Measures of Bone Morphology in the Medial and Lateral Condyles of the Metacarpus in Beef Cross Dairy Cattle at 8–12 and 24 Months of Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. pQCT Scanning
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubin, C.T.; Lanyon, L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef]
- Gibson, M.; Dittmer, K.; Hickson, R.; Back, P.; Rogers, C. Bone Morphology and Strength in the Mid-Diaphysis of the Humerus and Metacarpus in Dairy Calves Prior to Weaning. Animals 2020, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Rogers, C.; Dittmer, K.; Hickson, R.; Pettigrew, E.; Back, P. Can bone measures of the bovine metacarpus predict humeral bone structure? N. Z. J. Anim. Sci. Prod. 2019, 79, 8–12. [Google Scholar]
- Firth, E.C.; Rogers, C.W.; van Weeren, P.R.; Barneveld, A.; McIlwraith, C.W.; Kawcak, C.E.; Goodship, A.E.; Smith, R.K. Mild exercise early in life produces changes in bone size and strength but not density in proximal phalangeal, third metacarpal and third carpal bones of foals. Vet. J. 2011, 190, 383–389. [Google Scholar] [CrossRef]
- Akers, R.M.; Denbow, D.M. Bones and skeletal system. In Anatomy and Physiology of Domestic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 133–169. [Google Scholar]
- Sinclair, C.; Birch, H.L.; Smith, R.K.W.; Goodship, A.E. Skeletal physiology: Responses to exercise and training. In Equine Sports Medicine and Surgery, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; W.B. Saunders: Edinburgh, NY, USA, 2014; pp. 145–165. [Google Scholar] [CrossRef]
- Bouza-Rodríguez, J.B.; Miramontes-Sequeiros, L.C. Three-dimensional biomechanical analysis of the bovine humerus. Appl. Bionics Biomech. 2014, 11, 13–24. [Google Scholar] [CrossRef][Green Version]
- Bartosiewicz, L. Sexual dimorphism of long bone growth in cattle. Acta Vet. Hung. 1984, 32, 135–146. [Google Scholar]
- Guilbert, H.R.; Gregory, P.W. Some features of growth and development of Hereford cattle. J. Anim. Sci. 1952, 11, 3–16. [Google Scholar] [CrossRef]
- Berg, R.T.; Andersen, B.B.; Liboriussen, T. Growth of bovine tissues 4. Genetic influences on patterns of bone growth and distribution in young bulls. J. Anim. Sci. 1978, 27, 71–77. [Google Scholar] [CrossRef]
- Rakestraw, P.C. Fractures of the humerus. J. Vet. Clin. Food Anim. Pract. 1996, 12, 153–168. [Google Scholar] [CrossRef]
- Greenough, P.R.; Weaver, A.D.; MacCallum, F.J. Lameness in Cattle; Oliver & Boyd: Edinburgh, UK, 1972. [Google Scholar]
- Hickson, R.; Balcomb, C.; Fraser, K.; Lopez-Villalobos, K.P.; Morris, S. The effect of breed on the onset of puberty in heifers. In Proceedings of the Association for the Advancement of Animal Breeding and Genetics, Perth, WA, Australia, 19–21 July 2011; pp. 51–54. [Google Scholar]
- Gupta, S.K.; Singh, P.; Shinde, K.P.; Lone, S.; Kumar, N.; Kumar, A. Strategies for attaining early puberty in cattle and buffalo: A review. Agric. Rev. 2016, 37, 160–167. [Google Scholar] [CrossRef]
- Handcock, R.C.; Jenkinson, C.M.; Laven, R.; McNaughton, L.R.; Lopez-Villalobos, N.; Back, P.J.; Hickson, R.E. Linear versus seasonal growth of dairy heifers decreased age at puberty but did not affect first lactation milk production. N. Z. J. Agric. Res. 2021, 64, 83–100. [Google Scholar] [CrossRef]
- Singh, D.; Sanyal, S.; Chattopadhyay, N. The role of estrogen in bone growth and formation: Changes at puberty. Cell Health Cytoskelet. 2010, 3, 1–12. [Google Scholar] [CrossRef][Green Version]
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef]
- Purchas, R.W.; Burnham, D.L.; Morris, S.T. Effects of growth potential and growth path on tenderness of beef longissimus muscle from bulls and steers. J. Anim. Sci. 2002, 80, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Telldahl, Y. Ageing cattle: The use of radiographic examinations on cattle metapodials from eketorp ringfort on the island of Öland in Sweden. PLoS ONE 2015, 10, e0137109. [Google Scholar] [CrossRef][Green Version]
- Meaker, H.; Liebenberg, G. Live and carcass characteristics of bulls and steers castrated at three different ages. S. Afr. J. Anim. Sci. 1982, 12, 375–378. [Google Scholar]
- Keller, A.; Clauss, M.; Muggli, E.; Nuss, K. Even-toed but uneven in length: The digits of artiodactyls. Zoology 2009, 112, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Nacambo, S.; Hässig, M.; Lischer, C.; Nuss, K. Difference in the Length of the Medial and Lateral Metacarpal and Metatarsal Condyles in Calves and Cows—A Post—Mortem Study. Anat. Histol. Embryol. 2007, 36, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Van der Tol, P.; Metz, J.; Noordhuizen-Stassen, E.; Back, W.; Braam, C.; Weijs, W. The pressure distribution under the bovine claw during square standing on a flat substrate. J. Dairy Sci. 2002, 85, 1476–1481. [Google Scholar] [CrossRef]
- Van der Tol, P.P.J.; Metz, J.H.M.; Noordhuizen-Stassen, E.N.; Back, W.; Braam, C.R.; Weijs, W.A. The Vertical Ground Reaction Force and the Pressure Distribution on the Claws of Dairy Cows While Walking on a Flat Substrate. J. Dairy Sci. 2003, 86, 2875–2883. [Google Scholar] [CrossRef]
- Muir, P.; Fugle, C.; Ormond, A. Calf rearing using a once-a-day milk feeding system: Current best practice. In Proceedings of the New Zealand Grassland Association, West Coast, New Zealand, 5–7 November 2002; pp. 21–24. [Google Scholar]
- Pike, S.; Schreurs, N.; Hickson, R.; Hunt, J.; Kenyon, P.; Garrick, D.; Blair, H.; Morris, S. Brief Communication: Meat quality of light-weight, yearling steers of dairy origin. N. Z. J. Anim. Sci. Prod. 2019, 79, 156–158. [Google Scholar]
- Firth, E.C.; Rogers, C.; Doube, M.; Jopson, N.B. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 6. Bone parameters in the third metacarpal and third metatarsal bones. N. Z. Vet. J. 2005, 53, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Gibson, M.; Hickson, R.; Back, P.; Dittmer, K.; Schreurs, N.; Rogers, C. The Effect of Sex and Age on Bone Morphology and Strength in the Metacarpus and Humerus in Beef-Cross-Dairy Cattle. Animals 2021, 11, 694. [Google Scholar] [CrossRef]
- Bijen, L.; Gibson, M.J.; Back, P.J.; Hickson, R.E.; Van Knegsel, A.; Rogers, C.W. Effect of breed and paddock activity on bone mass and strength in three different beef cattle breeds. N. Z. J. Anim. Sci. Prod. 2020, 80, 29–33. [Google Scholar]
- Logan, A.A.; Nielsen, B.D.; Robison, C.I.; Manfredi, J.M.; Buskirk, D.D.; Schott, H.C.; Hiney, K.M. Calves, as a model for juvenile horses, need only one sprint per week to experience increased bone strength. J. Anim. Sci. 2019, 97, 3300–3312. [Google Scholar] [CrossRef]
- Riggs, C.M.; Whitehouse, G.H.; Boyde, A. Structural variation of the distal condyles of the third metacarpal and third metatarsal bones in the horse. Equine Vet. J. 1999, 31, 130–139. [Google Scholar] [CrossRef]
- Gibson, M.J.; Adams, B.R.; Back, P.J.; Hickson, R.E.; Dittmer, K.E.; Rogers, C.W. Live Weight and Bone Growth from Birth to 23 Months of Age in Holstein-Friesian, Jersey and Crossbred Heifers. Dairy 2022, 3, 333–344. [Google Scholar] [CrossRef]
- Gibson, M.; Rogers, C.; Hickson, R.; Dittmer, K.; Back, P. Live weight and bone growth from birth to 15 months of age in pure-bred and cross-bred Jersey and Friesian heifers. N. Z. J. Anim. Sci. Prod. 2021, 81, 45–50. [Google Scholar]
- Gilsanz, V.; Roe, T.F.; Gibbens, D.T.; Schulz, E.E.; Carlson, M.E.; Gonzalez, O.; Boechat, M.I. Effect of sex steroids on peak bone density of growing rabbits. Am. J. Physiol. 1988, 255, E416–E421. [Google Scholar] [CrossRef] [PubMed]
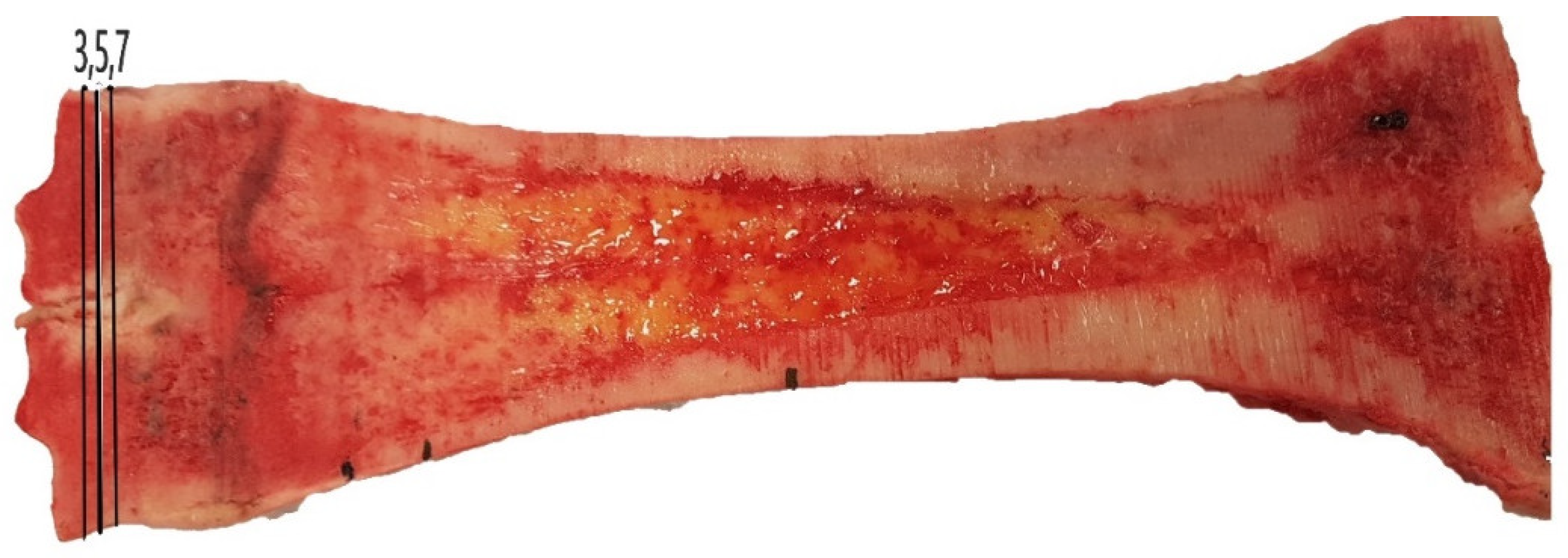
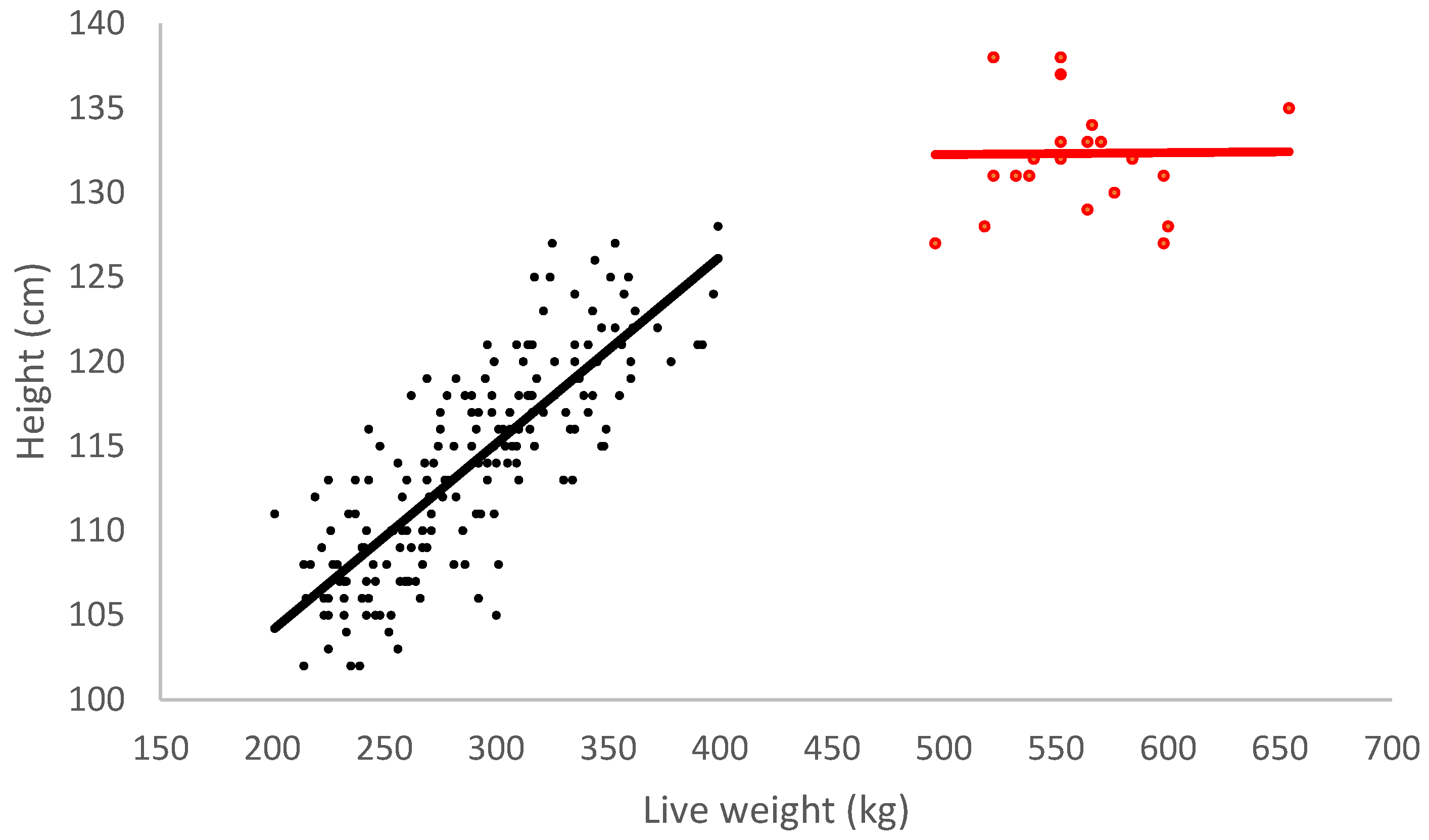
| Age (Months) | 8–12 | 20 (24 Month Age Group) | 24 | p-Value |
|---|---|---|---|---|
| n | 59 | 22 | 22 | |
| Weight (kg) | 302.1 ± 5.7 | 580.1 ± 9.2 | <0.001 | |
| Height (cm) | 114.1 ± 0.4 | 132.3 ± 1.3 | <0.001 | |
| Metacarpus length (mm) | 204.7 ± 0.8 | 221.6 ± 1.4 | <0.001 |
| Measure | Intercept | Coefficient | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 8–12 m | 24 m | 8–12 m | 24 m | Age | Body Weight | Age × Body Weight interaction | R2 | ||
| Height (cm) | 82.0 | 131.7 | 0.1 | 0.001 | <0.001 | <0.001 | <0.001 | 0.81 | |
| Bone length (mm) | 183.5 | 137.9 | 0.07 | 0.10 | 0.010 | <0.001 | 0.025 | 0.74 | |
| 3-mm | |||||||||
| Total bone area (mm2) | Medial | 582.5 | 139.9 | 0.7 | 1.5 | 0.044 | <0.001 | 0.067 | 0.70 |
| Lateral | 651.5 | 305.8 | 0.6 | 1.2 | 0.101 | <0.001 | 0.117 | 0.69 | |
| Total bone density (mg/cm3) | Medial | 436.6 | 938.6 | 0.4 | −0.4 | <0.001 | 0.962 | <0.001 | 0.69 |
| Lateral | 417.6 | 826.2 | 0.4 | −0.2 | 0.002 | 0.319 | 0.009 | 0.76 | |
| Total bone content (mg/mm) | Medial | 223.7 | 334.8 | 0.8 | 0.6 | 0.328 | <0.001 | 0.417 | 0.91 |
| Lateral | 247.8 | 331.4 | 0.7 | 0.7 | 0.447 | <0.001 | 0.871 | 0.93 | |
| Trabecular bone area (mm2) | Medial | 717.6 | −383.7 | −0.3 | 1.5 | 0.001 | 0.067 | 0.004 | 0.33 |
| Lateral | 785.6 | −131.4 | −0.3 | 1.0 | 0.009 | 0.304 | 0.039 | 0.51 | |
| Trabecular bone density (mg/cm3) | Medial | 488.1 | 640.6 | 0.1 | −0.2 | 0.029 | 0.780 | 0.017 | 0.12 |
| Lateral | 468.1 | 658.0 | 0.2 | −0.2 | 0.016 | 0.914 | 0.020 | 0.41 | |
| Cortical/subcortical bone area (mm2) | Medial | −135.1 | 523.1 | 1.1 | −0.02 | <0.001 | 0.005 | 0.004 | 0.88 |
| Lateral | −134.0 | 437.2 | 1.0 | 0.2 | 0.007 | 0.003 | 0.060 | 0.91 | |
| Cortical/subcortical bone density (mg/cm3) | Medial | 501.2 | 941.9 | 0.6 | −0.2 | 0.003 | 0.132 | 0.004 | 0.70 |
| Lateral | 458.9 | 834.0 | 0.7 | −0.02 | 0.034 | 0.048 | 0.038 | 0.69 | |
| Cortical/subcortical bone content (mg/mm) | Medial | −130.5 | 496.3 | 0.9 | −0.1 | <0.001 | 0.021 | 0.002 | 0.88 |
| Lateral | −127.2 | 363.3 | 0.8 | 0.2 | 0.008 | 0.006 | 0.081 | 0.91 | |
| Percentage of cortical/subcortical bone area | Medial | −0.1 | 1.0 | 0.001 | −0.0008 | <0.001 | 0.532 | <0.001 | 0.75 |
| Lateral | −0.1 | 0.8 | 0.001 | −0.0005 | <0.001 | 0.266 | 0.004 | 0.82 | |
| 5 mm | |||||||||
| Total bone area (mm2) | Medial | 652.1 | 483.4 | 0.8 | 1.6 | 0.041 | <0.001 | 0.078 | 0.66 |
| Lateral | 699.1 | 444.7 | 0.7 | 1.1 | 0.219 | <0.001 | 0.292 | 0.65 | |
| Total bone density (mg/cm3) | Medial | 393.4 | 892.3 | 0.5 | −0.4 | <0.001 | 0.573 | <0.001 | 0.74 |
| Lateral | 374.8 | 808.9 | 0.5 | −0.2 | 0.002 | 0.211 | 0.004 | 0.78 | |
| Total bone content (mg/mm) | Medial | 222.2 | −107.0 | 0.9 | 0.7 | 0.365 | <0.001 | 0.424 | 0.92 |
| Lateral | 231.6 | 427.0 | 0.8 | 0.5 | 0.075 | <0.001 | 0.163 | 0.93 | |
| Trabecular bone area (mm2) | Medial | 808.2 | −256.1 | −0.3 | 1.4 | 0.003 | 0.108 | 0.015 | 0.43 |
| Lateral | 839.7 | −49.7 | −0.3 | 1.0 | 0.011 | 0.307 | 0.049 | 0.52 | |
| Trabecular bone density (mg/cm3) | Medial | 444.0 | 638.9 | 0.2 | −0.2 | 0.010 | 0.742 | 0.005 | 0.28 |
| Lateral | 422.4 | 633.0 | 0.3 | −0.1 | 0.016 | 0.436 | 0.012 | 0.46 | |
| Cortical/subcortical bone area (mm2) | Medial | −156.2 | 424.7 | 1.1 | 0.2 | 0.004 | <0.001 | 0.021 | 0.90 |
| Lateral | −143.6 | 494.4 | 1.0 | 0.1 | 0.003 | 0.008 | 0.025 | 0.90 | |
| Cortical/subcortical bone density (mg/cm3) | Medial | 452.6 | 934.8 | 0.7 | −0.2 | 0.005 | 0.099 | 0.004 | 0.69 |
| Lateral | 424.5 | 832.1 | 0.8 | −0.02 | 0.025 | 0.030 | 0.021 | 0.69 | |
| Cortical/subcortical bone content (mg/mm) | Medial | −144.3 | 415.3 | 0.9 | 0.1 | 0.002 | 0.007 | 0.016 | 0.90 |
| Lateral | −133.7 | 414.5 | 0.8 | 0.1 | 0.003 | 0.018 | 0.037 | 0.90 | |
| Percentage of cortical/subcortical bone area | Medial | −0.1 | 0.9 | 0.001 | −0.001 | <0.001 | 0.327 | <0.001 | 0.81 |
| Lateral | −0.1 | 0.8 | 0.001 | −0.0005 | <0.001 | 0.266 | 0.003 | 0.83 | |
| 7 mm | |||||||||
| Total bone area (mm2) | Medial | 686.1 | 163.1 | 0.8 | 1.7 | 0.044 | <0.001 | 0.097 | 0.61 |
| Lateral | 721.4 | 483.3 | 0.7 | 1.0 | 0.238 | <0.001 | 0.377 | 0.61 | |
| Total bone density (mg/cm3) | Medial | 462.4 | 916.8 | 0.2 | −0.5 | 0.041 | 0.560 | 0.082 | 0.35 |
| Lateral | 358.4 | 459.7 | 0.6 | 0.3 | 0.647 | 0.051 | 0.476 | 0.31 | |
| Total bone content (mg/mm) | Medial | 368.0 | 727.6 | 0.5 | −0.1 | 0.299 | 0.523 | 0.382 | 0.37 |
| Lateral | 283.3 | 101.4 | 0.7 | 0.9 | 0.561 | 0.007 | 0.734 | 0.36 | |
| Trabecular bone area (mm2) | Medial | 815.5 | −177.5 | −0.1 | 1.3 | 0.008 | 0.077 | 0.039 | 0.41 |
| Lateral | 820.1 | −91.8 | −0.1 | 1.1 | 0.005 | 0.087 | 0.035 | 0.52 | |
| Trabecular bone density (mg/cm3) | Medial | 408.3 | 674.9 | 0.2 | −0.3 | 0.011 | 0.672 | 0.009 | 0.22 |
| Lateral | 380.6 | 527.8 | 0.4 | −0.02 | 0.182 | 0.109 | 0.077 | 0.26 | |
| Cortical/subcortical bone area (mm2) | Medial | 77.5 | 581.0 | 0.4 | −0.2 | 0.304 | 0.864 | 0.511 | 0.40 |
| Lateral | −62.1 | −112.9 | 0.8 | 0.9 | 0.912 | 0.057 | 0.960 | 0.34 | |
| Cortical/subcortical bone density (mg/cm3) | Medial | 567.4 | 947.2 | 0.4 | −0.3 | 0.126 | 0.855 | 0.173 | 0.34 |
| Lateral | 488.8 | 540.8 | 0.6 | 0.4 | 0.830 | 0.029 | 0.564 | 0.26 | |
| Cortical/subcortical bone content (mg/mm) | Medial | 57.8 | 556.1 | 0.3 | −0.3 | 0.248 | 0.941 | 0.459 | 0.40 |
| Lateral | −70.7 | −173.6 | 0.7 | 0.8 | 0.796 | 0.049 | 0.839 | 0.33 | |
| Percentage of cortical/subcortical bone area | Medial | 0.1 | 0.8 | 0.0003 | −0.0007 | 0.188 | 0.625 | 0.321 | 0.25 |
| Lateral | −0.04 | 0.1 | 0.0008 | 0.0004 | 0.705 | 0.179 | 0.648 | 0.26 | |
| 8–12 Months | 24 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| LSMean | p-Value | LSMean | p-Value | |||||
| Lateral | Medial | Body Weight | Condyle | Lateral | Medial | Body Weight | Condyle | |
| 3 mm | ||||||||
| Total bone area (mm2) | 838.4 ± 7.5 | 799.5 ± 7.5 | <0.001 | <0.001 | 1025.6 ± 16.8 | 994.4 ± 16.8 | <0.001 | 0.196 |
| Total bone density (mg/cm3) | 552.9 ± 5.2 | 570.6 ± 5.2 | <0.001 | 0.017 | 708.6 ± 7.5 | 687.8 ± 7.5 | 0.026 | 0.059 |
| Total bone content (mg/mm) | 462.8 ± 3.9 | 456.5 ± 3.9 | <0.001 | 0.266 | 724.8 ± 8.7 | 681.3 ± 8.7 | 0.001 | <0.001 |
| Trabecular bone area (mm2) | 681.3 ± 13.1 | 612.6 ± 13.1 | 0.103 | <0.001 | 457.6 ± 21.6 | 481.1 ± 21.6 | 0.003 | 0.447 |
| Trabecular bone density (mg/cm3) | 523.1 ± 3.0 | 530.1 ± 3.0 | 0.001 | 0.103 | 561.7 ± 3.9 | 539.1 ± 3.9 | 0.022 | <0.001 |
| Cortical/subcortical bone area (mm2) | 157.1 ± 7.9 | 186.9 ± 7.9 | <0.001 | 0.009 | 568.0 ± 12.3 | 513.3 ± 12.3 | 0.645 | 0.003 |
| Cortical/subcortical bone density (mg/cm3) | 662.3 ± 7.1 | 688.9 ± 7.1 | <0.001 | 0.009 | 824.0 ± 5.1 | 823.9 ± 5.1 | 0.243 | 0.984 |
| Cortical/subcortical bone content (mg/mm) | 108.3 ± 6.6 | 132.5 ± 6.6 | <0.001 | 0.011 | 468.9 ± 11.9 | 423.4 ± 11.9 | 0.898 | 0.010 |
| Percentage of cortical/subcortical bone area | 0.2 ± 0.01 | 0.2 ± 0.01 | <0.001 | 0.003 | 0.6 ± 0.02 | 0.5 ± 0.02 | 0.029 | 0.109 |
| 5 mm | ||||||||
| Total bone area (mm2) | 892.9 ± 7.7 | 888.8 ± 7.7 | <0.001 | 0.703 | 1057.7 ± 17.2 | 1075.3 ± 17.2 | <0.001 | 0.474 |
| Total bone density (mg/cm3) | 531.9 ± 5.2 | 545.2 ± 5.2 | <0.001 | 0.072 | 688.5 ± 8.0 | 680.6 ± 8.0 | 0.058 | 0.487 |
| Total bone content (mg/mm) | 474.2 ± 4.0 | 484.0 ± 4.0 | <0.001 | 0.083 | 726.6 ± 9.0 | 729.0 ± 9.0 | <0.001 | 0.852 |
| Trabecular bone area (mm2) | 745.1 ± 13.1 | 722.2 ± 13.1 | 0.159 | 0.220 | 516.2 ± 24.2 | 533.5 ± 24.2 | 0.012 | 0.617 |
| Trabecular bone density (mg/cm3) | 503.7 ± 3.3 | 512.3 ± 3.3 | <0.001 | 0.069 | 549.5 ± 4.1 | 534.4 ± 4.1 | 0.037 | 0.013 |
| Cortical/subcortical bone area (mm2) | 147.9 ± 7.4 | 166.6 ± 7.4 | <0.001 | 0.075 | 541.5 ± 14.8 | 541.8 ± 14.8 | 0.603 | 0.989 |
| Cortical/subcortical bone density (mg/cm3) | 655.3 ± 7.6 | 669.9 ± 7.6 | <0.001 | 0.176 | 817.6 ± 5.7 | 820.2 ± 5.7 | 0.289 | 0.751 |
| Cortical/subcortical bone content (mg/mm) | 100.9 ± 6.2 | 115.7 ± 6.2 | <0.001 | 0.093 | 443.8 ± 14.3 | 445.2 ± 14.3 | 0.846 | 0.946 |
| Percentage of cortical/subcortical bone area | 0.2 ± 0.01 | 0.2 ± 0.01 | <0.001 | 0.103 | 0.5 ± 0.02 | 0.5 ± 0.02 | 0.093 | 0.774 |
| 7 mm | ||||||||
| Total bone area (mm2) | 928.7 ± 8.2 | 941.5 ± 8.2 | <0.001 | 0.272 | 1075.6 ± 18.0 | 1122.9 ± 18.0 | <0.001 | 0.071 |
| Total bone density (mg/cm3) | 527.6 ± 8.0 | 535.5 ± 8.0 | 0.002 | 0.490 | 612.5 ± 16.9 | 636.7 ± 16.9 | 0.724 | 0.318 |
| Total bone content (mg/mm) | 500.6 ± 11.5 | 516.7 ± 11.5 | 0.001 | 0.327 | 634.5 ± 26.3 | 683.2 ± 26.3 | 0.386 | 0.199 |
| Trabecular bone area (mm2) | 783.0 ± 12.4 | 783.4 ± 12.4 | 0.566 | 0.984 | 573.3 ± 25.4 | 594.8 ± 25.4 | 0.011 | 0.553 |
| Trabecular bone density (mg/cm3) | 486.6 ± 3.9 | 494.1 ± 3.9 | <0.001 | 0.174 | 517.6 ± 7.0 | 519.0 ± 7.0 | 0.268 | 0.889 |
| Cortical/subcortical bone area (mm2) | 182.5 ± 16.7 | 192.6 ± 16.7 | 0.028 | 0.671 | 382.0 ± 37.4 | 451.4 ± 37.4 | 0.647 | 0.197 |
| Cortical/subcortical bone density (mg/cm3) | 681.1 ± 9.4 | 676.0 ± 9.4 | 0.001 | 0.700 | 757.5 ± 16.4 | 787.7 ± 16.4 | 0.870 | 0.199 |
| Cortical/subcortical bone content (mg/mm) | 131.7 ± 14.5 | 139.1 ± 14.5 | 0.047 | 0.722 | 303.1 ± 33.0 | 365.0 ± 33.0 | 0.684 | 0.191 |
| Percentage of cortical/subcortical bone area | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.088 | 0.612 | 0.4 ± 0.04 | 0.4 ± 0.04 | 0.781 | 0.316 |
| 8–12 Months | 24 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LSMean | p-Value | LSMean | p-Value | |||||||
| 3 mm | 5 mm | 7 mm | Body Weight | Scan | 3 mm | 5 mm | 7 mm | Body Weight | Scan | |
| Medial | ||||||||||
| Total bone area (mm2) | 799.5 ± 8.4 a | 888.8 ± 8.4 b | 941.5 ± 8.4 c | <0.001 | <0.001 | 994.4 ± 18.2 a | 1075.3 ± 18.2 b | 1122.9 ± 18.2 b | <0.001 | <0.001 |
| Total bone density (mg/cm3) | 570.6 ± 6.3 b | 545.2 ± 6.3 a | 535.5 ± 6.3 a | <0.001 | <0.001 | 687.8 ± 10.8 b | 680.6 ± 10.8 b | 636.7 ± 10.8 a | 0.010 | 0.003 |
| Total bone content (mg/mm) | 456.5 ± 7.8 a | 484.0 ± 7.8 b | 516.7 ± 7.8 c | <0.001 | <0.001 | 681.3 ± 17.4 | 729.0 ± 17.4 | 683.2 ± 17.4 | 0.126 | 0.099 |
| Trabecular bone area (mm2) | 612.6 ± 13.3 a | 722.2 ± 13.3 b | 783.4 ± 13.3 c | 0.161 | <0.001 | 481.1 ± 23.2 a | 533.5 ± 23.2 ab | 594.8 ± 23.2 b | <0.001 | 0.004 |
| Trabecular bone density (mg/cm3) | 530.1 ± 3.2 c | 513.3 ± 3.2 b | 494.1 ± 3.2 a | <0.001 | <0.001 | 539.1 ± 4.3 b | 534.4 ± 4.3 b | 519.0 ± 4.3 a | 0.002 | 0.005 |
| Cortical/subcortical bone area (mm2) | 186.9 ± 12.3 | 166.6 ± 12.3 | 192.6 ± 12.3 | <0.001 | 0.293 | 513.3 ± 22.4 ab | 541.8 ± 22.4 b | 451.4 ± 22.4 a | 0.970 | 0.018 |
| Cortical/subcortical bone density (mg/cm3) | 688.9 ± 8.0 | 669.9 ± 8.0 | 676.0 ± 8.0 | <0.001 | 0.236 | 823.9 ± 9.4 b | 820.2 ± 9.4 b | 787.7 ± 9.4 a | 0.111 | 0.015 |
| Cortical/subcortical bone content (mg/mm) | 132.5 ± 10.6 | 115.7 ± 10.6 | 139.1 ± 10.6 | <0.001 | 0.280 | 423.4 ± 20.5 b | 445.2 ± 20.5 b | 365.0 ± 20.5 a | 0.661 | 0.021 |
| Percentage of cortical/subcortical bone area | 0.24 ± 0.01 | 0.19 ± 0.01 | 0.21 ± 0.01 | <0.001 | 0.088 | 0.52 ± 0.02 b | 0.51 ± 0.02 b | 0.41 ± 0.02 a | 0.036 | <0.001 |
| Lateral | ||||||||||
| Total bone area (mm2) | 838.4 ± 7.2 a | 892.9 ± 7.2 b | 928.7 ± 7.2 c | <0.001 | <0.001 | 1025.6 ± 16.0 | 1057.7 ± 16.0 | 1075.6 ± 16.0 | <0.001 | 0.090 |
| Total bone density (mg/cm3) | 552.9 ± 6.1 b | 531.9 ± 6.1 a | 527.6 ± 6.1 a | <0.001 | 0.009 | 708.6 ± 12.2 b | 688.5 ± 12.2 b | 612.5 ± 12.2 a | 0.789 | <0.001 |
| Total bone content (mg/mm) | 462.8 ± 7.0 a | 474.2 ± 7.0 a | 500.6 ± 7.0 b | <0.001 | <0.001 | 724.8 ± 16.0 b | 726.6 ± 16.0 b | 634.5 ± 16.0 a | 0.005 | <0.001 |
| Trabecular bone area (mm2) | 681.3 ± 12.4 a | 745.1 ± 12.4 b | 783.0 ± 12.4 c | 0.111 | <0.001 | 457.6 ± 24.1 a | 516.2 ± 24.1 ab | 573.3 ± 24.1 b | 0.005 | 0.005 |
| Trabecular bone density (mg/cm3) | 523.1 ± 3.6 c | 503.7 ± 3.6 b | 486.6 ± 3.6 a | <0.001 | <0.001 | 561.7 ± 5.9 b | 549.5 ± 5.9 b | 517.6 ± 5.9 a | 0.218 | <0.001 |
| Cortical/subcortical bone area (mm2) | 157.1 ± 10.7 | 147.9 ± 10.7 | 182.5 ± 10.7 | <0.001 | 0.061 | 568.0 ± 25.8 b | 541.5 ± 25.8 b | 382.0 ± 25.8 a | 0.318 | <0.001 |
| Cortical/subcortical bone density (mg/cm3) | 662.3 ± 8.2 | 655.3 ± 8.2 | 681.1 ± 8.2 | <0.001 | 0.071 | 824.0 ± 11.2 b | 817.6 ± 11.2 b | 757.5 ± 11.2 a | 0.510 | <0.001 |
| Cortical/subcortical bone content (mg/mm) | 108.3 ± 9.1 ab | 100.9 ± 9.1 a | 131.7 ± 9.1 b | <0.001 | 0.048 | 468.9 ± 22.8 b | 443.8 ± 228 b | 303.1 ± 22.8 a | 0.306 | <0.001 |
| Percentage of cortical/subcortical bone area | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.19 ± 0.01 | <0.001 | 0.228 | 0.56 ± 0.03 b | 0.52 ± 0.03 b | 0.35 ± 0.03 a | 0.626 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, M.J.; Hickson, R.E.; Dittmer, K.E.; Back, P.J.; Rogers, C.W. Measures of Bone Morphology in the Medial and Lateral Condyles of the Metacarpus in Beef Cross Dairy Cattle at 8–12 and 24 Months of Age. Ruminants 2022, 2, 297-307. https://doi.org/10.3390/ruminants2030020
Gibson MJ, Hickson RE, Dittmer KE, Back PJ, Rogers CW. Measures of Bone Morphology in the Medial and Lateral Condyles of the Metacarpus in Beef Cross Dairy Cattle at 8–12 and 24 Months of Age. Ruminants. 2022; 2(3):297-307. https://doi.org/10.3390/ruminants2030020
Chicago/Turabian StyleGibson, Michaela J., Rebecca E. Hickson, Keren E. Dittmer, Penny J. Back, and Chris W. Rogers. 2022. "Measures of Bone Morphology in the Medial and Lateral Condyles of the Metacarpus in Beef Cross Dairy Cattle at 8–12 and 24 Months of Age" Ruminants 2, no. 3: 297-307. https://doi.org/10.3390/ruminants2030020
APA StyleGibson, M. J., Hickson, R. E., Dittmer, K. E., Back, P. J., & Rogers, C. W. (2022). Measures of Bone Morphology in the Medial and Lateral Condyles of the Metacarpus in Beef Cross Dairy Cattle at 8–12 and 24 Months of Age. Ruminants, 2(3), 297-307. https://doi.org/10.3390/ruminants2030020






