Ruminal Bacterial Communities and Metabolome Variation in Beef Heifers Divergent in Feed Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collections
2.2. DNA Extraction, Sequencing, and Analysis
2.3. Microbial Sequence Processing
2.4. Metabolomics Processing and Analysis
2.5. Statistical Analyses
2.5.1. Bacteria and Archaea
2.5.2. Metabolomics
3. Results
3.1. Microbiome
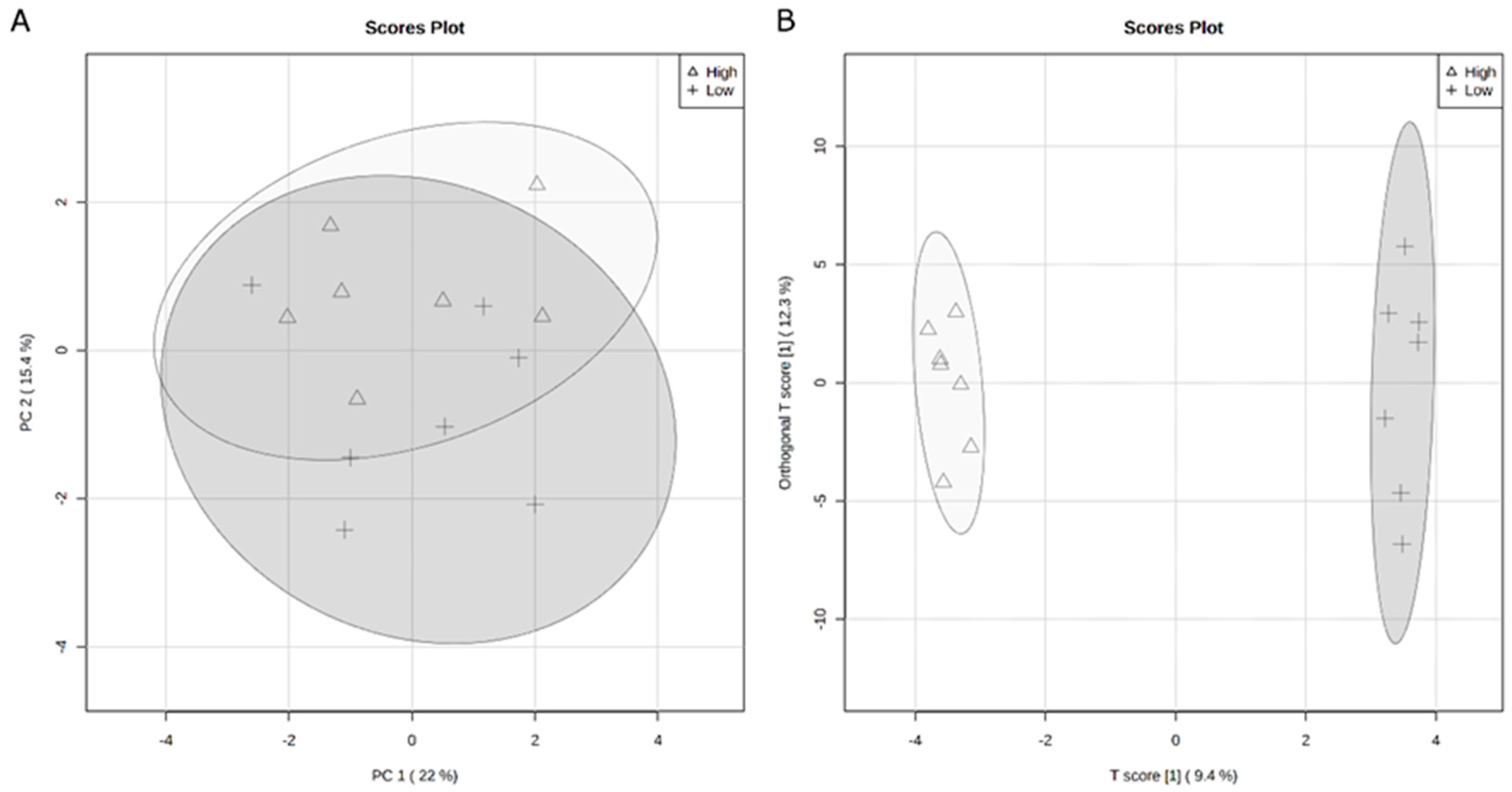
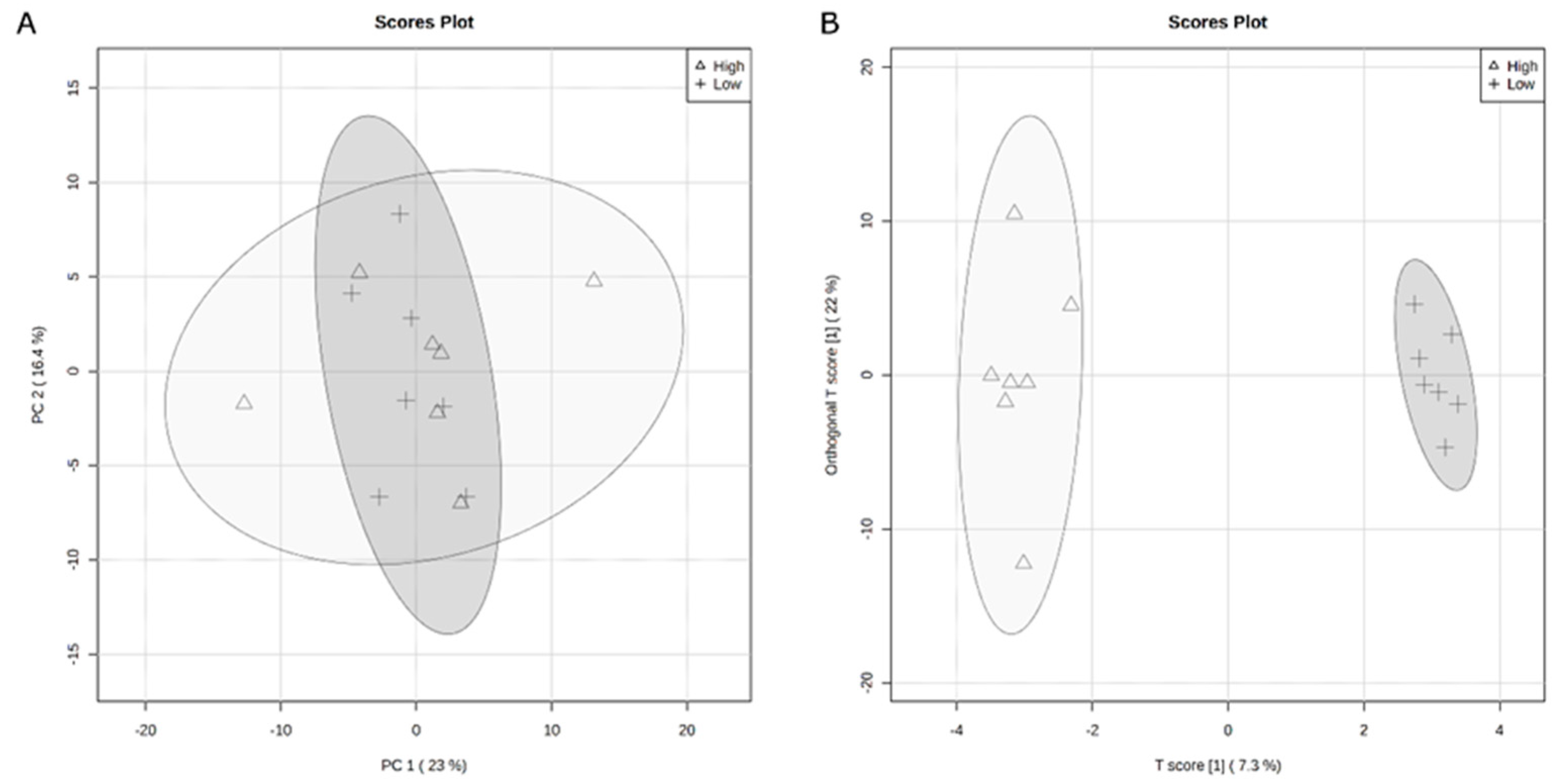
3.2. Rumen Metabolome
3.3. Serum Metabolome
3.4. Rumen Microbiome and Metabolome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, C. Cattle & Beef Statistics and Information. Available online: https://www.ers.usda.gov/topics/animal-products/cattle-beef/sector-at-a-glance (accessed on 23 March 2022).
- Herd, R.M.; Archer, J.A.; Arthur, P.F. Reducing the Cost of Beef Production through Genetic Improvement in Residual Feed Intake: Opportunity and Challenges to Application. J. Anim. Sci. 2003, 81, E9–E17. [Google Scholar]
- Montaño-Bermudez, M.; Nielsen, M.K.; Deutscher, G.H. Energy Requirements for Maintenance of Crossbred Beef Cattle with Different Genetic Potential for Milk. J. Anim. Sci. 1990, 68, 2279–2288. [Google Scholar] [CrossRef] [Green Version]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Fontanesi, L. Metabolomics and Livestock Genomics: Insights into a Phenotyping Frontier and Its Applications in Animal Breeding. Anim. Front. 2016, 6, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Young, J.W. Gluconeogenesis in Cattle: Significance and Methodology. J. Dairy Sci. 1977, 60, 1–15. [Google Scholar] [CrossRef]
- Hungate, R.E. The Rumen and Its Microbes; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1483263622. [Google Scholar]
- Myer, P.R.; Smith, T.P.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen Microbiome from Steers Differing in Feed Efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef] [Green Version]
- McCann, J.C.; Wiley, L.M.; Forbes, T.D.; Rouquette, F.M., Jr.; Tedeschi, L.O. Relationship between the Rumen Microbiome and Residual Feed Intake-Efficiency of Brahman Bulls Stocked on Bermudagrass Pastures. PLoS ONE 2014, 9, e91864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGovern, E.; Kenny, D.A.; McCabe, M.S.; Fitzsimons, C.; McGee, M.; Kelly, A.K.; Waters, S.M. 16S RRNA Sequencing Reveals Relationship between Potent Cellulolytic Genera and Feed Efficiency in the Rumen of Bulls. Front. Microbiol. 2018, 9, 1842. [Google Scholar] [CrossRef] [PubMed]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Miller, M.E.B.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific Microbiome-Dependent Mechanisms Underlie the Energy Harvest Efficiency of Ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, K.A.; McCormick, C.A.; Odt, C.L.; Weimer, P.J.; Suen, G. Ruminal Bacterial Community Composition in Dairy Cows Is Dynamic over the Course of Two Lactations and Correlates with Feed Efficiency. Appl. Environ. Microbiol. 2015, 81, 4697–4710. [Google Scholar] [CrossRef] [Green Version]
- Pitta, D.W.; Indugu, N.; Kumar, S.; Vecchiarelli, B.; Sinha, R.; Baker, L.D.; Bhukya, B.; Ferguson, J.D. Metagenomic Assessment of the Functional Potential of the Rumen Microbiome in Holstein Dairy Cows. Anaerobe 2016, 38, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.J.; Conant, G.C.; Lamberson, W.R.; Cockrum, R.R.; Austin, K.J.; Rule, D.C.; Cammack, K.M. Diet and Feed Efficiency Status Affect Rumen Microbial Profiles of Sheep. Small Rumin. Res. 2017, 156, 12–19. [Google Scholar] [CrossRef]
- Koch, R.M.; Swiger, L.A.; Chambers, D.; Gregory, K.E. Efficiency of Feed Use in Beef Cattle. J. Anim. Sci. 1963, 22, 486–494. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved Extraction of PCR-Quality Community DNA from Digesta and Fecal Samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit RRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor Revision to V4 Region SSU RRNA 806R Gene Primer Greatly Increases Detection of SAR11 Bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Afgan, E.; Baker, D.; Batut, B.; Van Den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Krueger, F. Trim Galore. A Wrapper Tool Cutadapt FastQC Consistently Apply Qual. Adapt. Trimming FastQ Files 2015, 516, 517. [Google Scholar]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Clemmons, B.A.; Mihelic, R.I.; Beckford, R.C.; Powers, J.B.; Melchior, E.A.; McFarlane, Z.D.; Cope, E.R.; Embree, M.M.; Mulliniks, J.T.; Campagna, S.R. Serum Metabolites Associated with Feed Efficiency in Black Angus Steers. Metabolomics 2017, 13, 147. [Google Scholar] [CrossRef]
- Kamphorst, J.J.; Fan, J.; Lu, W.; White, E.; Rabinowitz, J.D. Liquid Chromatography–High Resolution Mass Spectrometry Analysis of Fatty Acid Metabolism. Anal. Chem. 2011, 83, 9114–9122. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic Analysis via Reversed-Phase Ion-Pairing Liquid Chromatography Coupled to a Stand Alone Orbitrap Mass Spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS Data Processing with MAVEN: A Metabolomic Analysis and Visualization Engine. Curr. Protoc. Bioinforma. 2012, 37, 1–14. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An Effective Distance Metric for Microbial Community Comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.B.; Gotoh, T.; Greenwood, P.L. Current Situation and Future Prospects for Global Beef Production: Overview of Special Issue. Asian-Australas. J. Anim. Sci. 2018, 31, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA ERS. Cattle & Beef Statistics & Information; US Department of Agriculture: Washington, DC, USA, 2015.
- Ruzzo, E.K.; Capo-Chichi, J.-M.; Ben-Zeev, B.; Chitayat, D.; Mao, H.; Pappas, A.L.; Hitomi, Y.; Lu, Y.-F.; Yao, X.; Hamdan, F.F. Deficiency of Asparagine Synthetase Causes Congenital Microcephaly and a Progressive Form of Encephalopathy. Neuron 2013, 80, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, N.N.; Hui, S.; Ghergurovich, J.M.; Fan, J.; Intlekofer, A.M.; White, R.M.; Rabinowitz, J.D.; Thompson, C.B.; Zhang, J. As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metab. 2018, 27, 428–438.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, A.C.I. Production of Volatile Fatty Acids in the Rumen: Methods of Measurement. Nutr. Abstr. Rev. 1964, 34, 339–352. [Google Scholar] [PubMed]
- Pathak, A.K. Various Factors Affecting Microbial Protein Synthesis in the Rumen. Vet. World 2008, 1, 186. [Google Scholar]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, K.; Daitoku, H.; Shimamoto, Y.; Matsuzaki, H.; Hirota, K.; Ishida, J.; Fukamizu, A. Bile Acids Regulate Gluconeogenic Gene Expression via Small Heterodimer Partner-Mediated Repression of Hepatocyte Nuclear Factor 4 and Foxo1. J. Biol. Chem. 2004, 279, 23158–23165. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Penner, G.B.; Li, M.; Oba, M.; Guan, L.L. Changes in Bacterial Diversity Associated with Epithelial Tissue in the Beef Cow Rumen during the Transition to a High-Grain Diet. Appl. Environ. Microbiol. 2011, 77, 5770–5781. [Google Scholar] [CrossRef] [Green Version]
- Tizioto, P.C.; Coutinho, L.L.; Decker, J.E.; Schnabel, R.D.; Rosa, K.O.; Oliveira, P.S.; Souza, M.M.; Mourão, G.B.; Tullio, R.R.; Chaves, A.S. Global Liver Gene Expression Differences in Nelore Steers with Divergent Residual Feed Intake Phenotypes. BMC Genom. 2015, 16, 242. [Google Scholar] [CrossRef] [Green Version]
- Weingarden, A.R.; Chen, C.; Bobr, A.; Yao, D.; Lu, Y.; Nelson, V.M.; Sadowsky, M.J.; Khoruts, A. Microbiota Transplantation Restores Normal Fecal Bile Acid Composition in Recurrent Clostridium Difficile Infection. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G310–G319. [Google Scholar] [CrossRef] [Green Version]
- Devlin, A.S.; Fischbach, M.A. A Biosynthetic Pathway for a Prominent Class of Microbiota-Derived Bile Acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J.T.; Brosnan, M.E. Branched-Chain Amino Acids: Enzyme and Substrate Regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [CrossRef]
- Li, J.Y.; Suzuki, K.; Koike, Y.; Chen, D.S.; Yonezawa, T.; Nishihara, M.; Manabe, N. Effects of Dietary Supplementation with Branched-Chain Amino Acids (BCAAs) during Nursing on Plasma BCAA Levels and Subsequent Growth in Cattle. Asian-Australas. J. Anim. Sci. 2005, 18, 1440–1444. [Google Scholar] [CrossRef]
- Early, R.J.; Thompson, J.R.; Christopherson, R.J.; Sedgwick, G.W. Branched-Chain Alpha-Keto Acid Exchange across the Portal-Drained Viscera and Hindlimb of Fed and Fasted Steers. Can. J. Anim. Sci. 1984, 64, 276–277. [Google Scholar] [CrossRef]
- Chalupa, W. Degradation of Amino Acids by the Mixed Rumen Microbial Population. J. Anim. Sci. 1976, 43, 828–834. [Google Scholar] [CrossRef]
- Wu, G. Dietary Requirements of Synthesizable Amino Acids by Animals: A Paradigm Shift in Protein Nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Long, Y.C. Crosstalk between Cystine and Glutathione Is Critical for the Regulation of Amino Acid Signaling Pathways and Ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef]
- Kizil, O.; Akar, Y.; Saat, N.; Kizil, M.; Yuksel, M. The Plasma Lipid Peroxidation Intensity (MDA) and Chain-Breaking Antioxidant Concentrations in the Cows with Clinic or Subclinic Mastitis. Rev. Med. Vet. 2007, 158, 529–533. [Google Scholar]
- Nkrumah, J.D.; Okine, E.K.; Mathison, G.W.; Schmid, K.; Li, C.; Basarab, J.A.; Price, M.A.; Wang, Z.; Moore, S.S. Relationships of Feedlot Feed Efficiency, Performance, and Feeding Behavior with Metabolic Rate, Methane Production, and Energy Partitioning in Beef Cattle1. J. Anim. Sci. 2006, 84, 145–153. [Google Scholar] [CrossRef]
- Alexandre, P.A.; Kogelman, L.J.A.; Santana, M.H.A.; Passarelli, D.; Pulz, L.H.; Fantinato-Neto, P.; Silva, P.L.; Leme, P.R.; Strefezzi, R.F.; Coutinho, L.L.; et al. Liver Transcriptomic Networks Reveal Main Biological Processes Associated with Feed Efficiency in Beef Cattle. BMC Genom. 2015, 16, 1073. [Google Scholar] [CrossRef] [Green Version]
- Richardson, E.C.; Herd, R.M. Biological Basis for Variation in Residual Feed Intake in Beef Cattle. 2. Synthesis of Results Following Divergent Selection. Aust. J. Exp. Agric. 2004, 44, 431–440. [Google Scholar] [CrossRef]
- Connor, E.E.; Kahl, S.; Elsasser, T.H.; Parker, J.S.; Li, R.W.; Van Tassell, C.P.; Baldwin, R.L.; Barao, S.M. Enhanced Mitochondrial Complex Gene Function and Reduced Liver Size May Mediate Improved Feed Efficiency of Beef Cattle during Compensatory Growth. Funct. Integr. Genom. 2010, 10, 39–51. [Google Scholar] [CrossRef]
- Abo-Ismail, M.K.; Kelly, M.J.; Squires, E.J.; Swanson, K.C.; Bauck, S.; Miller, S.P. Identification of Single Nucleotide Polymorphisms in Genes Involved in Digestive and Metabolic Processes Associated with Feed Efficiency and Performance Traits in Beef Cattle1,2. J. Anim. Sci. 2013, 91, 2512–2529. [Google Scholar] [CrossRef]
- Clemmons, B.A.; Voy, B.H.; Myer, P.R. Altering the Gut Microbiome of Cattle: Considerations of Host-Microbiome Interactions for Persistent Microbiome Manipulation. Microb. Ecol. 2019, 77, 523–536. [Google Scholar] [CrossRef]
- Archer, J.A.; Richardson, E.C.; Herd, R.M.; Arthur, P.F. Potential for Selection to Improve Efficiency of Feed Use in Beef Cattle: A Review. Aust. J. Agric. Res. 1999, 50, 147–162. [Google Scholar] [CrossRef]
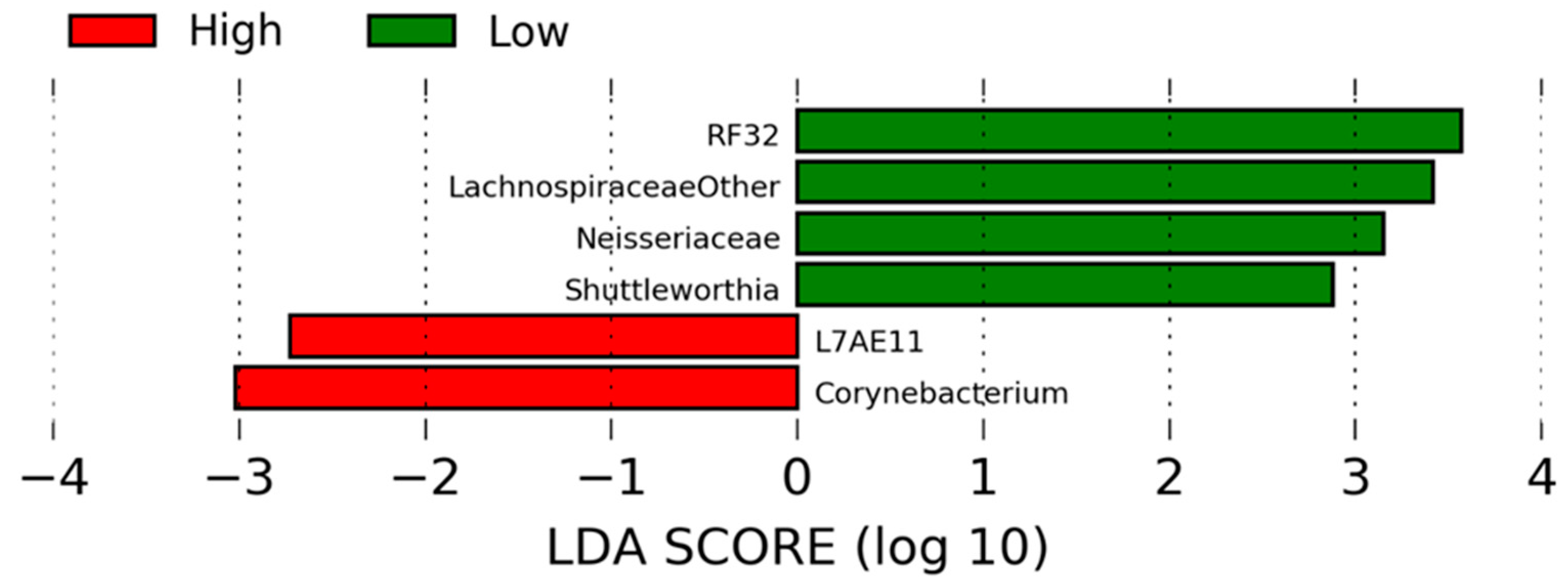
| Taxa Level | Taxon | High-RFI 1 | Low-RFI 1 | p-Value | FDR 2 |
|---|---|---|---|---|---|
| Family | Lachnospiraceae (Other) | 2.94 × 10−3 (7.07 × 10−4) | 7.82 × 10−3 (1.71 × 10−3) | 0.04 | 0.98 |
| Family | Desulfobulbaceae5 | 0.00 | 2.61 × 10−5 (1.11 × 10−5) | 0.05 | 0.98 |
| Family | Neisseriaceae3 | 3.37 × 10−5 (2.65 × 10−5) | 1.09 × 10−4 (3.20 × 10−5) | 0.02 | 0.98 |
| Genus | Shuttleworthia4 | 1.35 × 10−4 (2.48 × 10−5) | 5.89 × 10−4 (1.58 × 10−4) | 0.02 | 0.98 |
| Genus | Corynebacterium | 3.49 × 10−4 (4.89 × 10−5) | 1.53 × 10−4 (5.14 × 10−5) | 0.02 | 0.98 |
| Genus | p-75-a5 | 2.75 × 10−4 (7.19 × 10−5) | 1.11 × 10−4 (3.16 × 10−5) | 0.04 | 0.98 |
| Genus | L7A-E11 | 2.15 × 10−4 (8.59 × 10−5) | 1.49 × 10−5 (7.28 × 10−6) | <0.001 | 0.98 |
| Metric | High-RFI 1 | Low-RFI 1 | p-Value |
|---|---|---|---|
| Good’s Coverage | 0.95 (0.00) | 0.95 (0.00) | 0.74 |
| Observed OTU | 2366.83 (148.53) | 2195.63 (87.38) | 0.31 |
| Chao1 | 4658.78 (315.16) | 4536.07 (252.66) | 0.76 |
| Faith’s Phylogenetic Diversity | 121.40 (5.39) | 113.03 (3.28) | 0.19 |
| Shannon’s Diversity Index | 8.01 (0.31) | 7.80 (0.16) | 0.52 |
| Simpson’s Evenness E | 0.02 (0.01) | 0.02 (0.00) | 0.64 |
| Metric 1 | Test Statistic | p-Value |
|---|---|---|
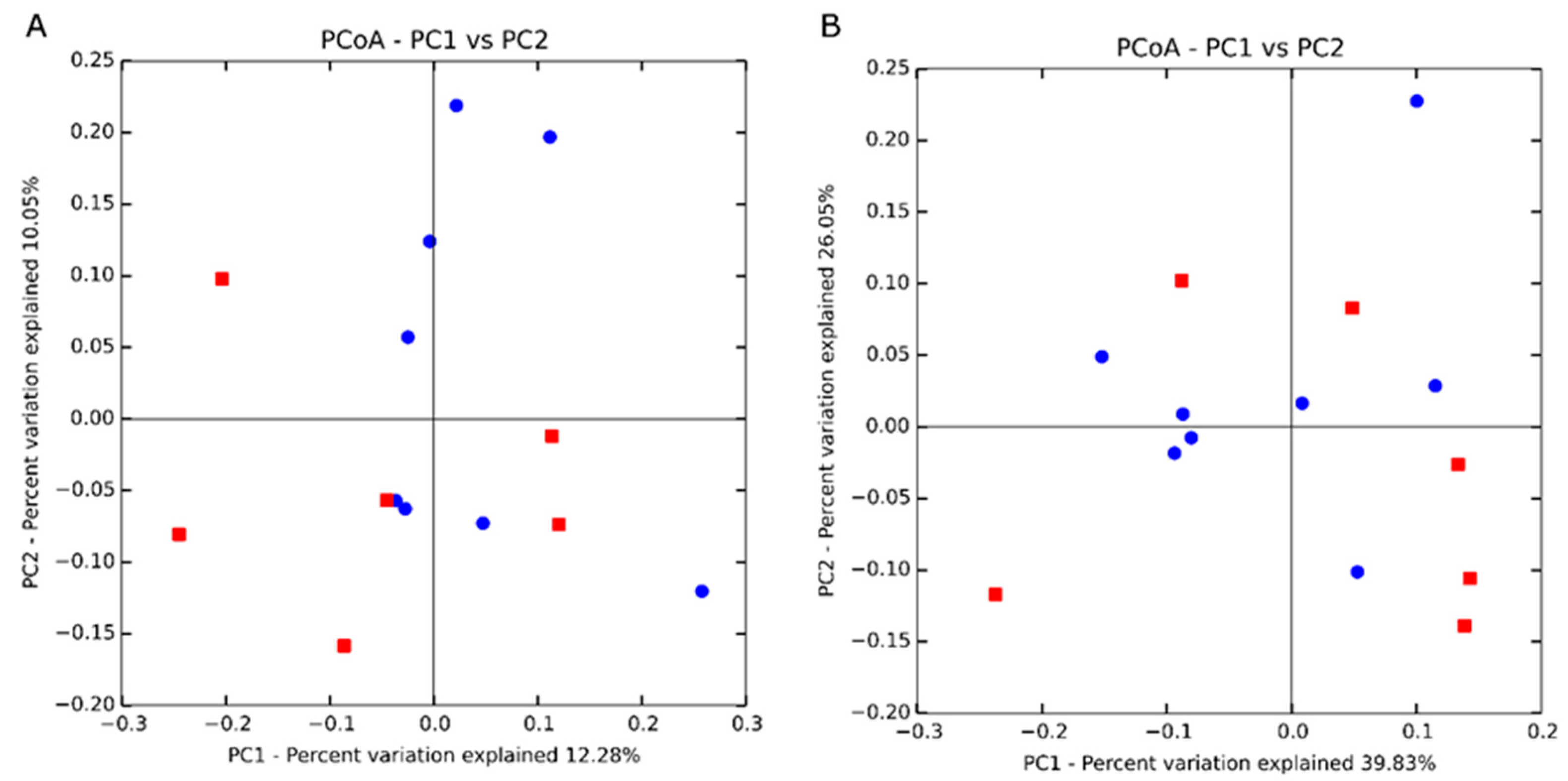
| PERMANOVA 2–weighted | 1.30 4 | 0.22 |
| PERMANOVA 2–unweighted | 1.17 4 | 0.03 |
| ANOSIM 3–weighted | 0.08 5 | 0.22 |
| ANOSIM 3–unweighted | 0.16 5 | 0.09 |
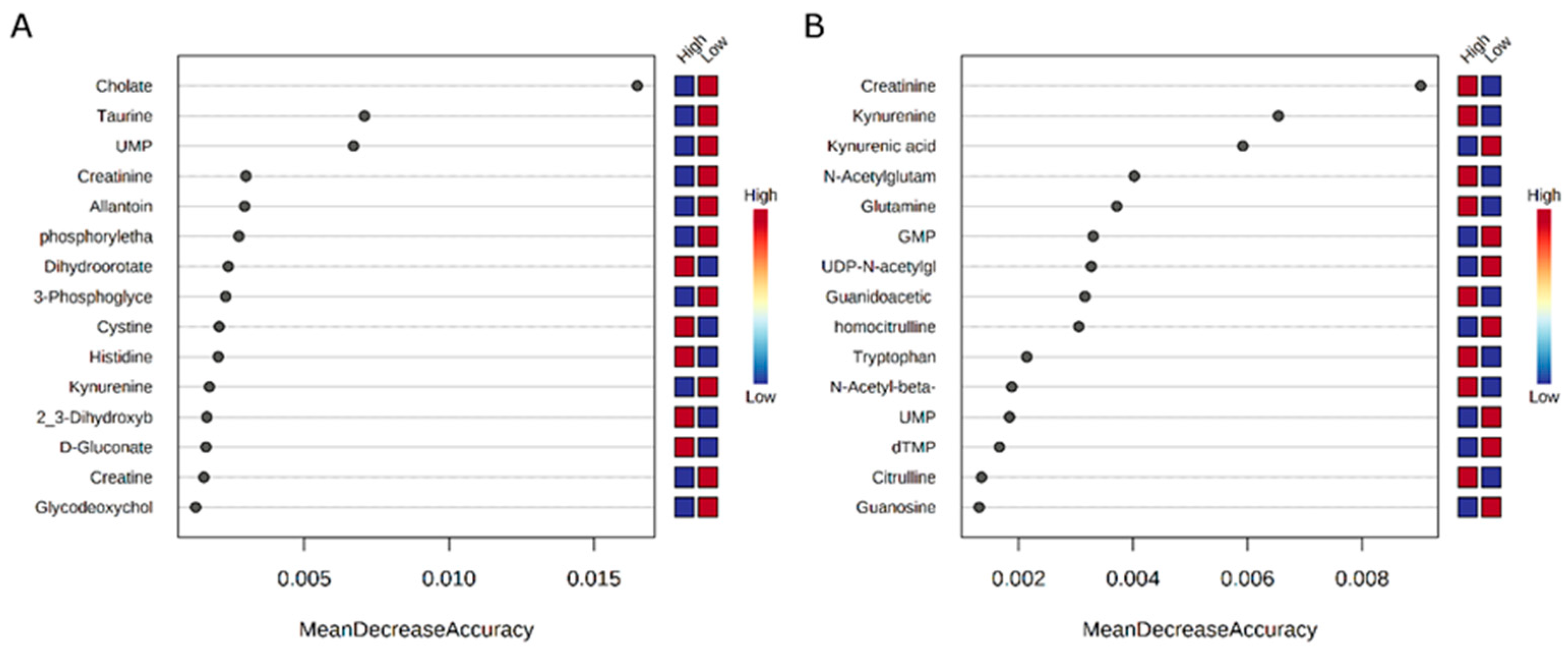
| Metabolite | High-RFI 1 | Low-RFI 1 | Fold Change |
|---|---|---|---|
| UDP-N-acetylglucosamine | 8.44 × 102 (8.44 × 102) | 3.23 × 105 (1.83 × 105) | 0.0032488 |
| NAD | 1.18 × 102 (1.18 × 102) | 1.84 × 104 (1.09 × 104) | 0.01375 |
| Taurine | 2.22 × 103 (1.54 × 103) | 1.35 × 105 (1.01 × 105) | 0.020692 |
| Cholate | 4.83 × 105 (1.36 × 105) | 7.08 × 106 (2.82 × 106) | 0.075272 |
| Creatine | 1.89 × 103 (6.28 × 102) | 3.77 × 104 (2.75 × 104) | 0.076414 |
| Taurodeoxycholate | n.d. | 5.48 × 105 (3.36 × 105) | 0.081299 |
| Glycodeoxycholate | n.d. | 4.29 × 105 (3.10 × 105) | 0.12439 |
| Cystathionine | 1.08 × 104 (5.76 × 103) | 2.03 × 103 (8.88 × 102) | 8.004 |
| Arginine | 9.39 × 103 (3.90 × 103) | 1.07 × 105 (6.57 × 104) | 0.13458 |
| IMP | 6.48 × 103 (2.24 × 103) | 4.42 × 104 (3.51 × 104) | 0.14821 |
| UMP | 4.93 × 104 (1.15 × 104) | 2.59 × 105 (1.08 × 105) | 0.19245 |
| UDP | n.d. | 7.78 × 102 (6.71 × 102) | 0.195 |
| phosphorylethanolamine | 3.88 × 102 (1.76 × 102) | 3.68 × 103 (1.96 × 103) | 0.20153 |
| cAMP | 4.04 × 105 (3.79 × 105) | 1.25 × 106 (9.49 × 105) | 0.24847 |
| Asparagine | 4.18 × 104 (7.51 × 103) | 1.53 × 105 (6.18 × 104) | 0.32909 |
| Octulose bisphosphate | 5.12 × 105 (1.67 × 105) | 1.89 × 105 (7.19 × 104) | 2.6493 |
| Creatinine | 6.67 × 104 (9.29 × 103) | 7.68 × 104 (3.23 × 104) | 0.39336 |
| Homocysteine | 1.59 × 102 (1.59 × 102) | 9.04 × 102 (7.49 × 102) | 0.42019 |
| Cysteine | 2.22 × 104 (7.15 × 103) | 1.01 × 104 (3.22 × 103) | 2.1765 |
| FAD | 2.81 × 103 (2.81 × 103) | 1.04 × 103 (6.76 × 102) | 2.1052 |
| 3-Phosphoglycerate | 9.84 × 105 (1.77 × 105) | 2.10 × 106 (3.99 × 105) | 0.49568 |
| Metabolite | High-RFI 1 | Low-RFI 1 | Fold Change |
|---|---|---|---|
| IMP | 2.75 × 103 (1.31 × 103) | 6.12 × 105 (4.99 × 105) | 0.0050715 |
| UDP-N-acetylglucosamine | 1.52 × 103 (1.03 × 103) | 1.09 × 105 (6.45 × 104) | 0.013976 |
| NAD | 2.24 × 102 (1.64 × 102) | 1.19 × 104 (7.07 × 103) | 0.023292 |
| UMP | 3.69 × 104 (7.16 × 103) | 3.87 × 105 (1.67 × 105) | 0.094823 |
| GMP | 2.17 × 104 (5.49 × 103) | 1.38 × 105 (6.40 × 104) | 0.1514 |
| dTMP | 2.46 × 104 (2.09 × 103) | 9.26 × 104 (3.17 × 104) | 0.29886 |
| N-Acetylglucosamine 1/6-phosphate | 2.06 × 104 (1.93 × 104) | 4.35 × 103 (3.02 × 103) | 2.8714 |
| IDP | 2.41 × 105 (1.26 × 105) | 5.65 × 105 (2.83 × 105) | 0.35639 |
| Guanosine | 4.20 × 104 (3.29 × 103) | 1.29 × 105 (4.69 × 104) | 0.36182 |
| Fructose 1,6-bisphosphate | 1.23 × 105 (4.62 × 104) | 3.55 × 105 (1.46 × 105) | 0.37308 |
| cAMP | 9.52 × 102 (3.60 × 102) | 2.66 × 103 (2.35 × 103) | 0.38656 |
| Kynurenic acid | 6.59 × 104 (1.34 × 104) | 1.32 × 105 (1.89 × 104) | 0.47088 |
| Glutathione | 6.30 × 103 (2.24 × 103) | 4.05 × 103 (1.88 × 103) | 2.1148 |
| Taxon | Metabolite | p 1 | p-Value 2 |
|---|---|---|---|
| p-75-a5 | 2-Oxoisovalerate | −0.54 | 0.04 |
| 3-Phosphoglycerate | −0.53 | 0.04 | |
| Creatinine | −0.73 | <0.01 | |
| Cytidine | −0.61 | 0.01 | |
| Glutamine | −074 | <0.01 | |
| IMP | −0.63 | 0.01 | |
| N-acetylornithine | −0.64 | 0.01 | |
| Pimelic acid | −0.56 | 0.03 | |
| Valine | −0.52 | 0.05 | |
| Lachnospiraceae (Other) | 2-Oxoisovalerate | 0.71 | <0.01 |
| 3-Phosphoglycerate | 0.55 | 0.03 | |
| Arginine | 0.55 | 0.03 | |
| Creatinine | 0.52 | 0.05 | |
| Cysteine | 0.59 | 0.02 | |
| Cytidine | 0.56 | 0.03 | |
| Glutamine | 0.59 | 0.02 | |
| Phosphorylethanolamine | 0.56 | 0.03 | |
| Taurine | 0.69 | <0.01 | |
| UMP | 0.52 | 0.05 | |
| Corynebacterium | 2-Oxoisovalerate | −0.66 | <0.01 |
| 3-Phosphoglycerate | −0.53 | 0.04 | |
| Citrate | −0.55 | 0.03 | |
| Phosphorylethanolamine | −0.60 | 0.02 | |
| Succinate/Methylmalonate | −0.54 | 0.04 | |
| Taurine | −0.74 | <0.01 | |
| Neisseriaceae | Creatine | 0.71 | <0.01 |
| Cysteine | −0.78 | <0.001 | |
| Dihydroorotate | −0.56 | 0.03 | |
| FMN | −0.53 | 0.04 | |
| Glycodeoxycholate | 0.53 | 0.04 | |
| Hydroxyproline | −0.53 | 0.04 | |
| Nicotinate | −0.58 | 0.02 | |
| Phosphorylethanolamine | 0.55 | 0.03 | |
| Taurodeoxycholate | 0.52 | 0.05 | |
| UMP | 0.59 | 0.02 | |
| Xylose | −0.56 | 0.03 | |
| Shuttleworthia | 2,3-Dihydroxybenzoate | −0.57 | 0.03 |
| 2-Oxo-4-methylthiobutanoate | 0.53 | 0.04 | |
| 2-Oxoisovalerate | 0.59 | 0.02 | |
| Asparagine | 0.58 | 0.02 | |
| Taurine | 0.66 | <0.01 | |
| UDP | 0.59 | 0.02 | |
| Desulfobulbaceae | 2-Dehydro-D-gluconate | −0.57 | 0.03 |
| Cysteine | −0.55 | 0.04 | |
| Deoxyuridine | −0.56 | 0.03 | |
| FMN | −0.64 | <0.01 | |
| Histidine | −0.55 | 0.04 | |
| Hydroxyproline | −0.60 | 0.02 | |
| Methionine | −0.62 | 0.01 | |
| Methionine sulfoxide | −0.53 | 0.04 | |
| N-carbamoyl-L-aspartate | −0.53 | 0.04 | |
| Nicotinate | −0.59 | 0.02 | |
| Tyrosine | −0.62 | 0.01 | |
| Uracil | −0.53 | 0.04 | |
| Xylose | −0.56 | 0.03 | |
| L7A-E11 | Succinate/Methylmalonate | −0.72 | <0.01 |
| Taurine | −0.59 | 0.02 | |
| UDP-N-acetylglucosamine | −0.76 | <0.01 | |
| UMP | −0.66 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemmons, B.A.; Mulon, P.-Y.; Anderson, D.E.; Ault-Seay, T.B.; Henniger, M.T.; Schneider, L.G.; Staton, M.; Voy, B.H.; Donohoe, D.R.; Campagna, S.R.; et al. Ruminal Bacterial Communities and Metabolome Variation in Beef Heifers Divergent in Feed Efficiency. Ruminants 2022, 2, 282-296. https://doi.org/10.3390/ruminants2020019
Clemmons BA, Mulon P-Y, Anderson DE, Ault-Seay TB, Henniger MT, Schneider LG, Staton M, Voy BH, Donohoe DR, Campagna SR, et al. Ruminal Bacterial Communities and Metabolome Variation in Beef Heifers Divergent in Feed Efficiency. Ruminants. 2022; 2(2):282-296. https://doi.org/10.3390/ruminants2020019
Chicago/Turabian StyleClemmons, Brooke A., Pierre-Yves Mulon, David E. Anderson, Taylor B. Ault-Seay, Madison T. Henniger, Liesel G. Schneider, Meg Staton, Brynn H. Voy, Dallas R. Donohoe, Shawn R. Campagna, and et al. 2022. "Ruminal Bacterial Communities and Metabolome Variation in Beef Heifers Divergent in Feed Efficiency" Ruminants 2, no. 2: 282-296. https://doi.org/10.3390/ruminants2020019
APA StyleClemmons, B. A., Mulon, P.-Y., Anderson, D. E., Ault-Seay, T. B., Henniger, M. T., Schneider, L. G., Staton, M., Voy, B. H., Donohoe, D. R., Campagna, S. R., McLean, K. J., & Myer, P. R. (2022). Ruminal Bacterial Communities and Metabolome Variation in Beef Heifers Divergent in Feed Efficiency. Ruminants, 2(2), 282-296. https://doi.org/10.3390/ruminants2020019






