scholar] [CrossRef]
- Kiyothong, K.; Rowlinson, P.; Wanapat, M.; Khampa, S. Effect of Mycotoxin Deactivator Product Supplementation on Dairy Cows. Anim. Prod. Sci. 2012, 52, 832–841. [Google Scholar] [CrossRef]
- Biscoto, G.L.; Salvato, L.A.; Alvarenga, É.R.; Dias, R.R.S.; Pinheiro, G.R.G.; Rodrigues, M.P.; Pinto, P.N.; Freitas, R.P.; Keller, K.M. Mycotoxins in Cattle Feed and Feed Ingredients in Brazil: A Five-Year Survey. Toxins 2022, 14, 552. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for Preventing, Decontaminating and Minimizing the Toxicity of Mycotoxins in Feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding Mycotoxin Contamination across the Food Chain in Brazil: Challenges and Opportunities. Toxins 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Birzele, B.; Prange, A.; Krämer, J. Deoxynivalenol and Ochratoxin A in German Wheat and Changes of Level in Relation to Storage Parameters. Food Addit. Contam. 2000, 17, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D.W. Mycotoxin Production by Aspergillus, Fusarium and Penicillium Species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited Review: Remediation Strategies for Mycotoxin Control in Feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Vieira, D.J.C.; Fonseca, L.M.; Poletti, G.; Martins, N.P.; Grigoletto, N.T.S.; Chesini, R.G.; Tonin, F.G.; Cortinhas, C.S.; Acedo, T.S.; Artavia, I.; et al. Anti-Mycotoxin Feed Additives: Effects on Metabolism, Mycotoxin Excretion, Performance, and Total Tract Digestibility of Dairy Cows Fed Artificially Multi-Mycotoxin-Contaminated Diets. J. Dairy. Sci. 2024, 107, 7891–7903. [Google Scholar] [CrossRef]
- Chiminelli, I.; Spicer, L.J.; Maylem, E.R.S.; Caloni, F. Emerging Mycotoxins and Reproductive Effects in Animals: A Short Review. J. Appl. Toxicol. 2022, 42, 1901–1909. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; Lizarraga Pérez, E.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).