Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption
Abstract
1. Introduction
2. Bioactive Components in Microalgae and Their Health Benefits
2.1. Proteins, Peptides, and Amino Acids
2.2. Lipids and Polyunsaturated Fatty Acids
2.3. Polysaccharides
2.4. Vitamins
2.5. Pigments
2.6. Carotenoids
2.7. Astaxanthin
2.8. Chlorophyll
2.9. Phycobiliproteins
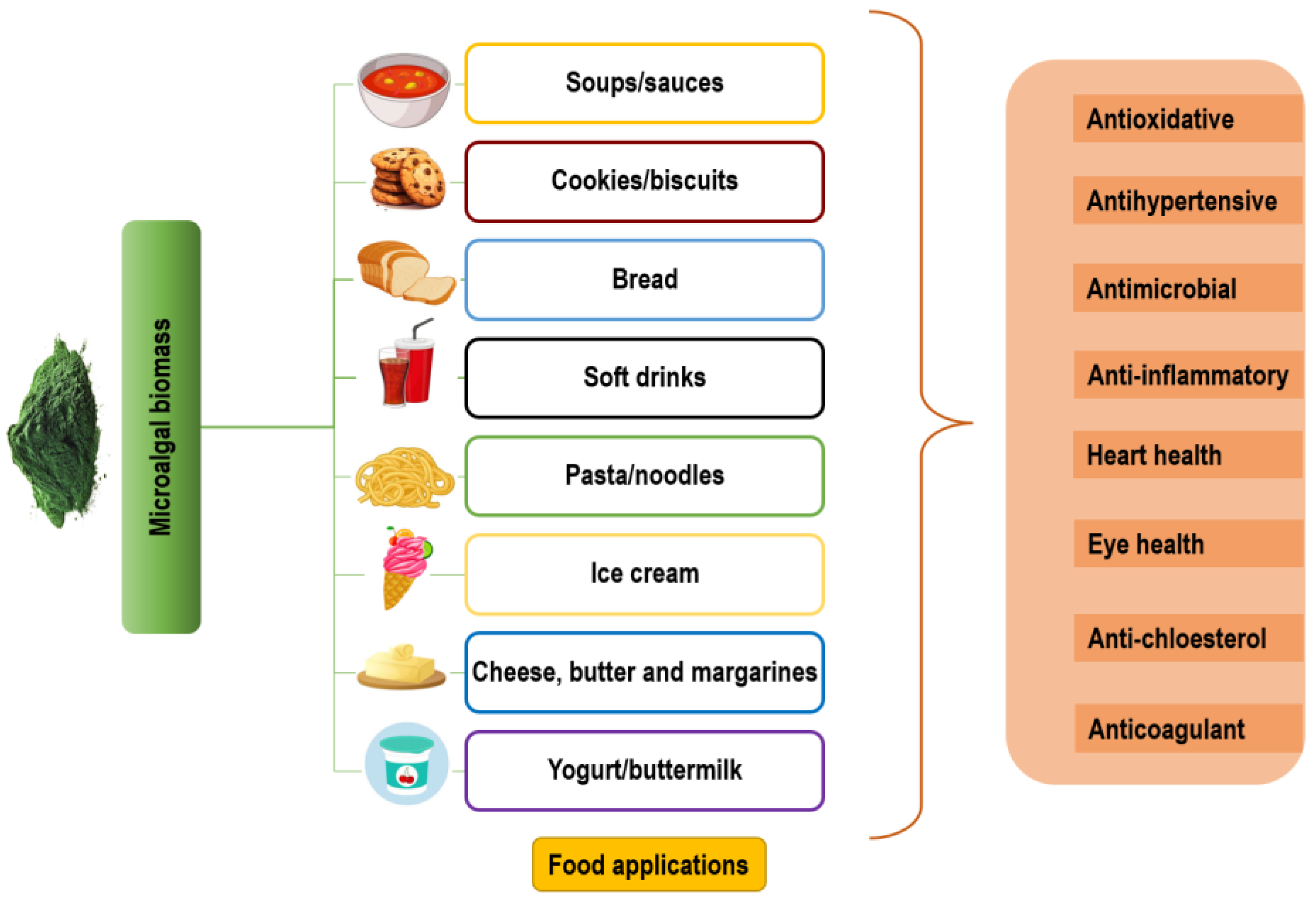
3. Application of Microalgae in the Food Industry
3.1. Functional Foods and Nutraceuticals
3.2. Microalgae-Enriched Foods and Their Health Benefits
3.3. Challenges in Processing and Consumer Acceptance
3.4. Consumer Trends and Innovations in Microalgae-Based Foods
4. Consumer Acceptance, Sustainability Issues, and Safety Concerns in Microalgae
4.1. Consumer Acceptance of Microalgae-Based Foods
4.2. Antinutritional Factors in Microalgae
4.3. Sustainability and Market Potential of Microalgae
4.4. Regulatory and Safety Concerns
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii is a potential food supplement with the capacity to outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Ortiz-Tena, J.G.; Rühmann, B.; Schieder, D.; Sieber, V. Revealing the diversity of algal monosaccharides: Fast carbohydrate fingerprinting of microalgae using crude biomass and showcasing sugar distribution in Chlorella vulgaris by biomass fractionation. Algal Res. 2016, 17, 227–235. [Google Scholar] [CrossRef]
- Choi, H.I.; Sung, Y.J.; Hong, M.E.; Han, J.; Min, B.K.; Sim, S.J. Reconsidering the potential of direct microalgal biomass utilization as end-products: A review. Renew. Sustain. Energy Rev. 2022, 155, 111930. [Google Scholar] [CrossRef]
- Katiyar, R.; Arora, A. Health-promoting functional lipids from the microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses, and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive composition; health benefits, safety, and prospects as potential high-value ingredients for the functional food industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Hernández, M.G.; Nallely, C.S.; Martínez-Roldán, A.D.; Rodríguez-Rosales, M.D.; Valle-Cervantes, S.; Rutiaga-Quiñones, O.M. Current perspectives on sustainable food product formulation by microalgae cultivation. In The Food-Energy-Water Triangle for Sustainable Development; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 121–144. [Google Scholar]
- Afzaal, M.; Imran, A.; Iqbal, S.S.; Akhtar, A.B.; Hayat, M.U.; Sharif, N.T.; Nawaz, R.; Naeem, U.; Batool, A.; Nawaz, S. Scaling up microalgal cultures for sustainable and nutritional food production. In Algae Biotechnology for Biomedical and Nutritional Applications; Academic Press: Cambridge, MA, USA, 2025; pp. 295–323. [Google Scholar]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Algae Product Market Report. Available online: https://www.factmr.com/report/845/algae-products-market (accessed on 20 March 2025).
- Kratzer, R.; Murkovic, M. Food ingredients and nutraceuticals from microalgae: Main product classes and biotechnological production. Foods 2021, 10, 1626. [Google Scholar] [CrossRef]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and healthier dairy products through the addition of microalgae: A review. Foods 2022, 11, 755. [Google Scholar] [CrossRef]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key challenges for the commercial expansion of ingredients from algae into human food products. Algal Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- Villaró, S.; Viñas, I.; Lafarga, T. Consumer acceptance and attitudes toward microalgae and microalgal-derived products as food. In Cultured Microalgae for the Food Industry; Academic Press: Cambridge, MA, USA, 2021; pp. 367–385. [Google Scholar]
- Bürck, M.; Ramos, S.D.; Braga, A.R. Enhancing the biological effects of bioactive compounds from microalgae through advanced processing techniques: Pioneering ingredients for next-generation food production. Foods 2024, 13, 1811. [Google Scholar] [CrossRef]
- Cruz, J.D.; Vasconcelos, V. Legal aspects of microalgae in the European food sector. Foods 2023, 13, 124. [Google Scholar] [CrossRef]
- MU, N.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent advances in microalgal bioactives for food, feed, and healthcare products: Commercial potential, market space, and sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; da Costa, W.K.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as a source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- De Oliveira, A.P.; Bragotto, A.P. Microalgae-based products: Food and public health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Salehipour-Bavarsad, F.; Nematollahi, M.A.; Pistocchi, R.; Pezzolesi, L. Algal food safety: Possible contaminations, challenges of harmonized quality assessments, and suggested recommendations for the nascent industry of microalgae-based products. Algal Res. 2024, 81, 103579. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- O’Brien, E.C.; Tsoi, K.Y.; Ma, R.C.; Hanson, M.; Hod, M.; McAuliffe, F.M. Nutrition through the life cycle: Pregnancy. In Encyclopedia of Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–74. [Google Scholar]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, Y.; Tibbetts, S.M.; Berrue, F.; McGinn, P.J.; MacQuarrie, S.P.; Puttaswamy, A.; Patelakis, S.; Schmidt, D.; Melanson, R.; MacKenzie, S.E. A rat study to evaluate the protein quality of three green microalgal species and the impact of mechanical cell wall disruption. Foods 2020, 9, 1531. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Ulrikh, E.; Ivanova, S.; Prosekov, A.; Dolganyuk, V. Production, purification, and study of the amino acid composition of microalgae proteins. Molecules 2020, 26, 2767. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.; Leite, M.D.; Albino, L.F.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Agyei, D.; Potumarthi, R.; Danquah, M.K. Food-derived multifunctional bioactive proteins and peptides: Sources and production. In Biotechnology of Bioactive Compounds: Sources and Applications; Gupta, V.K., Tuohy, M.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 483–505. [Google Scholar]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ACE-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Ko, S.C.; Kim, D.; Jeon, Y.J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Shih, M.F.; Cherng, J.Y. Protective effects of Chlorella-derived peptide against UVC-induced cytotoxicity through inhibition of caspase-3 activity and reduction of the expression of phosphorylated FADD and cleaved PARP-1 in skin fibroblasts. Molecules 2012, 17, 9116–9128. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Reinado, R.; Escobar-Niño, A.; Fajardo, C.; Morano, I.M.; Amil-Ruiz, F.; Martinez-Rodríguez, G.; Fuentes-Almagro, C.; Capilla, V.; Tomás-Cobos, L.; Soriano-Romaní, L.; et al. Development of a new antiproliferative compound against human tumor cells from the marine microalga Nannochloropsis gaditana by applied proteomics. Int. J. Mol. Sci. 2020, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.; Panjaitan, F.C.; Chang, Y.W. Prediction of bioactive peptides from Chlorella sorokiniana proteins using proteomic techniques in combination with bioinformatics analyses. Int. J. Mol. Sci. 2019, 20, 1786. [Google Scholar] [CrossRef] [PubMed]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.; Chang, Y.W. Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci. Nutr. 2019, 7, 2381–2390. [Google Scholar] [CrossRef]
- Vo, T.S.; Ryu, B.; Kim, S.K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Food 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Safitri, N.M.; Herawati, E.Y.; Hsu, J.L. Antioxidant activity of purified active peptide derived from Spirulina platensis enzymatic hydrolysates. Res. J. Life Sci. 2017, 4, 119–128. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Fassi, E.M.; Boschin, G.; Bartolomei, M.; Bollati, C.; Roda, G.; Arnoldi, A.; Grazioso, G.; Lammi, C. Investigation of Chlorella pyrenoidosa protein as a source of novel angiotensin I-converting enzyme (ACE) and dipeptidyl peptidase-IV (DPP-IV) inhibitory peptides. Nutrients 2021, 13, 1624. [Google Scholar] [CrossRef]
- Sheih, I.C.; Fang, T.J.; Wu, T.K. Isolation and characterization of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Chen, M.F.; Zhang, Y.Y.; Di He, M.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Qian, Z.J. Antioxidant peptide purified from enzymatic hydrolysates of Isochrysis zhanjiangensis and its protective effect against ethanol-induced oxidative stress in HepG2 cells. Biotechnol. Bioprocess Eng. 2019, 24, 308–317. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; O-Nam, K.; Ko, J.Y.; Lee, J.H.; Kang, M.C.; Kim, D.; Lee, J.B.; Lee, J.S.; Jeon, Y.J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Afify, A.E.; El Baroty, G.S.; El Baz, F.K.; Abd El Baky, H.H.; Murad, S.A. Scenedesmus obliquus: Antioxidant and antiviral activity of proteins hydrolyzed by three enzymes. J. Genet. Eng. Biotechnol. 2018, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Dhandwal, A.; Bashir, O.; Malik, T.; Salve, R.V.; Dash, K.K.; Amin, T.; Shams, R.; Wani, A.W.; Shah, Y.A. Sustainable microalgal biomass as a potential functional food and its applications in the food industry: A comprehensive review. Environ. Sci. Pollut. Res. 2024, 7, 1–9. [Google Scholar] [CrossRef]
- Tajuddin, N.; Shaikh, A.; Hassan, A. Prescription omega-3 fatty acid products: Considerations for patients with diabetes mellitus. Diabetes Metab. Syndr. Obes. 2016, 9, 109–118. [Google Scholar]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs production and use in food and feed industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Li, J.; Yin, H.; Bibus, D.M.; Byelashov, O.A. The role of Omega-3 docosapentaenoic acid in pregnancy and early development. Eur. J. Lipid Sci. Technol. 2016, 118, 1692–1701. [Google Scholar] [CrossRef]
- Dunstan, J.; Simmer, K.; Dixon, G.; Prescott, S.L. Cognitive assessment of children at age 2.5 years after maternal fish oil supplementation in pregnancy: A randomized controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F45–F50. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Bassett, C.M.; McCullough, R.; Pierce, G.N. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef]
- Shanab, S.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential sources of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chaves, H.; Singh, R.B.; Khan, S.; Wilczynska, A.; Takahashi, T. High omega-6/omega-3 fatty acid ratio diets and risk of non-communicable diseases: Is the tissue the main issue? In The Role of Functional Food Security in Global Health; Academic Press: Cambridge, UK, 2019; pp. 217–259. [Google Scholar]
- Morgese, M.G.; Mhillaj, E.; Francavilla, M.; Bove, M.; Morgano, L.; Tucci, P.; Trabace, L.; Schiavone, S. Chlorella sorokiniana extract improves short-term memory in rats. Molecules 2016, 21, 1311. [Google Scholar] [CrossRef] [PubMed]
- Babuskin, S.; Krishnan, K.R.; Saravana Babu, P.A.; Sivarajan, M.; Sukumar, M. Functional foods enriched with marine microalga Nannochloropsis oculata as a source of ω-3 fatty acids. Food Technol. Biotechnol. 2014, 52, 292–299. [Google Scholar]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef]
- De Souza, M.P.; Sanchez-Barrios, A.; Rizzetti, T.M.; Benitez, L.B.; Hoeltz, M.; de Souza Schneider, R.D.; de Farias Neves, F. Concepts and trends for extraction and application of microalgae carbohydrates. In Microalgae—From Physiology to Application; IntechOpen: London, UK, 2019. [Google Scholar]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae—Nutritious, sustainable aqua- and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. Biotechnol. 1990, 5, 259–263. [Google Scholar]
- Watanabe, F.; Takenaka, S.; Kittaka-Katsura, H.; Ebara, S.; Miyamoto, E. Characterization and bioavailability of vitamin B12 compounds from edible algae. J. Nutr. Sci. Vitaminol. 2002, 48, 325–331. [Google Scholar] [CrossRef]
- Merchant, R.E.; Phillips, T.W.; Udani, J. Nutritional supplementation with Chlorella pyrenoidosa lowers serum methylmalonic acid in vegans and vegetarians with a suspected vitamin B12 deficiency. J. Med. Food 2015, 18, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Del Mondo, A.; Smerilli, A.; Sané, E. Challenging microalgal vitamins for human health. Microb. Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- FAO. Manual on the Production and Use of Live Food for Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; Available online: https://www.fao.org/4/w3732e/w3732e07.htm (accessed on 18 February 2025).
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J. Food Compos. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Tarento, T.D.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a source of vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Parmar, R.S.; Singh, C. A comprehensive study of eco-friendly natural pigment and its applications. Biochem. Biophys. Rep. 2018, 13, 22–26. [Google Scholar] [CrossRef]
- Mulders, K.J.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Menegol, T.; Rech, R.; Mercali, G.D.; Marczak, L.D. Carotenoid and lipid extraction from HeteroChlorella luteoviridis using moderate electric field and ethanol. Process Biochem. 2016, 51, 1636–1643. [Google Scholar] [CrossRef]
- Sui, Y.; Mazzucchi, L.; Acharya, P.; Xu, Y.; Morgan, G.; Harvey, P.J. A comparison of β-carotene, phytoene, and amino acids production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina CCAP 19/30 using different light wavelengths. Foods 2021, 10, 2824. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high-performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Market Research Reports. The Global Market for Carotenoids; Report Code: FOD025G; BCC Publishing: Boston, MA, USA, 2022; Available online: https://www.bccresearch.com/market-research/food-and-beverage/the-global-market-for-carotenoids.html (accessed on 11 February 2024).
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein, and carbohydrate in industrial applications: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar]
- Rodrigues, D.B.; Menezes, C.R.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Bioactive pigments from microalgae Phormidium autumnale. Food Res. Int. 2015, 77, 273–279. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Utilization of algae for biofuel; bio-products and bioremediation. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Maria, A.G.; Graziano, R.; Nicolantonio, D.O. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef]
- Riccioni, G.; D’Orazio, N.; Palumbo, N.; Bucciarelli, V.; di Ilio, E.; Bazzano, L.A.; Bucciarelli, T. Relationship between plasma antioxidant concentrations and carotid intima-media thickness: The asymptomatic carotid atherosclerotic disease in Manfredonia study. Eur. J. Prev. Cardiol. 2009, 16, 351–357. [Google Scholar] [CrossRef]
- Butler, T.; Golan, Y. Astaxanthin production from microalgae. In Microalgae Biotechnology for Food, Health and High-Value Products; Asraful Alam, M., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 175–242. [Google Scholar]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; De Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.M.; Zubeck, H.M. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J. Nutr. Food Sci. 2012, 2, 5. [Google Scholar] [CrossRef]
- Seyidoglu, N.; Inan, S.; Aydin, C. A prominent superfood: Spirulina platensis. In Superfood and Functional Food: The Development of Superfoods and Their Roles as Medicine; InTech: Zagreb, Croatia, 2017; pp. 1–27. [Google Scholar]
- Nagini, S.; Palitti, F.; Natarajan, A.T. Chemopreventive potential of chlorophyllin: A review of the mechanisms of action and molecular targets. Nutr. Cancer 2015, 67, 203–211. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification, and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef]
- Eltanahy, E.; Torky, A. Microalgae as cell factories: Food and feed-grade high-value metabolites. In Microalgal Biotechnology; Shekh, A., Schenk, P., Sarada, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–35. [Google Scholar]
- Paliwal, C.; Ghosh, T.; George, B.; Pancha, I.; Maurya, R.; Chokshi, K.; Ghosh, A.; Mishra, S. Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 2016, 15, 24–31. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Singh, P.R.; Kumar, D.; Sinha, R.P. Phycobiliproteins in microalgae: Occurrence, distribution, and biosynthesis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 43–68. [Google Scholar]
- Vieira, R.C.; Medeiros, J.A.; do Nascimento, M.A.; de Souza Abud, A.K.; Raymundo, A.; de Farias Silva, C.E. Microalgae as sustainable food: Incorporation as a strategy in the formulation of functional food. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–30. [Google Scholar]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76. [Google Scholar] [CrossRef]
- De Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of Spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Raymundo, A.; Bandarra, N. Spirulina maxima and Diacronema vlkianum microalgae in vegetable gelled desserts. Nutr. Food Sci. 2008, 38, 492–501. [Google Scholar] [CrossRef]
- Malik, P.; Kempanna, C.; Murthy, N.; Anjum, A. Quality characteristics of yoghurt enriched with Spirulina powder. Mysore J. Agric. Sci. 2013, 47, 354–359. [Google Scholar]
- Mohamed, A.G.; Abo-El-Khair, B.E.; Shalaby, S.M. Quality of novel healthy processed cheese analogue enhanced with marine microalgae Chlorella vulgaris biomass. World Appl. Sci. J. 2013, 23, 914–925. [Google Scholar]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Hlaing, S.A.; Sadiq, M.B.; Anal, A.K. Enhanced yield of Scenedesmus obliquus biomacromolecules through medium optimization and development of microalgae-based functional chocolate. J. Food Sci. Technol. 2020, 57, 1090–1099. [Google Scholar] [CrossRef]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal proteins and bioactives for food, feed, and other applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Castillejo, N.; Carrión-Monteagudo, M.D.; Artés, F.; Artés-Hernández, F. Nutritional and bioactive compounds of commercialized algae powders used as food supplements. Food Sci. Technol. Int. 2018, 24, 172–182. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity, and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Finney, K.F.; Pomeranz, Y.; Bruinsma, B.L. Use of algae Dunaliella as a protein supplement in bread. Cereal Chem. 1984, 61, 402–406. [Google Scholar]
- Burcu, A.; Avsaroglu, E.; Isik, O.; Özyurt, G.; Kafkas, E.; Etyemez, M. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 30–38. [Google Scholar]
- Figueira, F.D.; Crizel, T.D.; Silva, C.R.; Salas-Mellado, M.D. Elaboration of gluten-free bread enriched with the microalgae Spirulina platensis. Braz. J. Food Technol. 2011, 14, 308–316. [Google Scholar]
- Robertson, R.C.; Mateo, M.R.; O’Grady, M.N.; Guihéneuf, F.; Stengel, D.B.; Ross, R.P.; Fitzgerald, G.F.; Kerry, J.P.; Stanton, C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016, 37, 237–246. [Google Scholar] [CrossRef]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Geppert, J.; Kraft, V.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid supplementation in vegetarians effectively increases omega-3 index: A randomized trial. Lipids 2005, 40, 807–814. [Google Scholar] [CrossRef]
- Sahni, P.; Aggarwal, P.; Sharma, S.; Singh, B. Nuances of microalgal technology in food and nutraceuticals: A review. Nutr. Food Sci. 2019, 49, 866–885. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as coloring source in traditional butter cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Yu, S.; Blennow, A.; Bojko, M.; Madsen, F.; Olsen, C.E.; Engelsen, S.B. Physico-chemical characterization of floridean starch of red algae. Starch-Stärke 2002, 54, 66–74. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary characterization, antioxidant properties, and production of chrysolaminarin from marine diatom Odontella aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in foods and its physiological functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, V.P.B.; De Souza, E.L.; De Albuquerque, T.M.R.; Da Costa Sassi, C.F.; Dos Santos Lima, M.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The pros and cons of using algal polysaccharides as prebiotics. Front. Nutr. 2020, 7, 571548. [Google Scholar] [CrossRef]
- Gouda, M.; Tadda, M.A.; Zhao, Y.; Farmanullah, F.; Chu, B.; Li, X.; He, Y. Microalgae bioactive carbohydrates as a novel sustainable and eco-friendly source of prebiotics: Emerging health functionality and recent technologies for extraction and detection. Front. Nutr. 2022, 9, 806692. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of Spirulina microalgae. Mini-Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- EUFIC. Protein Pioneers Leading the Way in Alternative Protein Project. Available online: https://www.eufic.org/en/newsroom/article/protein-pioneers-leading-the-way-in-alternative-protein-project (accessed on 18 February 2025).
- Alehosseini, E.; McSweeney, P.L.; Miao, S. Recent updates on plant protein-based dairy cheese alternatives: Outlook and challenges. Crit. Rev. Food Sci. Nutr. 2025, 1, 1–15. [Google Scholar] [CrossRef]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Effect of microalgae addition on mineral content, colour, and mechanical properties of breadsticks. Food Funct. 2019, 10, 4685–4692. [Google Scholar] [CrossRef]
- Tohamy, M.M.; Ali, M.A.; Shaaban, H.A.; Mohamad, A.G.; Hasanain, A.M. Production of functional spreadable processed cheese using Chlorella vulgaris. Acta Sci. Pol. Technol. Aliment. 2018, 17, 347–358. [Google Scholar] [PubMed]
- Hernández-López, I.; Abadias, M.; Prieto-Santiago, V.; Chic-Blanco, Á.; Ortiz-Solà, J.; Aguiló-Aguayo, I. Exploring the nutritional potential of microalgae in the formulation of bakery products. Foods 2023, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Nefertari, C.G.; Elisa, C.C.; Guadalupe, C.M.; Carolina, F.G.; Javier, P.P.; Del Carmen, R.O.; David, G.O.; Monzerrath, O.S.; de Abril Alexandra, S.M.; Raúl, R.H. Bio-Based Bakery Products: A Present Insight on Its Nutritive Potential. In Biological Outlook to Improve the Nutritive Quality of Bakery Products; Springer: Singapore, 2025; pp. 1–20. [Google Scholar]
- ProFuture. Available online: https://www.pro-future.eu/news/profuture-update-which-results-have-we-achieved-so-far?utm_source (accessed on 20 March 2025).
- Ang, W.S.; Kee, P.E.; Lan, J.C.W.; Chen, W.; Chang, J.; Khoo, K.S. Unveiling the rise of microalgae-based foods in the global market: Perspective views and way forward. Food Biosci. 2025, 66, 105390. [Google Scholar] [CrossRef]
- Wassmann, B.; Hartmann, C.; Siegrist, M. Novel microalgae-based foods: What influences Singaporean consumers’ acceptance? Food Qual. Prefer. 2024, 113, 105068. [Google Scholar] [CrossRef]
- Yesuraj, D.; Deepika, C.; Ravishankar, G.A.; Ranga Rao, A. Seaweed-based recipes for food, health-food applications, and innovative products including meat and meat analogs. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical, and Health Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 267–292. [Google Scholar]
- Lafarga, T.; Rodríguez-Bermúdez, R.; Morillas-España, A.; Villaró, S.; García-Vaquero, M.; Morán, L.; Sánchez-Zurano, A.; González-López, C.V.; Acién-Fernández, F.G. Consumer knowledge and attitudes towards microalgae as food: The case of Spain. Algal Res. 2021, 54, 102174. [Google Scholar] [CrossRef]
- Grasso, A.C.; Hung, Y.; Olthof, M.R.; Verbeke, W.; Brouwer, I.A. Older consumers’ readiness to accept alternative, more sustainable protein sources in the European Union. Nutrients 2019, 11, 1904. [Google Scholar] [CrossRef]
- Maehle, N.; Skjeret, F. Microalgae-based food: Purchase intentions and willingness to pay. Future Foods 2022, 6, 100205. [Google Scholar] [CrossRef]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ associations, perceptions, and acceptance of meat and plant-based meat alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef]
- Ahmad, A.; Hassan, S.W.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: Food security and circular economy. Bioengineered 2022, 13, 9521. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Svirčev, Z. Microalgae and cyanobacteria: Food for thought. J. Phycol. 2008, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Gnansounou, E. Protein extraction from biomass in a bioethanol refinery—Possible dietary applications: Use as animal feed and potential extension to human consumption. Bioresour. Technol. 2011, 102, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Gille, A.; Louis, S.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Bioavailability and Safety of Nutrients from the Microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 Mice. Nutrients 2018, 10, 965. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Kong, F. Microalgae biotechnology: Methods and applications. Bioengineering 2024, 11, 965. [Google Scholar] [CrossRef]
- Chemsafe. Microalgae-Based Novel Foods in the EU. Available online: https://www.chemsafe-consulting.com/en/2024/07/16/microalgae-based-novel-foods-in-eu/?utm_source (accessed on 20 March 2025).
- Su, M.; Bastiaens, L.; Verspreet, J.; Hayes, M. Applications of microalgae in foods, pharma, and feeds and their use as fertilizers and biostimulants: Legislation and regulatory aspects for consideration. Foods 2022, 12, 3878. [Google Scholar] [CrossRef]
- Algae Novel Food. Available online: https://www.algae-novel-food.com/?utm_source (accessed on 20 March 2025).
- Renganathan, P.; Gaysina, L.A.; Jaime, R.; Carlos, J.; Rueda, E.O. Phycoremediated microalgae and cyanobacteria biomass as biofertilizer for sustainable agriculture: A holistic biorefinery approach to promote circular bioeconomy. Biomass 2024, 4, 1047–1077. [Google Scholar] [CrossRef]
- Gelagutashvili, E.; Bagdavadze, N.; Gongadze, A.; Gogebashvili, M.; Ivanishvili, N. Effect of Ag(I), Ni(II), Zn(II) ions on Irradiated Spirulina platensis. arXiv 2021. [Google Scholar] [CrossRef]
- Uzochukwu, J.S.; Nkeiruka, N.C.; Odinaka, N.J.; Olajide, O.G. Fabrication and optimization of alginate membranes for improved wastewater treatment. Arch. Case Rep. 2025, 9, 026–043. [Google Scholar]
| Microalgal Species | Protein Content (%) | Essential Amino Acids (g 100 g Protein−1 DW) | Non-Essential Amino Acids (g 100 g Protein−1 DW) | References | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thr | Val | Met | Iso | Leu | Phe | His | Lys | Arg | Try | Asp | Ser | Glu | Pro | Gly | Ala | Cys | Tyr | |||
| Acutodesmus obliquus | 40.34 | 5.28 | 5.88 | 1.64 | 3.48 | 8.38 | 4.34 | 2.04 | 5.7 | 7.40 | 1.97 | 1.00 | 5.02 | 12.62 | 5.90 | 6.32 | 8.34 | 1.08 | 4.35 | [30] |
| Arthrospira platensis | - | 4.57 | 7.81 | 1.93 | 4.48 | 9.81 | 7.85 | 2.19 | 7.11 | 6.02 | 1.16 | 10.12 | 3.31 | 14.36 | 5.17 | 5.25 | 11.48 | 1.94 | 7.85 | [31] |
| Botryococcus braunii | 39.9 | 3.7 | 4.4 | 2.5 | 3.4 | 7.1 | 4.4 | 1.5 | 4.7 | 20.5 | 2.2 | 8.7 | 3.5 | 12.7 | 4.6 | 4.9 | 6.4 | 1.4 | 2.8 | [2] |
| Chlorella vulgaris | - | 4.16 | 6.37 | 2.52 | 3.36 | 8.41 | 6.17 | 1.52 | 5.35 | 7.33 | 0.21 | 8.54 | 4.34 | 10.28 | 5.08 | 7.14 | 10.82 | 1.47 | 4.34 | [31] |
| Chlorella pyrenoidosa | - | 3.45 | 5.17 | 3.30 | 6.20 | 3.44 | 3.83 | 1.64 | 8.14 | 5.91 | - | 8.12 | 2.79 | 7.87 | - | 9.73 | 5.08 | 2.82 | 1.22 | [3] |
| Chlorella sorokiniana | 5.02 | 4.29 | 5.80 | 1.87 | 3.61 | 9.19 | 5.37 | 1.77 | 5.19 | 8.79 | 2.33 | 1.03 | 5.09 | 11.82 | 5.35 | 5.82 | 8.42 | 1.02 | 4.43 | [30] |
| Dunaliella salina | - | 5.16 | 7.23 | 2.79 | 4.09 | 9.58 | 6.98 | 1.73 | 5.99 | 8.16 | 0.18 | 9.56 | 4.81 | 12.41 | 5.23 | 8.71 | 10.99 | 1.63 | 4.86 | [31] |
| Nannochloropsis granulata | 33.5 | 5.4 | 7.1 | 3.5 | 5.6 | 11.0 | 6.2 | 2.3 | 8.5 | 7.4 | 2.8 | 11.4 | 5.6 | 14.1 | 11.2 | 7.5 | 7.1 | 1.6 | 4.2 | [2] |
| Nostoc sp. | - | 5.31 | 7.15 | 2.23 | 3.68 | 9.41 | 7.15 | 2.01 | 6.47 | 6.15 | 1.02 | 9.18 | 3.16 | 12.38 | 5.28 | 6.54 | 9.88 | 1.54 | 6.84 | [30] |
| Phaeodactylum tricornutum | 39.6 | 4.8 | 5.1 | 2.7 | 4.6 | 7.0 | 4.8 | 1.5 | 6.4 | 5.7 | 2.6 | 11.6 | 4.8 | 18.8 | 7.1 | 5.5 | 7.3 | 1.5 | 3.4 | [2] |
| Pleurochrysis carterae | - | 5.67 | 7.55 | 2.41 | 4.22 | 9.93 | 7.69 | 1.89 | 7.24 | 6.88 | 1.14 | 9.19 | 3.48 | 15.17 | 5.12 | 7.02 | 11.51 | 2.03 | 7.69 | [31] |
| Porphyridium aerugineum | 31.6 | 5.8 | 7.3 | 3.7 | 7.1 | 11.9 | 6.3 | 1.9 | 8.0 | 8.6 | 3.3 | 15.0 | 7.0 | 15.6 | 5.0 | 7.0 | 8.4 | 2.2 | 5.8 | [2] |
| Tetraselmis chuii | 46.5 | 4.0 | 4.8 | 2.4 | 3.4 | 7.3 | 4.7 | 1.6 | 5.6 | 9.4 | 2.3 | 14.1 | 4.2 | 12.0 | 3.6 | 6.5 | 6.0 | 2.8 | 3.0 | [2] |
| Species | Enzyme Used for Hydrolysis | Peptide Sequence | Observations | References |
|---|---|---|---|---|
| Arthrospira maxima | Trypsin, α-chymotrypsin, and pepsin | LDAVNR MMLDF | Anti-inflammatory effect was shown in vitro; Suppressive effect on the release of histamine and production of interleukin-8 | [42] |
| Arthrospira platensis | Thermolysin | FSESSAPEQHY | Antioxidant | [43] |
| Chlorella ellipsoidea | Papain, trypsin, pepsin, and a-chymotrypsin | LNGDVW | Antioxidant | [37] |
| Chlorella pyrenoidosa | Pepsin and trypsin | FLKPLGSGK QIYTMGK FLFVAEAIYK QHAGTKAK | Anti-hypertensive and anti-diabetic effect; Inhibited the activity of angiotensin I-converting enzyme (ACE) and dipeptidyl peptidase-IV (DPP-IV) in vitro | [44] |
| Chlorella sorokiniana | Pepsin, bromelain, and papain | n/d | DPP IV and ACE inhibitory, antioxidant, anti-amnestic, and antithrombotic activity | [41] |
| Chlorella vulgaris | Pepsin | VECYGPNRPQF | Hendeca-peptide inhibit the activity of ACE; Regulated hypertension and water-fluid balance. | [45] |
| Isochrysis zhanjiangensis | Chymotrypsin | NDAEYGICGF | Antioxidant | [46] |
| Nannochloropsis oculata | Pepsin, trypsin, α-chymotrypsin, papain, alcalase, and neutrase | GMNNLTP LEQ | ACE inhibitory | [47] |
| Scenedesmus obliquus | Pepsin, trypsin, and papain | RKDAH | Antioxidant and antiviral activity | [48] |
| Tetradesmus obliquus | Alcalase | WPRGYFL GPDRPKFLGPF WYGPDRPKFL SDWDRF | Antioxidant and ACE-inhibitory | [49] |
| Commercial Industry | Microalgae Species | Area of Application | Location |
|---|---|---|---|
| Algae Tech. | - | Nutraceuticals, animal feed, and aquafeed | Seaford, VIC, Australia |
| Algomed | Chlorella sp. and Arthrospira sp. | Food supplements and nutraceuticals | Klötze, Germany |
| AlgoSource | Arthrospira maxima, Arthrospira platensis, Scenedesmus sp. | Nutraceuticals, cosmetics, and health | Guérande, France |
| Algatechnologies Ltd. | Haematococcus pluvialis, Phaeodactylum tricornutum, Porphyridium cruentum, and Nannochloropsis sp. | Nutrition, food and beverages, and cosmetics | Ketura, Israel |
| Aurora Algae Inc. | - | Pharmaceutical, nutrition, aquafeed, and fuels | Hayward, CA, USA |
| Allmicroalgae | Chlorella vulgaris, A. platensis, Tetraselmis chui, Nannochloropsis oceanica, Scenedesmus rubescens, P. tricornutum, and Chlorococcum amblystomatis | Dietary supplements, food, feed, and agro applications | Pataias, Portugal |
| BlueBioTech International GmbH | A. platensis and H. pluvialis | Nutraceuticals | Kollmar, Germany |
| Cyanotech Corporation | H. pluvialis and Arthrospira sp. | Functional foods | Kailua-Kona, HI, USA |
| Canadian Pacific Algae, Inc. | Marine phytoplankton | Functional foods and nutraceuticals | Nanaimo, BC, Canada |
| Parry nutraceuticals | Arthrospira sp., Chlorella sp., Dunaliella salina, and H. pluvialis | Astaxanthin, food supplements, and nutraceuticals | Tamil Nadu, India |
| Euglena Co., Ltd. | Euglena gracilis, Euglena mutabilis, Euglena sanguinea, and Euglena agilis | Nutritional supplements, biofuels, feed, fertilizers, and biomass plastics | Minato City, Japan |
| Fermentalg SA | - | Omega 3 fatty acids, coloring agents, antioxidants, and biopolymers | Libourne, France |
| Oilgae | - | Food, feed, nutraceuticals, pharmaceuticals, biofuel, biopolymers, cosmetics, paper, lubricants, and chemicals | Chennai, India |
| Sun Chlorella A | Chlorella spp. | Nutritional supplements, food, feed, and personal care | Kyoto, Japan |
| Subitec | Anabena sp., Aphanizomenon Flos-Aquae, C. vulgaris, Cyanobacterium aponinum, D. salina, H. pluvialis, Nannochloropsis sp., P. tricornutum, Scenedesmus sp., A. platensis | Food supplements, pharmaceutical, personal care, and animal feed | Köngen, Germany |
| Simris Alg. AB | Cyanobacterial species | Food supplements, nutraceuticals, and skin care | Hammenhog, Sweden |
| Solarvest BioEnergy Inc. | - | Nutraceuticals | Vancouver, BC, Canada |
| Mera Pharmaceuticals | - | Personal care and food supplements | Las Vegas, NV, USA |
| ZIVO Bioscience, Inc. | - | Food supplements | Bloomfield Hills, MI, USA |
| Taiwan Chlorella Manufacturing | Chlorella spp. | Algae-based foods (juice, noodles, and chocolates) and nutritional supplements | Taipei City, Taiwan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ruiz, F.E.; Andrade-Bustamante, G.; Holguín-Peña, R.J.; Renganathan, P.; Gaysina, L.A.; Sukhanova, N.V.; Puente, E.O.R. Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass 2025, 5, 25. https://doi.org/10.3390/biomass5020025
Martínez-Ruiz FE, Andrade-Bustamante G, Holguín-Peña RJ, Renganathan P, Gaysina LA, Sukhanova NV, Puente EOR. Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass. 2025; 5(2):25. https://doi.org/10.3390/biomass5020025
Chicago/Turabian StyleMartínez-Ruiz, Francisco Eleazar, Gabriela Andrade-Bustamante, Ramón Jaime Holguín-Peña, Prabhaharan Renganathan, Lira A. Gaysina, Natalia V. Sukhanova, and Edgar Omar Rueda Puente. 2025. "Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption" Biomass 5, no. 2: 25. https://doi.org/10.3390/biomass5020025
APA StyleMartínez-Ruiz, F. E., Andrade-Bustamante, G., Holguín-Peña, R. J., Renganathan, P., Gaysina, L. A., Sukhanova, N. V., & Puente, E. O. R. (2025). Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass, 5(2), 25. https://doi.org/10.3390/biomass5020025









