Assessing the Reliability of Optimized Residual Feed Intake Measurements in Beef Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Management, Feeding and Measurements
2.2. Modelling and Comparing RFI Values
3. Results
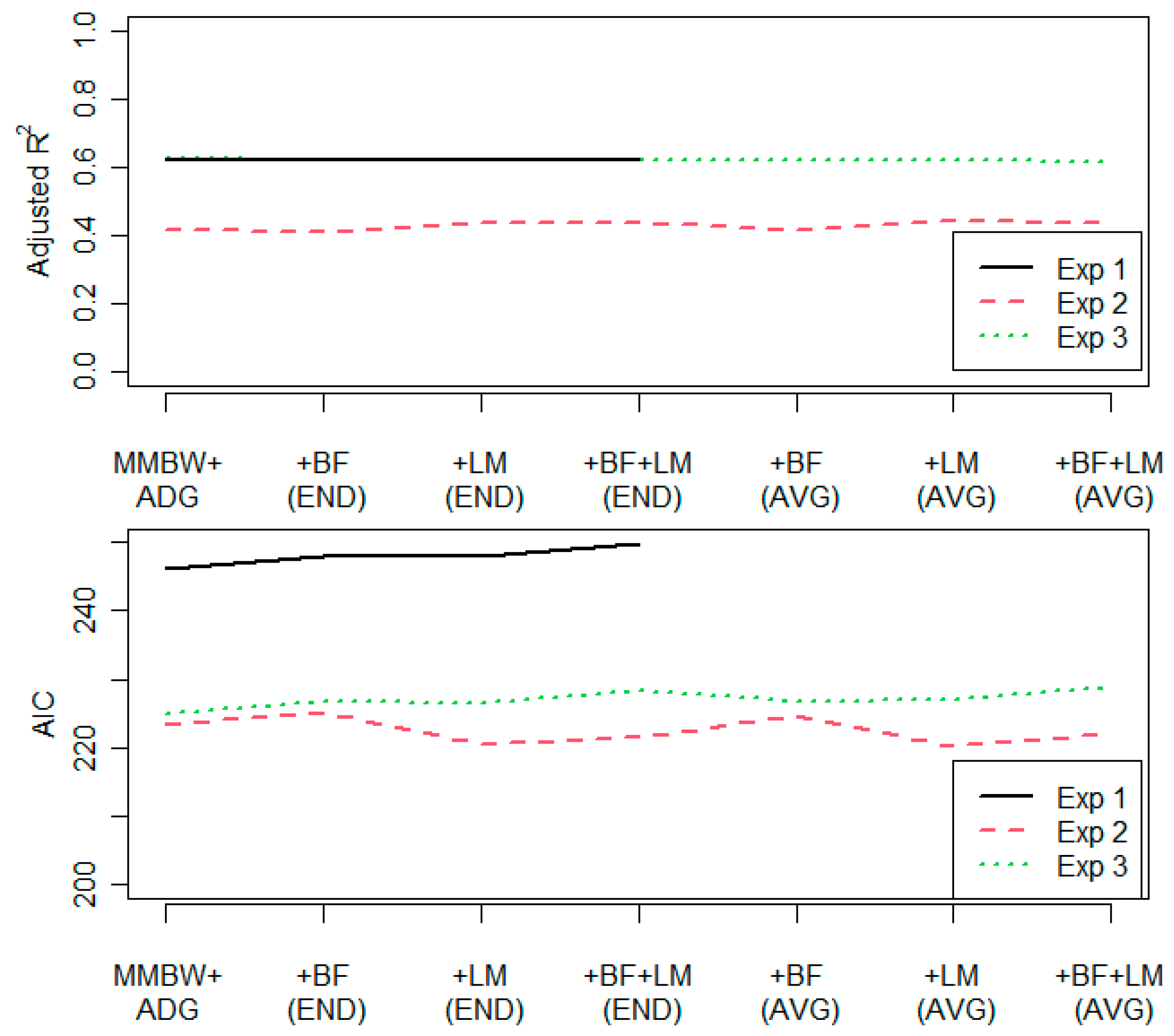
3.1. Additional Covariates
3.2. Reducing the Number of Times Weighed or the Duration of the Test Period
4. Discussion
4.1. Additional Covariates
4.2. Reducing the Number of Times Weighed or the Duration of the Test Period
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenny, D.A.; Fitzsimons, C.; Waters, S.M.; McGee, M. Invited Review: Improving Feed Efficiency of Beef Cattle–the Current State of the Art and Future Challenges. Animal 2018, 12, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.P.F.; Herd, R.M. Residual Feed Intake in Beef Cattle. Rev. Bras. Zootec. 2008, 37, 269–279. [Google Scholar] [CrossRef]
- Savietto, D.; Berry, D.P.; Friggens, N.C. Towards an Improved Estimation of the Biological Components of Residual Feed Intake in Growing Cattle. J. Anim. Sci. 2014, 92, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Manafiazar, G.; Basarab, J.; McKeown, L.; Stewart-Smith, J.; Baron, V.S.; MacNeil, M.; Plastow, G. Optimizing Feed Intake Recording and Feed Efficiency Estimation to Increase the Rate of Genetic Gain for Feed Efficiency in Beef Cattle. Can. J. Anim. Sci. 2017, 97, 456–465. [Google Scholar] [CrossRef]
- Judge, M.M.; Conroy, S.; Hegarty, P.J.; Cromie, A.R.; Fanning, R.; Kelly, D.; Crofton, E.; Berry, D.P. Eating Quality of the Longissimus Thoracis Muscle in Beef Cattle–Contributing Factors to the Underlying Variability and Associations with Performance Traits. Meat Sci. 2021, 172, 108371. [Google Scholar] [CrossRef]
- Mao, F.; Chen, L.; Vinsky, M.; Okine, E.; Wang, Z.; Basarab, J.; Crews, D.H.; Li, C. Phenotypic and Genetic Relationships of Feed Efficiency with Growth Performance, Ultrasound, and Carcass Merit Traits in Angus and Charolais Steers. J. Anim. Sci. 2013, 91, 2067–2076. [Google Scholar] [CrossRef]
- Culbertson, M.M.; Speidel, S.E.; Peel, R.K.; Cockrum, R.R.; Thomas, M.G.; Enns, R.M. Optimum Measurement Period for Evaluating Feed Intake Traits in Beef Cattle. J. Anim. Sci. 2015, 93, 2482–2487. [Google Scholar] [CrossRef]
- Archer, J.A.; Arthur, P.F.; Herd, R.M.; Parnell, P.F.; Pitchford, W.S. Optimum Postweaning Test for Measurement of Growth Rate, Feed Intake, and Feed Efficiency in British Breed Cattle. J. Anim. Sci. 1997, 75, 2024–2032. [Google Scholar] [CrossRef]
- Wang, Z.; Nkrumah, J.D.; Li, C.; Basarab, J.A.; Goonewardene, L.A.; Okine, E.K.; Crews, D.H.; Moore, S.S. Test Duration for Growth, Feed Intake, and Feed Efficiency in Beef Cattle Using the GrowSafe System. J. Anim. Sci. 2006, 84, 2289–2298. [Google Scholar] [CrossRef]
- Marzocchi, M.Z.; Sakamoto, L.S.; Canesin, R.C.; dos Santos Gonçalves Cyrillo, J.; Mercadante, M.E.Z. Evaluation of Test Duration for Feed Efficiency in Growing Beef Cattle. Trop. Anim. Health Prod. 2020, 52, 1533–1539. [Google Scholar] [CrossRef]
- De Castilhos, A.M.; Branco, R.H.; Razook, A.G.; Bonilha, S.F.M.; Mercadante, M.E.Z.; de Figueiredo, L.A. Test Post-Weaning Duration for Performance, Feed Intake and Feed Efficiency in Nellore Cattle. Rev. Bras. Zootec. 2011, 40, 301–307. [Google Scholar] [CrossRef]
- Ahlberg, C.M.; Allwardt, K.; Broocks, A.; Bruno, K.; McPhillips, L.; Taylor, A.; Krehbiel, C.R.; Calvo-Lorenzo, M.; Richards, C.J.; Place, S.E.; et al. Test Duration for Water Intake, ADG, and DMI in Beef Cattle. J. Anim. Sci. 2018, 96, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.; Castellan, N.J., Jr. Nonpareamteric Statistics for the Behavioral Sciences, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1988. [Google Scholar]
- Aldrighi, J.; Branco, R.H.; Cyrillo, J.N.d.S.G.; Magnani, E.; do Nascimento, C.F.; Bonilha, S.F.M.; Mercadante, M.E.Z. Ingestive Behavior and Temperament of Nellore Cattle Classified for Residual Feed Intake. Semin. Cienc. Agrar. 2019, 40, 457–468. [Google Scholar] [CrossRef]
- McKenna, C.; Keogh, K.; Porter, R.K.; Waters, S.M.; Cormican, P.; Kenny, D.A. An Examination of Skeletal Muscle and Hepatic Tissue Transcriptomes from Beef Cattle Divergent for Residual Feed Intake. Sci. Rep. 2021, 11, 8942. [Google Scholar] [CrossRef] [PubMed]
- Parsons, I.L.; Johnson, J.R.; Kayser, W.C.; Tedeschi, L.O.; Carstens, G.E. Characterization of Feeding Behavior Traits in Steers with Divergent Residual Feed Intake Consuming a High-Concentrate Diet. J. Anim. Sci. 2020, 98, skaa189. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, A.K.; Jaakkola, S.; Manni, K. Intake, Gain and Carcass Traits of Hereford and Charolais Bulls Offered Diets Based on Triticale, Barley and Grass Silages. Agric. Food Sci. 2020, 29, 318–330. [Google Scholar] [CrossRef]
- Luke Feed Tables and Nutrient Requirements. Available online: http://www.luke.fi/feedtables (accessed on 19 September 2022).
- DeVries, T.J.; von Keyserlingk, M.A.G.; Weary, D.M.; Beauchemin, K.A. Technical Note: Validation of a System for Monitoring Feeding Behavior of Dairy Cows. J. Dairy Sci. 2003, 86, 3571–3574. [Google Scholar] [CrossRef]
- Mendes, E.D.M.; Carstens, G.E.; Tedeschi, L.O.; Pinchak, W.E.; Friend, T.H. Validation of a System for Monitoring Feeding Behavior in Beef Cattle. J. Anim. Sci. 2011, 89, 2904–2910. [Google Scholar] [CrossRef]
- Huuskonen, A.K.; Pesonen, M. A Comparison of First-, Second- and Third-Cut Timothy Silages in the Diets of Finishing Beef Bulls. Agric. Food Sci. 2017, 26, 16–24. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: Boca Raton, FL, USA, 1994. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Bonferroni, C.E. Teoria Statistica Delle Classi e Calcolo Della Probabilità; Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze: Firenze, Italy, 1936; Volume 8. [Google Scholar]
- Basarab, J.A.; Price, M.A.; Aalhus, J.L.; Okine, E.K.; Snelling, W.M.; Lyle, K.L. Residual Feed Intake and Body Composition in Young Growing Cattle. Can. J. Anim. Sci. 2003, 83, 189–204. [Google Scholar] [CrossRef]
- Schenkel, F.S.; Miller, S.P.; Wilton, J.W. Genetic Parameters and Breed Differences for Feed Efficiency, Growth, and Body Composition Traits of Young Beef Bulls. Can. J. Anim. Sci. 2004, 84, 177–185. [Google Scholar] [CrossRef]
- Lancaster, P.A.; Carstens, G.E.; Ribeiro, F.R.B.; Tedeschi, L.O.; Crews, D.H. Characterization of Feed Efficiency Traits and Relationships with Feeding Behavior and Ultrasound Carcass Traits in Growing Bulls. J. Anim. Sci. 2009, 87, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, P.A.; Carstens, G.E.; Crews, D.H.; Welsh, T.H.; Forbes, T.D.A.; Forrest, D.W.; Tedeschi, L.O.; Randel, R.D.; Rouquette, F.M. Phenotypic and Genetic Relationships of Residual Feed Intake with Performance and Ultrasound Carcass Traits in Brangus Heifers. J. Anim. Sci. 2009, 87, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Montanholi, Y.R.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Caldwell, T.R.; Miller, S.P. On the Determination of Residual Feed Intake and Associations of Infrared Thermography with Efficiency and Ultrasound Traits in Beef Bulls. Livest. Sci. 2009, 125, 22–30. [Google Scholar] [CrossRef]
- Kelly, A.K.; McGee, M.; Crews, D.H.; Fahey, A.G.; Wylie, A.R.; Kenny, D.A. Effect of Divergence in Residual Feed Intake on Feeding Behavior, Blood Metabolic Variables, and Body Composition Traits in Growing Beef Heifers. J. Anim. Sci. 2010, 88, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Basarab, J.A.; Colazo, M.G.; Ambrose, D.J.; Novak, S.; McCartney, D.; Baron, V.S. Residual Feed Intake Adjusted for Backfat Thickness and Feeding Frequency Is Independent of Fertility in Beef Heifers. Can. J. Anim. Sci. 2011, 91, 573–584. [Google Scholar] [CrossRef]
- Archer, J.A.; Bergh, L. Duration of Performance Tests for Growth Rate, Feed Intake and Feed Efficiency in Four Biological Types of Beef Cattle. Livest. Prod. Sci. 2000, 65, 47–55. [Google Scholar] [CrossRef]
- Gilpin, A.R. Table for Conversion of Kendall’S Tau to Spearman’S Rho Within the Context of Measures of Magnitude of Effect for Meta-Analysis. Educ. Psychol. Meas. 1993, 53, 87–92. [Google Scholar] [CrossRef]
| Variable | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| Dry matter (DM), g/kg | 413 | 438 | 578 |
| Crude protein, g/kg DM | 123 | 118 | 120 |
| Neutral detergent fiber, g/kg DM | 404 | 351 | 423 |
| Metabolizable energy, MJ/kg DM | 11.8 | 11.5 | 11.1 |
| Metabolizable protein, g/kg DM | 86 | 85 | 82 |
| Timeline of Experiment 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Period or Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | End |
| Period start date | 26 Sep | 2 Oct | 9 Oct | 16 Oct | 23 Oct | 30 Oct | 6 Nov | 13 Nov | 20 Nov |
| Period end date | 1 Oct | 8 Oct | 15 Oct | 22 Oct | 29 Oct | 5 Nov | 12 Nov | 19 Nov | - |
| Period length, days (d) | 6 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | - |
| Weighing days | 1 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 |
| Comparisons | |||||||||
| S1 = Gold standard 1 | |||||||||
| S1: D 55 (d 1–55) W9 | 1 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 |
| S2: D 55 (d 1–55) W5 | 1 | 14 | 28 | 42 | 56 | ||||
| S3: D 55 (d 1–55) W3 | 1 | 28 | 56 | ||||||
| S4: D 49 (d 7–55) W8 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | |
| S5: D 42 (d 14–55) W7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | ||
| S6: D 35 (d 21–55) W6 2 | 21 | 28 | 35 | 42 | 49 | 56 | |||
| S7: D 28 (d 28–55) W5 | 28 | 35 | 42 | 49 | 56 | ||||
| S8: D 48 (d 1–48) W8 | 1 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | |
| S9: D 41 (d 1–41) W7 | 1 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| S10: D 34 (d 1–34) W6 3 | 1 | 7 | 14 | 21 | 28 | 35 | |||
| S11: D 27 (d 1–27) W5 | 1 | 7 | 14 | 21 | 28 | ||||
| S12: D 35 (d 21–55) W6 2 | 21 | 28 | 35 | 42 | 49 | 56 | |||
| S13: D 34 (d 1–34) W6 3 | 1 | 7 | 14 | 21 | 28 | 35 | |||
| S14: D 35 (d 14–48) W6 | 14 | 21 | 28 | 35 | 42 | 49 | |||
| S15: D 35 (d 7–41) W6 | 7 | 14 | 21 | 28 | 35 | 42 | |||
| Timeline of Experiment 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period, Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | End |
| Period start date | 13 Nov | 20 Nov | 26 Nov | 3 Dec | 10 Dec | 17 Dec | 23 Dec | 31 Dec | 7 Jan | 14 Jan |
| Period end date | 19 Nov | 25 Nov | 2 Dec | 9 Dec | 16 Dec | 22 Dec | 30 Dec | 6 Jan | 13 Jan | - |
| Period length, day (d) | 7 | 6 | 7 | 7 | 7 | 6 | 8 | 7 | 7 | - |
| Weighing days | 1 | 8 | 14 | 21 | 28 | 35 | 41 | 49 | 56 | 63 |
| Comparisons | ||||||||||
| S1 = Gold standard 1 | ||||||||||
| S1: D 62 (d 1–62) W10 | 1 | 8 | 14 | 21 | 28 | 35 | 41 | 49 | 56 | 63 |
| S2: D 62 (d 1–62) W5 | 1 | 14 | 28 | 41 | 63 | |||||
| S3: D 62 (d 1–62) W3 | 1 | 28 | 63 | |||||||
| S4: D55 (d 8–62) W9 | 8 | 14 | 21 | 28 | 35 | 41 | 49 | 56 | 63 | |
| S5: D 49 (d 14–62) W8 | 14 | 21 | 28 | 35 | 41 | 49 | 56 | 63 | ||
| S6: D 42 (d 21–62) W7 2 | 21 | 28 | 35 | 41 | 49 | 56 | 63 | |||
| S7: D 35 (d 28–62) W6 | 28 | 35 | 41 | 49 | 56 | 63 | ||||
| S8: D 55(d 1–55) W9 | 1 | 8 | 14 | 21 | 28 | 35 | 41 | 49 | 56 | |
| S9: D 48(d 1–48) W8 | 1 | 8 | 14 | 21 | 28 | 35 | 41 | 49 | ||
| S10: D 40 (d 1–40) W7 3 | 1 | 8 | 14 | 21 | 28 | 35 | 41 | |||
| S11: D 34 (d 1–34) W6 | 1 | 8 | 14 | 21 | 28 | 35 | ||||
| S12: D 42 (d 21–62) W7 2 | 21 | 28 | 35 | 41 | 49 | 56 | 63 | |||
| S13: D 40 (d 1–40) W7 3 | 1 | 8 | 14 | 21 | 28 | 35 | 41 | |||
| S14: D 42 (d 14–55) W7 | 14 | 21 | 28 | 35 | 41 | 49 | 56 | |||
| S15: D 41 (d 8–48) W7 | 8 | 14 | 21 | 28 | 35 | 41 | 49 | |||
| Experiment 1 | Experiment 2 | Experiment 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD 1 | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| At the beginning of the experiment | ||||||||||||
| Age, d | 230 | 17 | 164 | 265 | 188 | 7 | 176 | 218 | 204 | 18 | 164 | 261 |
| Live weight, kg | 325 | 55 | 198 | 486 | 265 | 24 | 215 | 341 | 347 | 56 | 235 | 548 |
| Ultrasound backfat, mm | 1.56 | 0.47 | 0.63 | 3.95 | 2.57 | 0.53 | 1.31 | 3.92 | ||||
| Ultrasound ribeye area, cm2 | 44 | 5 | 30 | 57 | 56 | 9 | 35 | 83 | ||||
| At the end of the experiment | ||||||||||||
| Live weight, kg | 396 | 59 | 264 | 584 | 328 | 27 | 267 | 398 | 432 | 61 | 310 | 644 |
| Ultrasound backfat, mm | 3.20 | 1.06 | 1.61 | 6.28 | 1.49 | 0.37 | 0.67 | 2.96 | 3.39 | 0.77 | 1.96 | 5.57 |
| Ultrasound ribeye area, cm2 | 60 | 10 | 39 | 82 | 48 | 5 | 33 | 61 | 68 | 9 | 44 | 87 |
| Average during the test period | ||||||||||||
| Daily gain, kg/d | 1.23 | 0.21 | 0.73 | 1.72 | 1.13 | 0.18 | 0.61 | 1.61 | 1.34 | 0.21 | 0.94 | 2.06 |
| Dry matter intake, kg/d | 8.50 | 1.18 | 3.91 | 11.84 | 7.83 | 0.97 | 4.56 | 10.32 | 8.63 | 1.19 | 6.49 | 12.03 |
| Metabolic body weight, kg | 82 | 10 | 59 | 111 | 71 | 5 | 55 | 84 | 87 | 10 | 67 | 119 |
| Experiment | Coefficient | Estimate | SE | p-Value |
|---|---|---|---|---|
| 1 | (Intercept) | 0.13 | 0.66 | 0.84 |
| ADG 1 | 1.37 | 0.38 | <0.001 *** | |
| MMBW 2 | 0.084 | 0.013 | <0.001 *** | |
| LM | −0.003 | 0.013 | 0.84 | |
| BF | −0.034 | 0.071 | 0.64 | |
| 2 | (Intercept) | 0.23 | 0.97 | 0.81 |
| ADG | 2.39 | 0.38 | <0.001 *** | |
| MMBW | 0.078 | 0.016 | <0.001 *** | |
| LM | −0.037 | 0.016 | 0.02 * | |
| BF | 0.17 | 0.18 | 0.33 | |
| 3 | (Intercept) | −0.53 | 0.73 | 0.47 |
| ADG | 1.88 | 0.39 | <0.001 *** | |
| MMBW | 0.071 | 0.013 | <0.001 *** | |
| LM | 0.008 | 0.014 | 0.55 | |
| BF | −0.028 | 0.100 | 0.78 |
| S | (Intercept) | ADG 1 | MMBW 2 | SE (Intercept) | SE (ADG) | SE (MMBW) | -Value |
|---|---|---|---|---|---|---|---|
| 1 | 0.010 | 2.592 | 0.069 | 1.113 | 0.442 | 0.017 | 0.42 |
| 2 | −0.205 | 2.707 | 0.070 | 1.127 | 0.459 | 0.017 | 0.42 |
| 3 | 0.027 | 2.671 | 0.067 | 1.137 | 0.451 | 0.017 | 0.41 |
| 4 | −0.332 | 2.362 | 0.079 | 1.161 | 0.446 | 0.017 | 0.39 |
| 5 | −0.673 | 2.238 | 0.088 | 1.228 | 0.432 | 0.017 | 0.37 |
| 6/12 | −0.230 | 2.012 | 0.087 | 1.302 | 0.414 | 0.018 | 0.32 |
| 7 | −0.109 | 1.753 | 0.092 | 1.345 | 0.348 | 0.018 | 0.30 |
| 8 | 0.252 | 2.297 | 0.072 | 1.045 | 0.373 | 0.016 | 0.45 |
| 9 | 0.692 | 2.422 | 0.064 | 1.160 | 0.408 | 0.018 | 0.41 |
| 10/13 | 0.922 | 1.850 | 0.069 | 1.016 | 0.299 | 0.016 | 0.46 |
| 11 | 0.593 | 1.499 | 0.078 | 1.221 | 0.318 | 0.019 | 0.34 |
| 14 | −0.601 | 2.136 | 0.091 | 1.209 | 0.379 | 0.017 | 0.39 |
| 15 | 0.041 | 2.105 | 0.080 | 1.147 | 0.379 | 0.017 | 0.41 |
| S | (Intercept) | ADG 1 | MMBW 2 | SE (Intercept) | SE (ADG) | SE (MMBW) | -Value |
|---|---|---|---|---|---|---|---|
| 1 | −0.515 | 1.841 | 0.077 | 0.714 | 0.380 | 0.008 | 0.63 |
| 2 | −0.204 | 1.678 | 0.076 | 0.712 | 0.384 | 0.009 | 0.62 |
| 3 | −0.131 | 1.699 | 0.074 | 0.708 | 0.373 | 0.009 | 0.62 |
| 4 | −1.591 | 2.574 | 0.079 | 0.963 | 0.493 | 0.011 | 0.55 |
| 5 | −0.365 | 2.045 | 0.074 | 0.705 | 0.373 | 0.009 | 0.64 |
| 6/12 | −0.163 | 2.354 | 0.067 | 0.788 | 0.389 | 0.010 | 0.60 |
| 7 | 0.408 | 1.688 | 0.074 | 1.072 | 0.474 | 0.013 | 0.40 |
| 8 | −0.491 | 1.555 | 0.081 | 0.928 | 0.455 | 0.010 | 0.50 |
| 9 | −0.435 | 1.467 | 0.083 | 0.714 | 0.330 | 0.008 | 0.62 |
| 10/13 | −0.068 | 1.227 | 0.082 | 0.808 | 0.338 | 0.009 | 0.53 |
| 11 | −0.277 | 0.796 | 0.090 | 0.942 | 0.366 | 0.011 | 0.46 |
| 14 | −0.362 | 1.591 | 0.082 | 0.812 | 0.396 | 0.009 | 0.56 |
| 15 | −0.797 | 1.495 | 0.088 | 0.948 | 0.398 | 0.011 | 0.50 |
| Standard | (Experiment 2) | (Experiment 3) | Kendall’s (Experiment 2) | Kendall’s (Experiment 3) |
|---|---|---|---|---|
| 2 | 0.98 | 0.99 | 0.89 | 0.91 |
| 3 | 0.97 | 0.98 | 0.86 | 0.88 |
| 4 | 0.90 | 0.63 | 0.75 | 0.49 |
| 5 | 0.87 | 0.95 | 0.68 | 0.82 |
| 6/12 | 0.84 | 0.74 | 0.65 | 0.56 |
| 7 | 0.79 | 0.60 | 0.60 | 0.43 |
| 8 | 0.91 | 0.67 | 0.76 | 0.51 |
| 9 | 0.78 | 0.98 | 0.62 | 0.88 |
| 10/13 | 0.84 | 0.86 | 0.66 | 0.67 |
| 11 | 0.82 | 0.82 | 0.63 | 0.64 |
| 14 | 0.88 | 0.82 | 0.70 | 0.63 |
| 15 | 0.86 | 0.65 | 0.68 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mononen, J.; Kostensalo, J.; Pesonen, M.; Huuskonen, A.; Manni, K. Assessing the Reliability of Optimized Residual Feed Intake Measurements in Beef Cattle. Ruminants 2022, 2, 407-419. https://doi.org/10.3390/ruminants2040028
Mononen J, Kostensalo J, Pesonen M, Huuskonen A, Manni K. Assessing the Reliability of Optimized Residual Feed Intake Measurements in Beef Cattle. Ruminants. 2022; 2(4):407-419. https://doi.org/10.3390/ruminants2040028
Chicago/Turabian StyleMononen, Jaakko, Joel Kostensalo, Maiju Pesonen, Arto Huuskonen, and Katariina Manni. 2022. "Assessing the Reliability of Optimized Residual Feed Intake Measurements in Beef Cattle" Ruminants 2, no. 4: 407-419. https://doi.org/10.3390/ruminants2040028
APA StyleMononen, J., Kostensalo, J., Pesonen, M., Huuskonen, A., & Manni, K. (2022). Assessing the Reliability of Optimized Residual Feed Intake Measurements in Beef Cattle. Ruminants, 2(4), 407-419. https://doi.org/10.3390/ruminants2040028








