Multifunctional Green-Synthesized Cu2O-Cu(OH)2 Nanocomposites Grown on Cu Microfibers for Water Treatment Applications
Abstract
1. Introduction
2. Materials and Methods
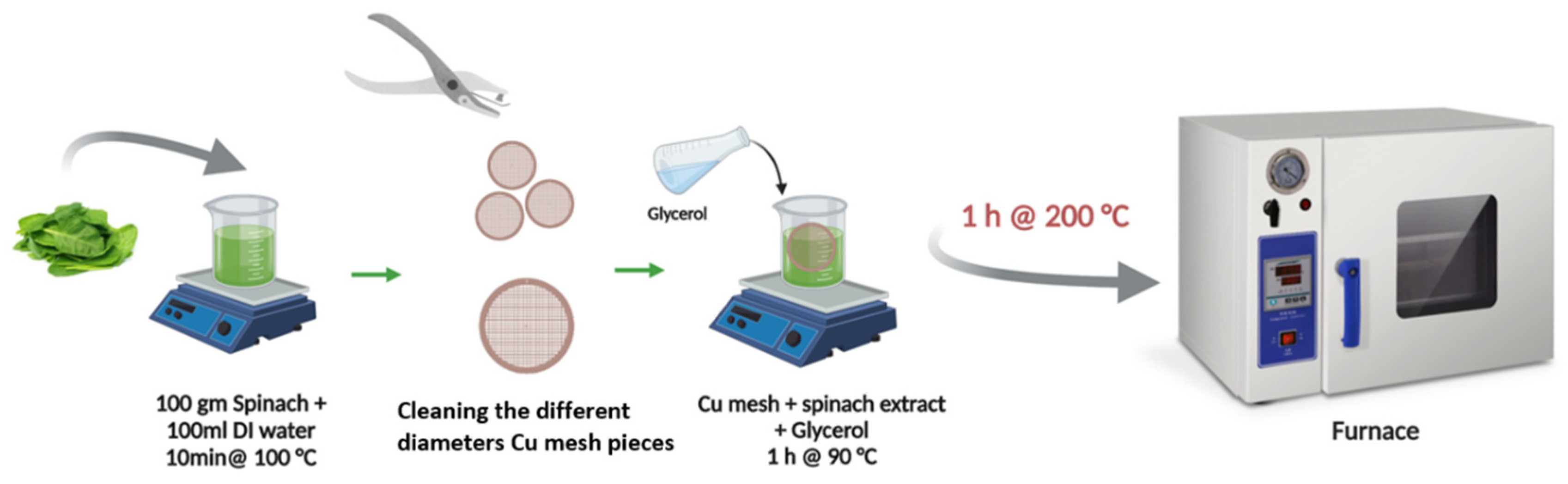
2.1. Synthesis of the Cu2O-Cu(OH)2@Cu Mesh
2.2. Characterization Techniques
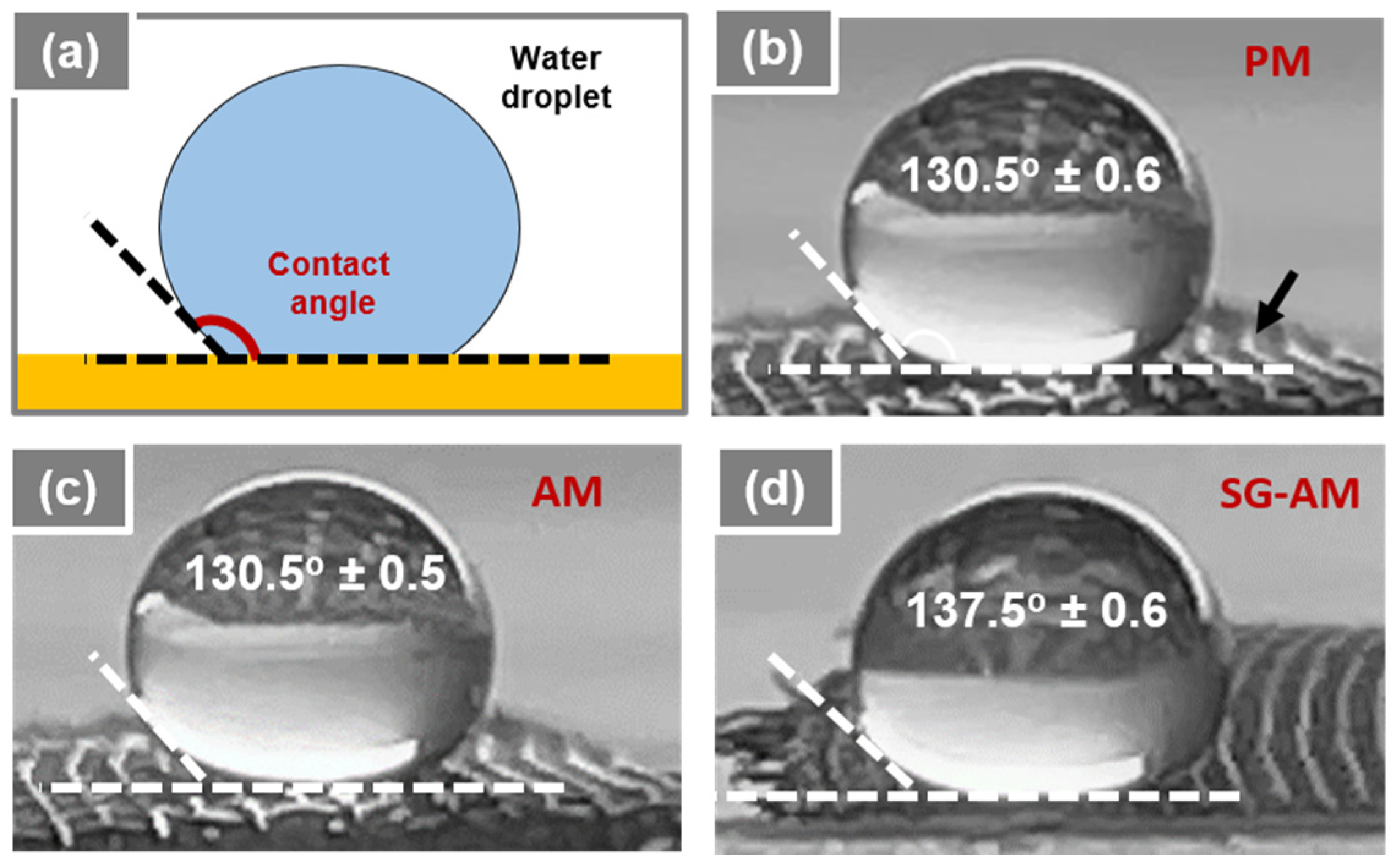
2.3. Contact Angle and Oil–Water Separation Measurements
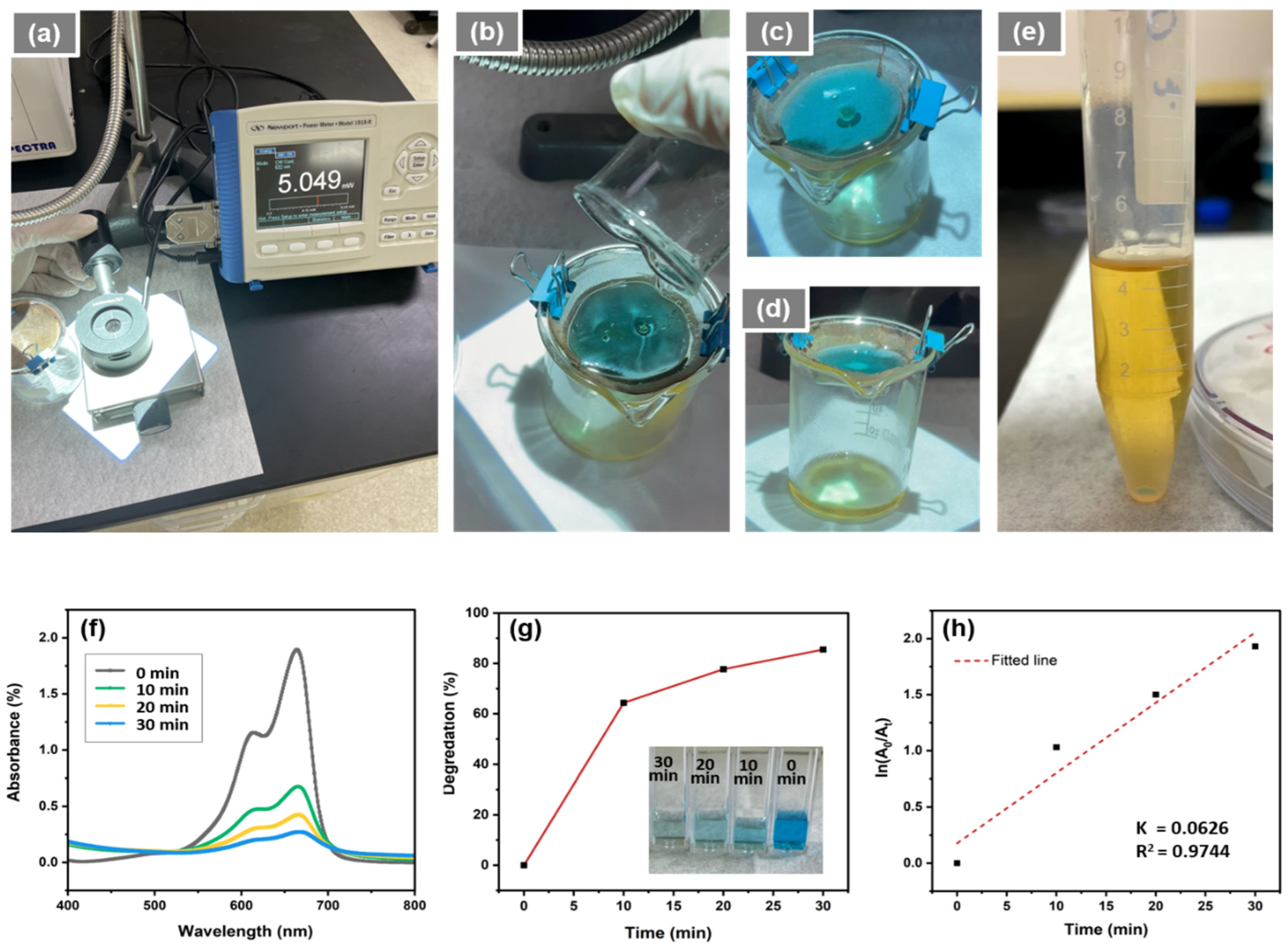
2.4. Photocatalytic Activity Assessment
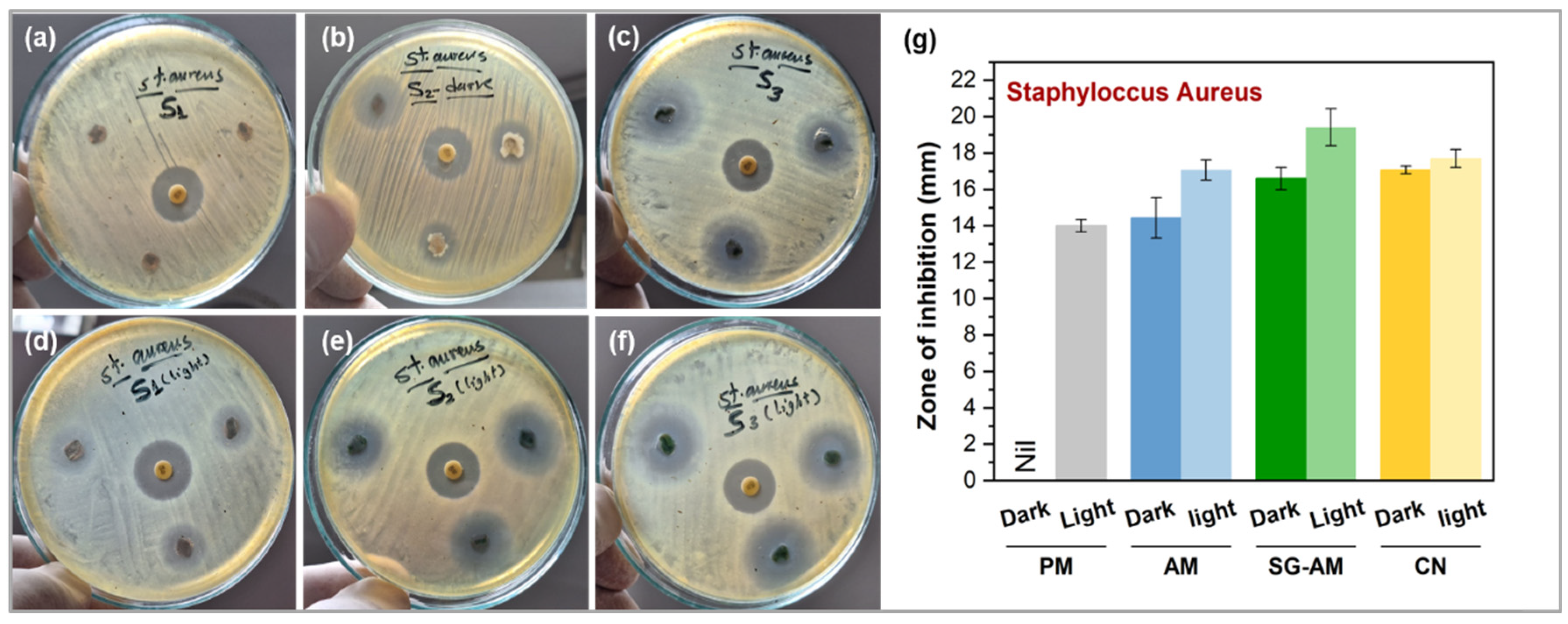
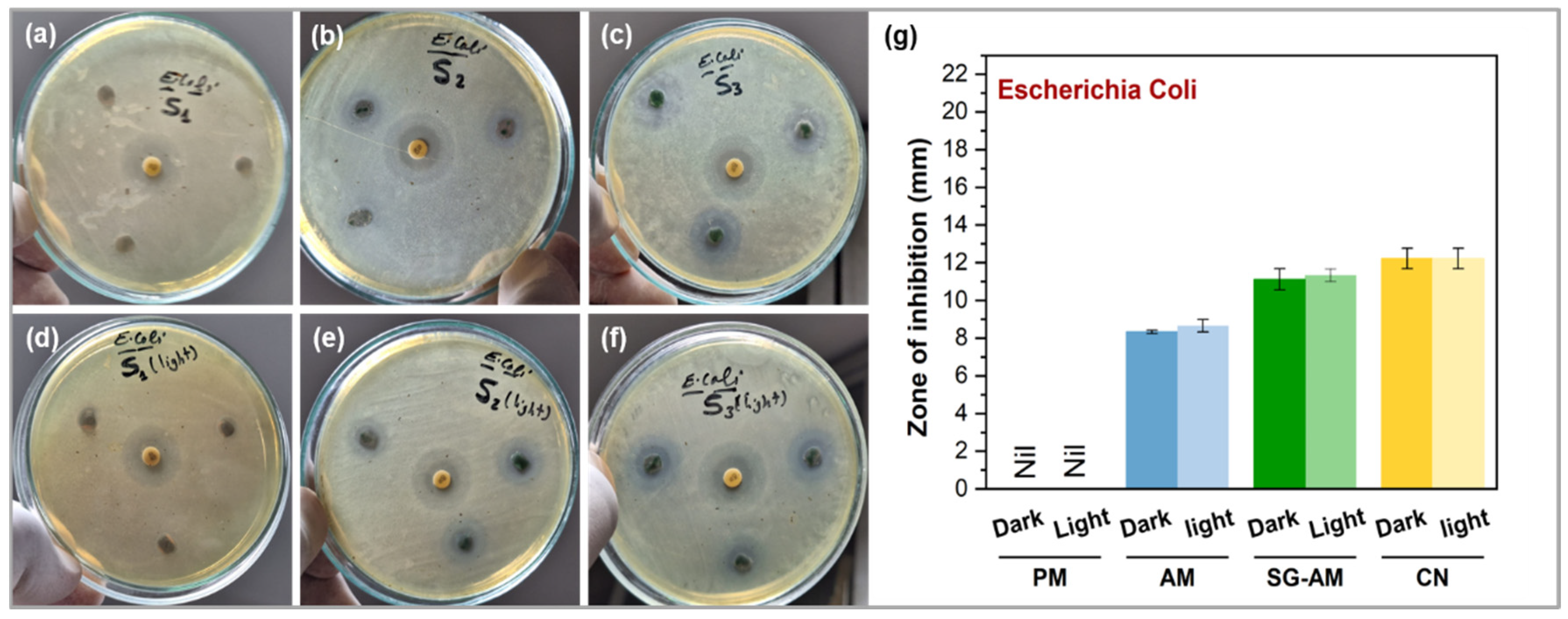
2.5. Antibacterial Activity Assessment
3. Results and Discussion
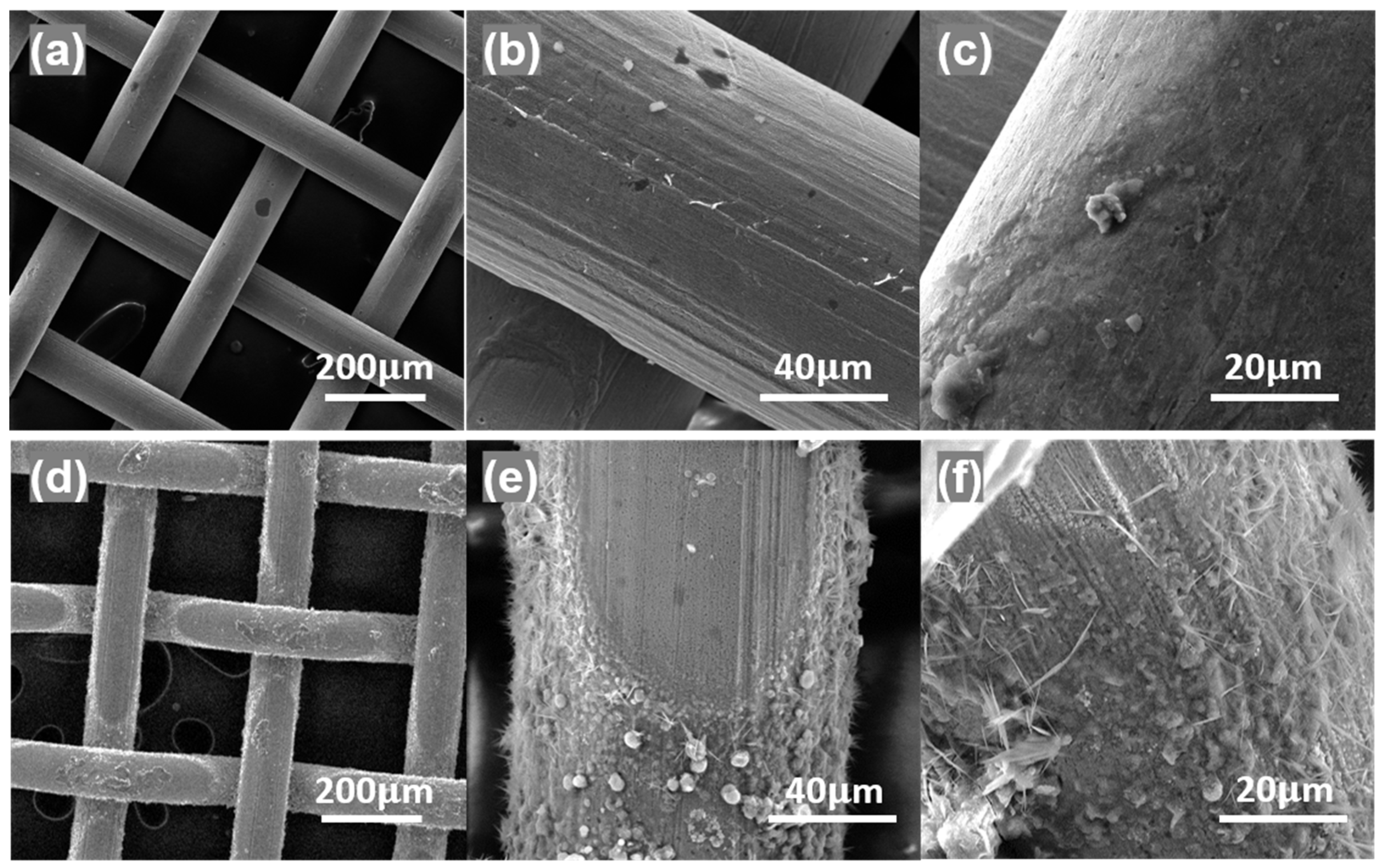
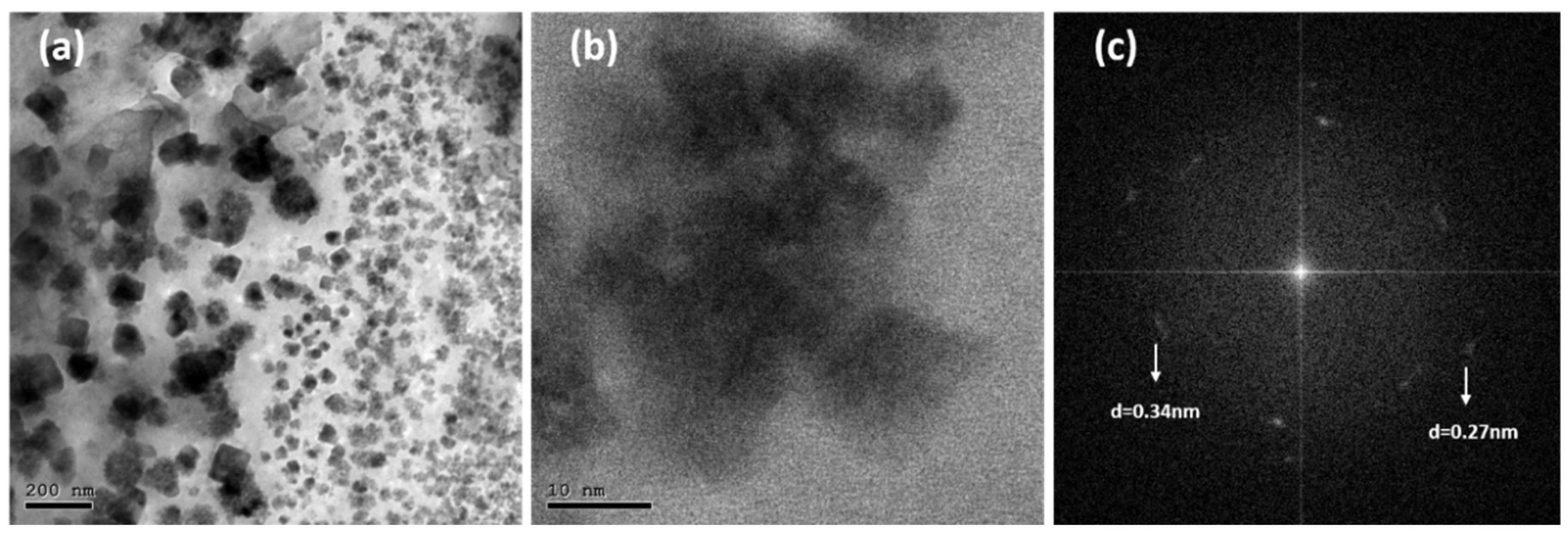
3.1. Structural and Morphological Properties
3.2. Water Treatment Application
3.3. Antibacterial Activity Against S. aureus
3.4. Antibacterial Activity Against E. coli
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNESCO. Imminent Risk of a Global Water Crisis, Warns the UN World Water Development Report 2023. Pressed on 22 March 2023. Available online: https://www.unesco.org/en/articles/imminent-risk-global-water-crisis-warns-un-world-water-development-report-2023 (accessed on 2 July 2025).
- Khan, S.; Sayed, M.; Sohail, M.; Shah, L.A.; Raja, M.A. Chapter 6—Advanced Oxidation and Reduction Processes. In Advances in Water Purification Techniques; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–164. [Google Scholar]
- Sadeghfar, F.; Ghaedi, M.; Zalipour, Z. Chapter 4—Advanced oxidation. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 225–324. [Google Scholar]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi B 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Sun, S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawhari, H.; Al-Murashi, R.; Abu Saba, L.; Alhebshi, N.; Altuwirqi, R. Effective degradation of MB under natural daylight using green synthesized Cu-Cu2O composite films. Mater. Lett. 2019, 254, 233–236. [Google Scholar] [CrossRef]
- Alhebshi, N.; Huang, H.; Ghandour, R.; Alghamdi, N.K.; Alharbi, O.; Aljurban, S.; He, J.-H.; Al-Jawhari, H. Green synthesized CuxO@Cu nanocomposites on a Cu mesh with dual catalytic functions for dye degradation and hydrogen evaluation. J. Alloys Compd. 2020, 848, 156284. [Google Scholar] [CrossRef]
- Murugadoss, G.; Rajesh Kumar, M.; Kuppusami, P. Synthesis of Cu2O-Cu(OH)2 nanocomposite from CuSCN precursor by a facile chemical precipitation method. Mater. Lett. 2021, 284, 128866. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Hu, Y.; Liu, Y.; Zhang, S.; Zhou, Y.; Zhang, Y.; Liu, Y.; Li, B. The preparation of Janus Cu(OH)2@Cu2O/Cu mesh and application in purification of oily wastewater. Mater. Res. Bull. 2020, 126, 110815. [Google Scholar] [CrossRef]
- Ye, S.; Cao, Q.; Wang, Q.; Wang, T.; Peng, Q. A highly efficient, stable, durable, and recyclable filter fabricated by femtosecond laser drilling of a titanium foil for oil-water separation. Sci. Rep. 2016, 6, 37591. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Al-Jawhari, H.A.; Alhebshi, N.A. Green Synthesized Cu2O-Cu(OH)2@Cu Nanocomposites with Fenton-like Catalytic Properties for the Degradation of Cationic and Anionic Dyes. Crystals 2022, 12, 1328. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R.; Safa, S.; Hasani, E. CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts. J. Mater. Chem. 2011, 21, 9634–9640. [Google Scholar] [CrossRef]
- Schio, A.L.; de Lima, M.S.; Frassini, R.; Scariot, F.J.; Cemin, F.; Elois, M.A.; Alvarez, F.; Michels, A.F.; Fongaro, G.; Roesch-Ely, M.; et al. Light, Copper, Action: Visible-Light Illumination Enhances Bactericidal Activity of Copper Particles. ACS Biomater. Sci. Eng. 2024, 10, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Tong, Z.-H.; Chen, J.-J.; Li, L.-L.; Yu, H.-Q. Morphology-dependent antimicrobial activity of Cu/CuxO nanoparticles. Ecotoxicology 2015, 24, 2067–2072. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Van, T.-K.; Pawar, A.U.; Kim, C.W.; Kang, Y.S. One-step transformation of Cu to Cu2O in alkaline solution. RSC Adv. 2014, 4, 18616–18620. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, Y.; Li, M.; Li, L.; Dong, L. In situ synthesis and assembly of copper oxide nanocrystals on copper foil via a mild hydrothermal process. J. Mater. Chem. 2006, 16, 192–198. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Li, X.; Liu, X.; Zhang, C.; Xu, H.; Zhao, X.S. Degradation of Organic Dyes over Fenton-Like Cu2O–Cu/C Catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Zhu, G.; Jin, Y.; Ge, M. Simple and green method for preparing copper nanoparticles supported on carbonized cotton as a heterogeneous Fenton-like catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 128978. [Google Scholar] [CrossRef]
- Crick, C.R.; Parkin, I.P. Preparation and characterisation of super-hydrophobic surfaces. Chemistry 2010, 16, 3568–3588. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sheng, Y.-J.; Tsao, H.-K. Facile fabrication of superhydrophobic copper mesh for oil/water separation and theoretical principle for separation design. J. Taiwan Inst. Chem. Eng. 2018, 87, 150–157. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Y.; Duan, C.; Wang, B. Fabrication of a superhydrophobic mesh based on PDMS/SiO2 nanoparticles/PVDF microparticles/KH-550 by one-step dip-coating method. RSC Adv. 2018, 8, 16251–16259. [Google Scholar] [CrossRef] [PubMed]
- Al-Rehaili, L.J.; Altuwirqi, R.M.; Aljarb, A.A.; Al-Jawhari, H.A. A superhydrophobic graphene@copper mesh irradiated by laser for efficient oil/water separation. Surf. Coat. Technol. 2023, 463, 129495. [Google Scholar] [CrossRef]
- Yu, Z.-P.; Zhan, B.; Dong, L.-M.; Jiang, W.; Song, Y.-Y.; Hu, S.-A. Self-Healing Structured Graphene Surface with Reversible Wettability for Oil–Water Separation. ACS Appl. Nano Mater. 2019, 2, 1505–1515. [Google Scholar] [CrossRef]
- Li, H.; Su, Z.; Hu, S.; Yan, Y. Free-standing and flexible Cu/Cu2O/CuO heterojunction net: A novel material as cost-effective and easily recycled visible-light photocatalyst. Appl. Catal. B 2017, 207, 134–142. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Kiwi, J. Polystyrene CuO/Cu2O uniform films inducing MB-degradation under sunlight. Catal. Today 2017, 284, 77–83. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Zarghami, Z.; Rahmatolahzadeh, R.; Ramezani, M. Solvent free synthesis of Cu-Cu2O nanocomposites via green thermal decomposition route. Adv. Powder Technol. 2017, 28, 2078–2086. [Google Scholar] [CrossRef]
- Xu, L.; Srinivasakannan, C.; Peng, J.; Zhang, L.; Zhang, D. Synthesis of Cu-CuO nanocomposite in microreactor and its application to photocatalytic degradation. J. Alloys Compd. 2017, 695, 263–269. [Google Scholar] [CrossRef]
- Jiang, D.; Xue, J.; Wu, L.; Zhou, W.; Zhang, Y.; Li, X. Photocatalytic performance enhancement of CuO-Cu2O heterostructures for photodegradation of organic dyes- Effects of CuO morphology. Appl. Catal. B 2017, 211, 199–204. [Google Scholar] [CrossRef]
- Bilal, M.; Ikram, M.; Shujah, T.; Haider, A.; Naz, S.; Ul-Hamid, A.; Naz, M.; Haider, J.; Shahzadi, I.; Nabgan, W. Chitosan-Grafted Polyacrylic Acid-Doped Copper Oxide Nanoflakes Used as a Potential Dye Degrader and Antibacterial Agent: In Silico Molecular Docking Analysis. ACS Omega 2022, 7, 41614–41626. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Gebreslassie, Y.T.; Gebremeskel, F.G. Green and cost-effective biofabrication of copper oxide nanoparticles: Exploring antimicrobial and anticancer applications. Biotechnol. Rep. 2024, 41, e00828. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Venkatesan, R.; Deepa, S.; Sana, S.S.; Arumugam, S.; Karami, A.M.; Vetcher, A.A.; Kim, S.-C. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci. Rep. 2023, 13, 18838. [Google Scholar] [CrossRef]
- Huong, L.M.; Nam, N.T.H.; Dat, N.T.; Dat, N.M.; Cong, C.Q.; Ngan, L.T.; Ngan, H.T.K.; An, H.; Tai, L.T.; Hung, P.N.P.; et al. Antibacterial mechanism of phyto-synthesized CuO-decorated ZnO nanostructure in relation to hydrogen peroxide generation under visible-light condition. Surf. Interfaces 2023, 40, 102988. [Google Scholar] [CrossRef]
| Bacteria | Illumination | Inhibition Zone (mm) | |||
|---|---|---|---|---|---|
| PM | AM | SG-AM | CN | ||
| S. aureus | Dark | nil | 14.44 ± 1.11 ** | 16.59 ± 0.61 ** | 17.07 ± 0.22 |
| Light | 14 ± 0.33 | 17.06 ± 0.59 * | 19.41 ± 1.02 * | 17.69 ± 0.49 | |
| E. coli | Dark | nil | 8.33 ± 0.09 ** | 11.11 ± 0.56 ** | 12.22 ± 0.55 |
| Light | nil | 8.66 ± 0.33 ** | 11.34 ± 0.33 ** | 12.22 ± 0.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jawhari, H.; Alhebshi, N.A.; Sait, R.; Altuwirqi, R.; Alrehaili, L.; Al-Ahmadi, N.; Elbialy, N. Multifunctional Green-Synthesized Cu2O-Cu(OH)2 Nanocomposites Grown on Cu Microfibers for Water Treatment Applications. Micro 2025, 5, 33. https://doi.org/10.3390/micro5030033
Al-Jawhari H, Alhebshi NA, Sait R, Altuwirqi R, Alrehaili L, Al-Ahmadi N, Elbialy N. Multifunctional Green-Synthesized Cu2O-Cu(OH)2 Nanocomposites Grown on Cu Microfibers for Water Treatment Applications. Micro. 2025; 5(3):33. https://doi.org/10.3390/micro5030033
Chicago/Turabian StyleAl-Jawhari, Hala, Nuha A. Alhebshi, Roaa Sait, Reem Altuwirqi, Laila Alrehaili, Noorah Al-Ahmadi, and Nihal Elbialy. 2025. "Multifunctional Green-Synthesized Cu2O-Cu(OH)2 Nanocomposites Grown on Cu Microfibers for Water Treatment Applications" Micro 5, no. 3: 33. https://doi.org/10.3390/micro5030033
APA StyleAl-Jawhari, H., Alhebshi, N. A., Sait, R., Altuwirqi, R., Alrehaili, L., Al-Ahmadi, N., & Elbialy, N. (2025). Multifunctional Green-Synthesized Cu2O-Cu(OH)2 Nanocomposites Grown on Cu Microfibers for Water Treatment Applications. Micro, 5(3), 33. https://doi.org/10.3390/micro5030033







