Abstract
Cistus ladanifer has been used in ruminants feeding systems, but there is no information about the seasonal variation in chemical composition and nutritional value of each morphological fraction during its growth cycle. This study characterized the morphological fractions of C. ladanifer (leaves, stems, flower buds, flowers, and seed heads) throughout the year in chemical composition, in vitro digestibility, and antioxidant activity. The leaves were the morphological fraction more variable throughout the seasons, being characterized by low protein level (48.5–114 g/kg dry matter (DM)), moderate cell-wall content (240–267 g NDF/kg DM), high levels of condensed tannins (CT) (131–246 g/kg DM), and low in vitro organic matter digestibility (IVOMD) (29.3–34.3%). The distinctive chemical composition among various morphological fractions allowed the identification of four distinct groups, the first composed by stems, the second by the leaves, the third by the seed heads, and the last one by the flower buds and flowers. All the morphological fractions are sources of bioactive compounds namely phenolic compounds. Leaves are the fraction with higher nutritive value, especially when collected during winter and spring.
1. Introduction
Shrubs are an essential component of the Silvo-Pastoral systems in the Mediterranean region, being considered important feeding resource for ruminants. During dry and cool seasons, when conventional forages are scarce and with low nutritive quality, expensive feed supplementation is required, hence shrubs can supply green material for grazing animals, providing nutrients and energy [1]. Shrubs are abundant and with higher quality than most of herbaceous plants during drought [2]. In the past decade, with the increase of the animal feed costs (e.g., price of cereal grains and soybean meal), several studies have explored the use of shrubs to replace conventional ingredients of animal diets [3,4]. Furthermore, shrubs contain secondary metabolites, as phenolic compounds, that present several biological activities with benefits for animal health and productivity and food quality, when used at low doses [4,5,6].
Cistus ladanifer L., known as rockrose, is a perennial shrub widespread in the forest and uncultivated areas of the western Mediterranean region (Portugal, Spain, south of France, north of Morocco) [7]. Cistus ladanifer can be considered a poor quality feed due to its poor nutritive value and high levels of antinutritional compounds, as condensed tannins (CT) [8,9]. The aerial parts (composed by leaves and soft stems) of C. ladanifer are characterized by low protein level (55–100 g/kg dry matter (DM)), moderate cell-wall content (318–410 g NDF/kg DM), high levels of phenolic compounds (55.1–106 g gallic acid equivalents (GAE)/kg DM) and CT (32.1–161 g/kg DM), and low in vitro organic matter digestibility (IVOMD) (24.9–41.8%) [9,10,11]. The aerial parts have higher levels of protein and lower cell-wall content during autumn and winter than during summer. Cistus ladanifer can be considered a valuable source of bioactive compounds for ruminants, due to its high level of phenolic compounds, as phenolic acids, CT, and terpenic compounds [9,12,13,14]. In summer, the aerial parts contain the highest levels of phenolic compounds, CT, and antioxidant activity [9,11]. Indeed, in the past decade, C. ladanifer has been explored as a CT source in lamb diets [15,16,17,18,19]. Previous work of our research team demonstrated that incorporation of C. ladanifer aerial part in lamb oil-supplemented diets improved the nutritional value of lipid fraction of meat, increasing the health favorable fatty acid, rumenic acid content, a conjugated linoleic acid isomer (CLA) [15]. Moreover, the inclusion of C. ladanifer aerial part in lamb diets enhanced the antioxidant potential of the meat, without compromising animal performance and meat sensorial properties [15,20,21]. Recently, Guerreiro et al. [16] showed that the incorporation of a CT extract from C. ladanifer (12.5 g CT/kg DM) in lamb diets improved in 25% the proportion of other healthy fatty acid, vaccenic acid, in intramuscular fat, without compromising animal growth performance and sensorial properties of the meat [22].
We have previously suggested that C. ladanifer can be used as a feed in ruminant diets, but only associated with other feeding resources to complement its nutritional imbalances [9]. The present work aims to increase knowledge about the chemical composition and nutritional value of C. ladanifer and their seasonal variations. So, the morphological fractions of C. ladanifer (leaves, stems, flower buds, flowers, and seed heads) were analyzed for proximate composition, phenolic compounds, including CT, tocopherols, in vitro digestibility, and antioxidant activity, throughout the year.
2. Materials and Methods
2.1. Plant Material Sampling
Between December 2015 and November 2016, six plants of C. ladanifer with 2–3 years of old were collected monthly in Ourique, Baixo Alentejo region, Southern Portugal (37°44′25.9″ N/8°21′27.6″ W). Cistus ladanifer plants were collected with an interval between two samplings of one month, with three collections for each season: winter (December, January and February), spring (March, April and May), summer (June, July and August), and autumn (September, October and November). The climate of the Baixo Alentejo region is of Mediterranean influence, characterized by hot, dry, and long summers and mild wet winters. In the sampling location and during the plant collection period was registered an annual mean temperature of 16.9 °C and total precipitation of 373 mm, as described by Jerónimo et al. [23]. The soils have low capacity for agricultural use, are mostly thin schist soils, with low drainage capacity and low organic matter content [24].
Samples were manually harvested with scissors and in laboratory each plant was immediately separated into each morphological fraction, as described by Jerónimo et al. [23]. Leaves and stems were collected in all sampling months. Seed heads were not present in plants in April. Between January and May were collected flower buds. flowers appeared in February and March, but in small quantity, so the six plants harvested were insufficient to carry out the analyses. So, for the evaluation of the composition of the flowers, more flowers were used than those present in the six plants collected in February and March, which were collected in contiguous C. ladanifer plants.
The C. ladanifer morphological fractions—leaves, stems, flower buds, flowers, and seed heads were dried in a ventilated oven at 40 °C (Dry-Big, J. P. Selecta, Barcelona, Spain) until constant weight, and then ground in a mill (1 mm) (Christy & Norris 8” Lab mill, Chelmsford, UK). For each morphological fraction in each month, material from six plants was pooled.
2.2. Chemical Composition
Ground samples of each morphological fraction were analyzed for dry matter (DM) [25], ash [26], Kjeldahl N [26], neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) [27], gross energy (GE) [28], and ether extract (EE) [29]. In vitro organic matter digestibility (IVOMD) was also determined [30]. Analyses were performed in duplicate.
2.3. Extract Preparation and Determination of Phenolic Compounds and Antioxidant Activity
The phenolic extracts of C. ladanifer were obtained according to Julkunen-Tiitto et al. [31] and Makkar et al. [32]. Briefly, 200 mg of ground samples were extracted with 10 mL of aqueous acetone (70%, v/v), in an ultrasonic bath, for 10 min. Extracts were centrifuged at 3800 rpm, at 4 °C, for 30 min, and the supernatants collected. The supernatants were stored at −20 °C and later used as “original extract” for total phenols and condensed tannins analysis.
2.3.1. Determination of Total Phenolic and Condensed Tannins Contents
Total phenols (TP) were quantified in the original extracts using the Folin–Ciocalteu’s reagents, described by Fialho et al. [33]. The concentration of TP was expressed as gallic acid equivalent (GAE) using gallic acid (Sigma Chemicals Co., Madrid, Spain) as standard.
Condensed tannins were measured using the butanol–HCl method, described by Porter et al. [34]. The concentration of CT was expressed as equivalents of C. ladanifer CT using as standard C. ladanifer CT purified in chromatographic column of Sephadex LH-20 (GE Healthcare Bio-Science, Uppsala, Sweden), according to Strumeyer and Malin [35]. Analyses were carried out in triplicate.
2.3.2. Evaluation of Antioxidant Activity
The antioxidant activity was evaluated by the ferric reducing antioxidant power assay (FRAP) and by the Trolox equivalent antioxidant capacity (TEAC).
Ferric reducing antioxidant power assay was performed following the methodology described by Fialho et al. [33]. Ferrous sulphate (FeSO4•7H2O, Merck KGAA, Darmstadt, Germany) was used as standard, and the results were expressed as µM Fe2+/g DM.
For the trolox equivalent antioxidant capacity assay, the procedure described by Fialho et al. [33] was used, and the results were expressed as µM of trolox/g of DM.
2.4. Determination of Tocopherol Content
The tocopherol content was quantified according to Sickel et al. [36] with some modifications. Briefly, a sample of 0.04 g of each morphological part of C. ladanifer was added with 1.5 mL of n-hexane, vortex-homogenized for 1 min and placed in an ultrasonic bath for 5 min at low temperature (<10 °C). This solution was stored overnight at −20 °C. The next day, the sample was centrifuged at 3500 rpm for 7 min and the supernatant was collected and stored at −20 °C until further analysis. The tocopherol quantification was performed using a Dionex Ultimate 3000 uHPLC (Thermo Fisher Scientific, Massachusetts, MA, USA) and a normal-phase silica column (Zorbax RX-Sil, with the corresponding 12.5 mm analytical guard column, 4.6 mm ID, 250 mm, 5 μm particle size, Agilent Technologies Inc., Palo Alto, CA, USA), using n-hexane/isopropanol (99:1 v/v) as the eluent. Twenty microliters of the extract were injected. The flow rate was 1.5 mL per minute. The fluorescence detector was calibrated with α-, β-, γ-, and δ-tocopherol standards in ethanol (Calbiochem, San Diego, CA, USA). Fluorescence of all components was excited at 295 nm and detected at 325 nm.
2.5. Statistical Analysis
The effect of season on the chemical composition, digestibility, and antioxidant activity of the morphological fractions was evaluated using the linear model of Proc MIXED option of SAS (SAS Institute Inc., Cary, NC, USA) according to the model:
where Yi is the observation, μ is the overall mean, Si is the season effect, and ei is the residual error. Samples were grouped per season, considering three collections in each season (three repetitions per season, corresponding to each month as indicated above). Data for leaves, stems, and seed heads were analyzed for the four seasons—winter, spring, summer, and autumn, while for flower buds only winter and spring were considered, and the flowers were collected only during spring. Least square means and standard error of the mean are presented in tables. Principal component analysis (PCA) was performed using Minitab Statistical Software (State College, PA, USA).
Yi = μ + Si + ei
3. Results
3.1. Seasonal Variation in the Morphological Composition of Cistus Ladanifer Plants
The morphological composition of C. ladanifer plants is presented in Table 1. The leaves and stems weight in the total plant was significantly affected by season (p = 0.002 and p = 0.003, respectively). The predominant morphological fraction throughout all seasons was the stems. Stems have highest weight in summer (753 g/kg DM) than in the other seasons, averaging 692 g/kg DM. Leaves were the second most predominant fraction, have the lowest weight in summer (214 g/kg DM). In winter, spring, and autumn the leaves weight in total plant is similar and average 270 g/kg DM. Seed heads and flower buds are minority fractions, each representing less than 4% of the aerial part of the plant. The flowers are in small quantity in February and March, 1.55 g/kg DM and 0.75 g/kg DM respectively, not having been considered for statistical analysis

Table 1.
Effect of the season on morphological composition (g/kg DM) of Cistus ladanifer plants.
3.2. Seasonal Variation in the Proximate Composition and In Vitro Digestibility of the Morphological Fractions of Cistus Ladanifer
The chemical composition and IVOMD of each morphological fraction of C. ladanifer are presented in Table 2. The proximate composition of leaves was more variable throughout the seasons than in other morphological fractions. The levels of DM, EE, and NDF leaves are similar among seasons (p > 0.05), but the ash, CP, ADF, and GE content were affected by season (p < 0.05). The ash content decreased (p = 0.013) from 53.0 g/kg DM in winter and spring to 38.4 g/kg DM in summer and autumn. The CP level was higher in winter (114 g/kg DM), reduced to 52 g/kg between winter and summer, and remained constant until autumn (averaging 55.2 g/kg DM) (p = 0.034). The ADF is lower in summer (186 g/kg DM) and gradually increased until spring (223 g/kg DM) (p = 0.021). The ADL content is similar among seasons. The GE content of leaves is lower in spring (19.3 MJ/kg DM) remaining constant during winter, summer, and autumn (averaging 20.3 MJ/kg DM) (p = 0.026) and the IVOMD is not variable between seasons (averaging 30%) (p > 0.05).

Table 2.
Effect of the season on chemical composition and in vitro organic matter digestibility of morphological parts of Cistus ladanifer plants.
In stems, only DM and ADL content were affected by season (p < 0.05), these compounds being higher in summer and autumn.
The chemical composition and nutritive value of the flower buds are similar in winter and spring, and in seed heads, only IVOMD was affected (p = 0.006) being lower in winter (18.1%).
3.3. Seasonal Variation in the Phenolic and Tocopherol Content and Antioxidant Activity of the Morphological Fractions of Cistus Ladanifer
The seasonal variation of total phenolic, CT, and tocopherol contents and antioxidant activity of each morphological fraction of C. ladanifer are presented in Table 3. In the leaves, the CT increased (p < 0.001) from 150 g/kg DM in winter and spring to 229 g/kg DM in summer and autumn. Leaves presented the highest (p = 0.008) γ-tocopherol content in spring (57.1 µg/g DM) compared to other seasons (21.1 µg/g DM). The TP content tended (p = 0.064) to be higher in summer (101 g GAE/kg DM) than in winter (65.1 g GAE/kg DM).

Table 3.
Effect of the season on total phenolic, condensed tannins, and tocopherol content and antioxidant activity of morphological parts of Cistus ladanifer plants.
In the reproductive organs (flower buds and seed heads), only the γ-tocopherol content was affected by season (p < 0.05). Both reproductive organs presented the lowest γ-tocopherol content in winter.
3.4. Chemical Composition, In Vitro Digestibility, Phenolic Content and Antioxidant Activity in Relation to the Morphological Fractions of Cistus Ladanifer
Results of principal components analysis (PCA) showed that the first two components explain 76.3% of variance (Figure 1), allowing the discrimination of the morphological fractions in function of their chemical composition, IVOMD, phenolic content, and antioxidant activity. As shown in Figure 1A, it was possible to identify the presence of four distinct groups, the first composed by stems, the second by the leaves, the third by the seed heads, and the last one by the flower buds and flowers. Leaves showed a higher association with phenolic composition (TP, CT), α-tocopherol, antioxidant activity, CP and IVOMD, whereas seed heads had great association with the lignin content, and the stems were more related with fiber content (Figure 1B).
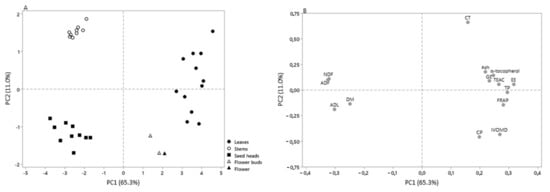
Figure 1.
Scores (A) and loadings (B) of the two first principal components (PC) computed using the chemical composition, in vitro digestibility, phenolic content, and antioxidant activity of C. ladanifer morphological fractions.
The first factor (PC 1) explained 65.3% of the variability, relating positively with ash, CP, EE, GE, IVOMD, phenolic content (TP, CT, and α-tocopherol), and antioxidant activity, and negatively with the cell-wall content and DM. The second factor (PC 2) accounted for the 11.0% of the variability, relating positively with the most of chemical parameters (except CP, ADL, and DM), phenolic content (except TP), TEAC, and negatively with IVOMD and FRAP.
Considering only the leaves data, the PCs explains 72.7% of the variability, allowing the discrimination of leaves in the function of their chemical composition, IVOMD, phenolic content, antioxidant activity, environmental temperatures, and precipitation (Figure 2). As shown in Figure 2A, it was possible to identify the presence of three distinct groups, the first composed by the leaves collected during winter, the second composed by leaves collected in spring, and the last group composed by leaves collected during summer and autumn. The first PC (PC1) accounted for 55.8% of the variability, relating positively with DM, EE, GE, phenolic content (except γ-tocopherol), and temperatures (mean, maximum, and minimum) and negatively with cell-wall content, digestibility, and precipitation. The second PC (PC2) explain 17.0% of the variability, relating positively with most of the chemical parameters and FRAP, and negatively with phenolic content (except CT), fiber content, and digestibility.
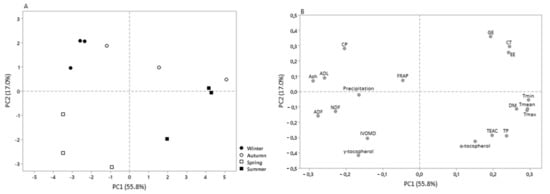
Figure 2.
Scores (A) and loadings (B) of the two first PCs computed using the chemical composition, in vitro digestibility, phenolic content, and antioxidant activity of C. ladanifer leaves, as well as data about temperature and precipitation among seasons.
4. Discussion
Cistus ladanifer is a resinous and fragrant evergreen shrub, and it is a dominant shrub species endemic to the western Mediterranean region, is resistant to strong drought and to poorly fertile and highly degraded soils [7]. Cistus ladanifer plants can reach 2 m in height, present branches very rigid wood covered by a sticky and viscous bark and lanceolate leaves welded at the base [7,37]. The stems and leaves are covered by labdanum gum, and present a unique large and terminal white flower, with a marron blotch at the base of each petal [37]. The seed heads of C. ladanifer are globular and lignified, with 6–12 valves, and each seed head produces a large number of seeds (approx. 250 per valve) [38,39].
The morphological fractions of C. ladanifer showed distinct chemical composition and nutritional value, which is confirmed by PCA, allowing to identify four distinct groups (Figure 1A): The first composed by leaves; the second by the stems; the third by the seed heads; and the last one by the flower buds and flowers. Leaves showed a higher association with phenolic compounds (TP, CT), α-tocopherol, antioxidant activity, CP, and IVOMD, whereas the seed heads had great association with the lignin content and the stems with fiber content (Figure 1B). The leaves, that represent about 25% of the aerial part of plant, represented the morphological fraction that presented the most similar chemical composition to the aerial parts described in previous works [9,10,11].
The chemical composition of aerial parts of C. ladanifer varied over the seasons, being strongly associated with the vegetative stage [9,11]. The time and duration of the vegetative development of plants varies with both climate conditions and location; however, it is well established that C. ladanifer vegetative growth starts after the first autumn rains, with the new plants to emerge and the new leaves to regrow, reducing during the summer dry season [37,40]. According to our results, leaves represented the more variable morphological fraction throughout the seasons, so we can assume that the leaves present an important contribution to the chemical composition of C. ladanifer aerial parts. The seasonal variation of the leaves chemical composition and nutritional value is evidenced by the PCA (Figure 2). Leaves collected during summer and autumn were more associated with phenolic compounds (TP and CT), α-tocopherol, TEAC, and EE. Leaves collected in spring had great association with fiber content, in vitro digestibility, and γ-tocopherol and leaves from winter were more related with CP and lignin content. Previous works that described the seasonal variation of the C. ladanifer aerial parts [9,11], showed that the aerial parts presented a higher CP and lower NDF contents during autumn and winter, whereas during spring and summer, the CP decreased and the NDF increased, as a result of plant maturation, in accordance with the phenological cycle of the C. ladanifer plants. In the present work, only leaves showed seasonal variation in the CP content, with higher CP content in winter decreasing until summer, and contrary to the previous findings, the CP content in the leaves remained low and equal during the summer and autumn. The apparently inconsistent findings are probably related to different environmental conditions, such as temperature, precipitation, light, soil type, and available nutrients that may have affected the plant vegetative development.
Higher cell-wall content in stems and seed heads than in leaves, flower buds, and flowers was expected. In stems, the lignin content increased progressively from winter and spring to autumn, which can be related to environmental factors, since high temperatures and water shortage during the summer lead to a more intense lignification of the cell-wall [41]. Contrarily, in leaves the ADF contents showed a steep decline from spring to summer and the lignin content presented the same tendency (p = 0.081). This result is in accordance with the results reported for other Cistus species [41,42] and could be due to the fact that the regrowth of new leaves in C. ladanifer plants only occurs at the end of summer. After flowering, the ovary is fully closed by sepals and during seed heads maturation, the pedicels elongate and lignify [37]. In early summer, the seed heads are mature and are exposed. During the summer, seed heads begin to open, and with the autumn wind and rain, the seeds are dispersed nearby [37]. In the present work, the seed heads were found throughout most of the year, with exception in April. So, the seed heads present in plants during winter and spring were formed in the previous reproductive cycle, which can explain why the lignin content remained unchanged throughout the seasons in seed heads.
The EE content varied according to each morphological fractions, and the leaves showed similar EE content (60–88 g/kg DM) with aerial parts described by Guerreiro et al. [9] (58–91 g/kg DM), but quite lower than the that described by Dentinho et al. [10] (92–95 g/kg DM).
Higher IVOMD was found in flowers (34.7%), flower buds (34.0%), and leaves (31.0%). Whereas, the stems showed the lowest digestibility (9.91%), as expected owing to high fiber and lignin contents [9,10,11]. Lignin accumulation of the plant cell-wall, together with the presence of other phenolic compounds such as CT, is the most principal factor which can limit digestibility [41].
The CT content vary widely across plant species, as well as in the different morphological fractions of plants and with the vegetative stage [43,44]. In the present study, leaves and stems showed the highest CT contents (179 and 161 g/kg DM for leaves and stems, respectively), and residual contents were found in the flowers (2.04 g/kg DM). In leaves, the CT content changed throughout the seasons in accordance with that previously observed in aerial parts [9], with higher content during summer and autumn than in winter and spring. It is well established that biosynthesis of secondary metabolites, as TP and CT, is related to environmental conditions and plant vegetative cycle, being stimulated by UV-light, hydric stress, and temperature [45], which explain the higher CT content in leaves during dry and hot seasons.
Tannins are generally considered as antinutritive and/or toxic compounds. However, evidence supports that dietary CT might have adverse, beneficial, or innocuous effects on ruminants depending on several factors, such as CT chemical structure and concentration in diets, basal diet composition, and factors related to the animal such as species and physiological stage [43,46,47,48]. Condensed tannins have been used in diets of ruminants to prevent bloating, control internal parasites, reduce methane production, improve digestive utilization of feed proteins and fatty acid composition of products [43,47,49]. In monogastric, improvement of the gastrointestinal health and animal performance due to CT is also reported [50,51]. Results from our research team suggest that levels of C. ladanifer CT up to 20 g/kg DM are safe without detrimental effects on lamb productivity [15,17,18]. Additionally, CT extract from C. ladanifer used as additive improved the soybean meal protein efficiency in lamb diets, since it increased the dietary protein rumen outflow that was available for absorption in the small intestine [22]. Furthermore, both C. ladanifer aerial part and its CT extract can also be used to induce beneficial changes in the ruminal fatty acids and thus in the fatty acid composition of edible products from ruminants [15,16,17,18,52].
The α-tocopherol was the predominant form, being identified in all morphological fractions of C. ladanifer, and remained constant throughout the year. As far as we know, only one study described the tocopherol content of C. ladanifer leaves, which reported an α- and γ-tocopherol content of 68 and 7.7 µg/g DM, respectively, lower than those presented in this study [53]. Contrary to our work, Guimarães et al. [53] found lower levels of β-tocopherol (1.02 µg/g DM) and did not identify the δ-tocopherol. In fact, in the present work, β-tocopherol was not identified in any morphological fraction and δ-tocopherol content of leaves ranged from 14.9 to 20.6 µg/g DM, presenting the same seasonal variation observed for γ-tocopherol, increasing during spring (p = 0.009; data not shown).
Vitamin E is considered a strong fat-soluble antioxidant, essential for the protection of polyunsaturated fatty acids against oxidative damage in both plants and animals [54]. Recent work of our research team demonstrated that C. ladanifer aerial part had a protective effect against lipid oxidation through increasing α-tocopherol concentration in the lamb muscle [21]. The increased level of α-tocopherol in the meat from lambs fed a diet containing 200 g/kg DM of soft stems and leaves of C. ladanifer may have derived directly from C. ladanifer [21]. Furthermore, is also known that polyphenolic compounds are able to interfere with vitamin E metabolism [55], so this increase in the lamb meat α-tocopherol levels can also be due to vitamin E recycling or sparing by C. ladanifer polyphenolic compounds. In fact, the high levels of phenolic compounds, both TP and CT, and α-tocopherol in leaves, can corroborate the conclusions suggested by Jerónimo et al. [21].
Thus, despite the low CP content and in vitro digestibility and high lignin content, aerial part of C. ladanifer has previously demonstrated beneficial effects when incorporated into ruminant diets, mainly due to its CT content. Furthermore, during periods of pasture and forage scarcity, the foliage of diverse Mediterranean trees and shrubs is an important source of nutrients to grazing ruminants, complementing the available feed resources. In general, the CP content of C. ladanifer leaves was higher or similar to the CP observed for the foliage of several Mediterranean shrubs and trees species also consumed by grazing ruminants, such as Quercus suber and Q. rotundifolia, Arbutus unedo and Pistacia lentiscus [10,56]. The aerial parts of C. ladanifer can be an important feed resource for grazing ruminants in the Mediterranean area and potentially can be used as a component of the diets of ruminants in the context of nutritional strategies to improve animal health and to improve the quality of ruminant products. However, their use requires care in order to avoid the potential negative effects on animal performance and the occurrence of metabolic disorders, and further studies are needed to determine which conditions are most favorable for the use of C. ladanifer as feed (e.g., the recommended dose).
5. Conclusions
Cistus ladanifer has low nutritive value but an important feeding resource as a complement to pasture and commercial concentrates. The leaves were the morphological fraction more variable throughout the seasons, with the highest CP content in winter while the CT was higher during summer and autumn. Moreover, due to its higher content of CP and in vitro digestibility leaves are the fraction with higher nutritive value, especially when collected during winter and spring. All the morphological fractions of C. ladanifer showed high contents of bioactive compounds namely phenolic compounds and tocopherol, giving this shrub species high antioxidant activity.
Author Contributions
Conceptualization, E.J.; methodology, D.S. and O.G.; writing—original draft preparation, O.G.; writing—review and editing, E.J. and M.T.P.D.; funding acquisition, E.J. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided through project “CistusRumen—Sustainable use of Rockrose (Cistus ladanifer L.) in small ruminants—Increase of the competitiveness and reduction of the environmental impact” (ALT20-03-0145-FEDER-000023) from the Alentejo2020 program, through of the European Regional Development Fund (ERDF); “CEBAL Technology Transfer Potentiation Program—Interface Highly Qualified Human Resources” (ALT20-05-3559-FSE-000076) co-funded by European Social Fund; and by National Funds through FCT—Foundation for Science and Technology through the projects UIDB/05183/2020 (MED) and UIDP/CVT/00276/2020 (CIISA). The authors would also like to thank the FCT for the research grant SFRH/BD/145814/2019 to D.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank to José Batista for cooperation in analytical determinations and Eng José Santos-Silva for cooperation in the review of the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bruno-Soares, A.M.; Matos, T.J.S.; Cadima, J. Nutritive Value of Cistus salvifolius Shrubs for Small Ruminants. Anim. Feed. Sci. Technol. 2011, 165, 167–175. [Google Scholar] [CrossRef]
- Papachristou, T.G.; Papanastasis, V.P. Forage Value of Mediterranean Deciduous Woody Fodder Species and Its Implication to Management of Silvo-Pastoral Systems for Goats. Agrofor. Syst. 1994, 27, 269–282. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The Effects of Dietary Consumption of Plants Secondary Compounds on Small Ruminants’ Products Quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Vandermeulen, S.; Ramírez-Restrepo, C.A.; Beckers, Y.; Claessens, H.; Bindelle, J. Agroforestry for Ruminants: A Review of Trees and Shrubs as Fodder in Silvopastoral Temperate and Tropical Production Systems. Anim. Prod. Sci. 2018, 58, 767–777. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The Effect of Condensed Tannins on the Nutrition and Health of Ruminants Fed Fresh Temperate Forages: A Review. Anim. Feed. Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Francis, G.; Becker, K. Bioactivity of Phytochemicals in Some Lesser-Known Plants and Their Effects and Potential Applications in Livestock and Aquaculture Production Systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef]
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus Ladanifer (Cistaceae): A Natural Resource in Mediterranean-Type Ecosystems. Planta 2017, 247, 1–12. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Moreira, O.C.; Pereira, M.S.; Bessa, R.J.B. The Use of a Tannin Crude Extract from Cistus ladanifer L. to Protect Soya-Bean Protein from Degradation in the Rumen. Animal 2007, 1, 645–650. [Google Scholar] [CrossRef]
- Guerreiro, O.; Dentinho, M.T.P.; Moreira, O.C.; Guerra, A.R.; Ramos, P.A.B.; Bessa, R.J.B.; Duarte, M.F.; Jerónimo, E. Potential of Cistus ladanifer L. (Rockrose) in Small Ruminant Diets—Effect of Season and Plant Age on Chemical Composition, in Vitro Digestibility and Antioxidant Activity. Grass Forage Sci. 2016, 71, 437–447. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Navas, D.; Potes, J. Chemical and Nutritional Evaluation of Food Complements for Large Cattle Breeding, in Montado de Azinho Area. Pastagens E Forrag. 2005, 26/27, 41–46. [Google Scholar]
- Castro, M.; Teixeira, A.; Fernández-Núñez, E. The Nutritive Value of Different Mediterranean Browse Species Used as Animal Feeds under Oak Silvopastoral Systems in Northern Portugal. Agrofor. Syst. 2021, 95, 269–278. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Saura, D.; Guillen, E.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae Aqueous Extracts Containing Ellagitannins Show Antioxidant and Antimicrobial Capacity, and Cytotoxic Activity against Human Cancer Cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Duenas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal Activity and Detailed Chemical Characterization of Cistus ladanifer Phenolic Extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alves, S.P.; Dentinho, M.T.; Martins, S.V.; Prates, J.A.; Vasta, V.; Santos-Silva, J.; Bessa, R.J. Effect of Grape Seed Extract, Cistus ladanifer L., and Vegetable Oil Supplementation on Fatty Acid Composition of Abomasal Digesta and Intramuscular Fat of Lambs. J. Agric. Food Chem. 2010, 58, 10710–10721. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Soldado, D.; Cachucho, L.; Almeida, J.M.; Francisco, A.; Santos-Silva, J.; Bessa, R.J.B.; Jeronimo, E. Inclusion of the Aerial Part and Condensed Tannin Extract from Cistus ladanifer L. in Lamb Diets–Effects on Growth Performance, Carcass and Meat Quality and Fatty Acid Composition of Intramuscular and Subcutaneous Fat. Meat Sci. 2020, 160, 107945. [Google Scholar] [CrossRef]
- Francisco, A.; Dentinho, M.T.; Alves, S.P.; Portugal, P.V.; Fernandes, F.; Sengo, S.; Jerónimo, E.; Oliveira, M.A.; Costa, P.; Sequeira, A.; et al. Growth Performance, Carcass and Meat Quality of Lambs Supplemented with Increasing Levels of a Tanniferous Bush (Cistus ladanifer L.) and Vegetable Oils. Meat Sci. 2015, 100, 275–282. [Google Scholar] [CrossRef]
- Francisco, A.; Alves, S.P.; Portugal, P.V.; Dentinho, M.T.; Jerónimo, E.; Sengo, S.; Almeida, J.; Bressan, M.C.; Pires, V.M.R.; Alfaia, C.M.; et al. Effects of Dietary Inclusion of Citrus Pulp and Rockrose Soft Stems and Leaves on Lamb Meat Quality and Fatty Acid Composition. Animal 2018, 12, 872–881. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, Ruminal Fermentation and Microbial Nitrogen Supply in Sheep Fed Soybean Meal Treated with Cistus ladanifer L. Tannins. Small Rumin. Res. 2014, 119, 57–64. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alfaia, C.M.M.; Alves, S.P.; Dentinho, M.T.P.; Prates, J.A.M.; Vasta, V.; Santos-Silva, J.; Bessa, R.J.B. Effect of Dietary Grape Seed Extract and Cistus ladanifer L. in Combination with Vegetable Oil Supplementation on Lamb Meat Quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef]
- Jerónimo, E.; Soldado, D.; Sengo, S.; Francisco, A.; Fernandes, F.; Portugal, A.P.V.; Alves, S.P.; Santos-Silva, J.; Bessa, R.J.B. Increasing the α-Tocopherol Content and Lipid Oxidative Stability of Meat through Dietary Cistus ladanifer L. in Lamb Fed Increasing Levels of Polyunsaturated Fatty Acid Rich Vegetable Oils. Meat Sci. 2020, 164, 108092. [Google Scholar] [CrossRef] [PubMed]
- Dentinho, M.T.P.; Paulos, K.; Francisco, A.; Belo, A.T.; Jerónimo, E.; Almeida, J.; Bessa, R.J.B.; Santos-Silva, J. Effect of Soybean Meal Treatment with Cistus ladanifer Condensed Tannins in Growth Performance, Carcass and Meat Quality of Lambs. Livest. Sci. 2020, 236, 104021. [Google Scholar] [CrossRef]
- Jerónimo, E.; Cachucho, L.; Soldado, D.; Guerreiro, O.; Bessa, R.J.B.; Alves, S.P. Fatty Acid Content and Composition of the Morphological Fractions of Cistus ladanifer L. And Its Seasonal Variation. Molecules 2020, 25, 1550. [Google Scholar] [CrossRef] [PubMed]
- Marta-Pedroso, C.; Freitas, H.; Domingos, T. A Estepe Cerealifera de Castro Verde. In Ecossistemas e Bem-estar Humano: Avaliação para Portugal do Millennium Ecosystem Assessment; Escolar Editora, Ed.; Escolar Editora: Lisbon, Portugal, 2009; pp. 559–583. [Google Scholar]
- International Organization for Standardization ISO 6496; Animal Feeding Stuffs–Determination of Moisture and the Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Goering, H.K.; van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications). In Agricultural Research Service Handbook no 379; ARS-USDA: Washington, DC USA, 1970. [Google Scholar]
- International Organization for Standardization ISO 9831; Animal Feeding Stuffs, Animal Products and Faeces or Urine–Determination of Gross Calorimetric Value–Bomb Calorimeter Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- International Organization for Standardization ISO 6492; Animal Feeding Stuffs–Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- Alexander, R.; Mcgowan, M. A Filtration Procedure for the in Vitro Determination of Digestibility of Herbage. J. Br. Grassl. Soc. 1966, 16, 140–147. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Boston, MA, USA; London, UK, 2003; ISBN 1402016328. [Google Scholar]
- Fialho, L.; Ramôa, S.; Parenzan, S.; Guerreiro, I.; Catronga, H.; Soldado, D.; Guerreiro, O.; García, V.G.; e Silva, P.O.; Jerónimo, E. Effect of Regulated Deficit Irrigation on Pomegranate Fruit Quality at Harvest and during Cold Storage. Agric. Water Manag. 2021, 251, 106869. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochem. 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Strumeyer, D.H.; Malin, M.J. Condensed Tannins in Grain-–Isolation, Fractionation, and Characterization. J. Agric. Food Chem. 1975, 23, 909–914. [Google Scholar] [CrossRef]
- Sickel, H.; Bilger, W.; Ohlson, M. High Levels of α-Tocopherol in Norwegian Alpine Grazing Plants. J. Agric. Food Chem. 2012, 60, 7573–7580. [Google Scholar] [CrossRef]
- Talavera, S.; Gibbs, P.E.; Herrera, J. Reproductive Biology of Cistus ladanifer (Cistaceae). Plant. Syst. Evol. 1993, 186, 123–134. [Google Scholar] [CrossRef]
- Bastida, F.; Talavera, S. Temporal and Spatial Patterns of Seed Dispersal in Two Cistus Species (Cistaceae). Ann. Bot. 2002, 89, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Demoly, J.P.; Montserrat, P. Cistus. Flora Iber. 1993, 3, 319–337. [Google Scholar]
- Cabezudo, B.; Navarro, T.; Pérez Latorre, A.; Nieto Caldera, J.M.; Orshan, G. Estudios Fenomorfológicos En La Vegetación Del Sur de España. I. Cistus L. (Phenomorphologic Studies in Vegetation of South of Spain. I. Cistus L.). Acta Bot. Malacit. 1992, 17, 229–237. [Google Scholar] [CrossRef]
- Ammar, H.; Lopez, S.; Gonzalez, J.S.; Ranilla, M.J. Chemical Composition and in Vitro Digestibility of Some Spanish Browse Plant Species. J. Sci. Food Agric. 2004, 84, 197–204. [Google Scholar] [CrossRef]
- Sfougaris, A.I.; Nastis, A.S.; Papageorgiou, N.K. Food Resources and Quality for the Introduced Cretan Wild Coat or Agrimi Capra Aegagrus Cretica on Atalandi Island, Greece, and Implications for Ecosystem Management. Biol. Conserv. 1996, 78, 239–245. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in Forage Plants and Their Role in Animal Husbandry and Environmental Sustainability: A Review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Jonker, A.; Yu, P. The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract. Int. J. Mol. Sci. 2017, 18, 1105. [Google Scholar] [CrossRef]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant. Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Waghorn, G. Beneficial and Detrimental Effects of Dietary Condensed Tannins for Sustainable Sheep and Goat Production-Progress and Challenges. Anim. Feed. Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Natalello, A.; Luciano, G.; Fondevila, M.; Priolo, A.; Toral, P.G. Ability of Tannins to Modulate Ruminal Lipid Metabolism and Milk and Meat Fatty Acid Profiles. Anim. Feed. Sci. Technol. 2020, 269, 114623. [Google Scholar] [CrossRef]
- Jerónimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Sales-Baptista, E.; Lopes, O.; Silva, F. Tannins in Ruminant Nutrition: Impact on Animal Performance and Quality of Edible Products. In Tannins: Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2016; pp. 121–168. [Google Scholar]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and Quebracho Tannins in Pig Nutrition: The Effects on Performance and Intestinal Health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and Challenges of Tannins as an Alternative to In-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.; Alves, S.P.; Portugal, P.V.; Pires, V.M.R.; Dentinho, M.T.; Alfaia, C.M.; Jeronimo, E.; Prates, J.A.M.; Santos-Silva, J.; Bessa, R.J.B. Effect of Feeding Lambs with a Tanniferous Shrub (Rockrose) and a Vegetable Oil Blend on Fatty Acid Composition of Meat Lipids. Animal 2016, 10, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C.F.R. Aromatic Plants as a Source of Important Phytochemicals: Vitamins, Sugars and Fatty Acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus Gunnii Leaves. Ind. Crops Prod. 2009, 30, 427–430. [Google Scholar] [CrossRef]
- Goffman, F.D.; Böhme, T. Relationship between Fatty Acid Profile and Vitamin e Content in Maize Hybrids (Zea Mays l.). J. Agric. Food Chem. 2001, 49, 4990–4994. [Google Scholar] [CrossRef]
- Frank, J.; Eliasson, C.; Leroy-Nivard, D.; Budek, A.; Lundh, T.; Vessby, B.; Åman, P.; Kamal-Eldin, A. Dietary Secoisolariciresinol Diglucoside and Its Oligomers with 3-Hydroxy-3-Methyl Glutaric Acid Decrease Vitamin E Levels in Rats. Br. J. Nutr. 2004, 92, 169–176. [Google Scholar] [CrossRef]
- Ammar, H.; Lopez, S.; Gonzalez, J.S. Assessment of the Digestibility of Some Mediterranean Shrubs by in Vitro Techniques. Anim. Feed. Sci. Technol. 2005, 119, 323–331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).