Abstract
The production of lactic acid (LA) through the microbial conversion of agro-industrial residuals is an important process in the biotechnology industry. The growth kinetics of 30 strains of lactic acid bacteria (LAB) isolated from agro-industrial residues were determined and nine strains were selected for microbioreactor fermentation. Lactiplantibacillus pentosus_70-1 (1.662) and L. pentosus_19-2 (1.563) showed the highest OD600 values, whereas the highest growth rates were observed for L. pentosus_19-2 (0.267 h−1) and Weissella soli_31 (0.256 h−1). The production of LA and acetic acid (AA), glucose consumption, and metabolic profiles were determined, without finding significant differences in the production of LA; however, W. soli_29 produced the highest amount of LA (20.833 gL−1) and was able to metabolize most of the studied carbohydrates. Based on these results, W. soli_29 was chosen for a 20 h fermentation in a 7 L bioreactor using both standard medium and milk whey supplemented medium. W. soli_29 produced 16.27 gL−1 and 7.21 gL−1 of LA in each of these mediums, respectively. These results show the underlying potential of Weissella strains for biotechnological applications. Additional analysis which should contemplate different agro-industrial residues and other conditions in bioreactors must be carried out.
1. Introduction
Lactic acid bacteria (LAB) are used in biorefineries for their ability to metabolize a large number of substrates to produce high-value compounds of industrial interest, including food ingredients and pharmaceutical precursors [1,2]. In addition to this, LAB have become promising cell factories due to their Generally Recognized as Safe (GRAS) or Qualified Presumption of Safety (QPS) statuses assigned by the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA), respectively. Metabolites, such as lactic acid (LA), bacteriocins, antioxidants, vitamins, and other inner- and outer-membrane compounds, are part of the products that make LAB attractive at a biotechnological level [3]. A significant number of LAB strains with biotechnological potential have been isolated from non-traditional sources [4], including fermented foods and agro-industrial wastes [5,6]. In Latin America (specifically in Costa Rica), the application of LAB in biorefineries has been little exploited to date, despite the diversity of microorganisms present in different microenvironments which could have great potential for the isolation of LAB [7].
LA is an organic molecule that is in growing demand worldwide due to its applications associated with the production of polylactic acid (PLA) [8]. The earliest uses of LA in the food field were related to acidulation and preservation, where LAB cells were incorporated into certain food products to promote natural LA production and control the growth of spoilage microorganisms during storage [9]. Today, the approach to using LA in food applications is similar. However, technological advances in strain selection, fermentation, purification, and formulation allow high-purity LA to be added directly to a food matrix. As a result, a large number of LAB strains have been studied; nevertheless, some genera remain less well explored and could have great potential in the biotechnology industry, as is the case with the genus Weissella.
The Food and Agriculture Organization of the United Nations (FAO-ONU) indicates that the economic loss related to food waste is estimated at USD 1 trillion per year. The beverage industries lead with the generation of around 26% of total food waste, followed by the dairy industry with 21%. Besides the economic threat, unused food waste contributes to environmental pollution [10,11]. Glucose, sucrose, and lactose are characterized as the primary carbon sources for LA production. However, there is a growing interest in using low-cost renewable carbon sources and taking advantage of by-products such as those obtained from the food industry, including cassava bagasse, sugar cane molasses, milk whey (MW), and starch residues [12]. The amount of MW obtained as a by-product of cheese manufacturing has been estimated at 1.9 × 108 ton/year. Due to the high Biochemical Oxygen Demand (BOD) and high Chemical Oxygen Demand (COD), MW depletes dissolved oxygen in water, posing a major risk to aquatic life [13]. Currently, several uses have been proposed to add value to MW, such as lactose recovery [14], whey-based beverages [15], and farm-animal feeding [16], among others.
Within the framework of bioeconomy, the use of agro-industrial waste is proposed for the production of high-added-value compounds. The accumulation of waste in agribusiness is a global concern. The linear system of the economy, which consists of extraction, manufacture, use, and disposal, has reached its maximum limit. For this reason, the circular economy has been introduced as a practice in the agro-industry. Therefore, the current research integrates a circular economy concept, since the LAB used in the experimental work were isolated from agro-industrial waste residues. The objective of this research was to evaluate LAB isolates for the production of metabolites of interest, selecting a strain for further fermentation in a 7 L bioreactor using standard medium and MW.
2. Materials and Methods
2.1. Lactic Acid Bacteria
A total of 30 LAB from agro-industrial waste were submitted by the Food Microbiology Collection of the Center for Research in Tropical Diseases (CIET) at the Faculty of Microbiology and the Bacteriology Collection at the National Center in Food Science and Technology (CITA), University of Costa Rica. All strains were previously identified by 16S rRNA sequencing [17], and the sequences were submitted to GenBank. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 14 June 2022) (Table 1). All accessions were Gram-stained and purity was confirmed. Cultures were stored in glycerol (20% (v/v)) at −80 °C until analysis.

Table 1.
Maximum optical density (OD600) and growth rate (h−1) * of lactic acid bacteria (LAB) strains isolated from agro-industrial residuals.
2.2. LAB Growth Curves
Growth kinetics (maximal growth and µ max) were determined for each of the 30 LAB isolates. A bacterial suspension was prepared for each bacterium in 10 mL of DeMan, Rogosa, and Sharpe broth (MRS) (Thermo Scientific™ Oxoid™, Lenexa, KS, USA) and incubated at 37 ± 1 °C overnight, which was diluted to attain an initial absorbance of 0.05 at 600 nm (OD600). A volume of 250 µL of each bacterial suspension was incubated at 37 °C using a 96-well microplate. Three replicates of each sample were made for each of three repetitions for each bacterium. MRS broth without inoculum was used as a control. Bacterial growth measurements (OD600) were performed every 15 min using a microplate reader (Biotek, Winnoski, VT, USA) [18].
2.3. Metabolite Production
A total of nine LAB strains that showed the best growth parameters were selected for microbioreactor fermentation (Applikon Biotechnology, Delft, Holland) with a fermentation volume of 6 mL in MRS medium (Oxoid™) with an initial pH of 6.8 and an initial absorbance (OD600) of 0.05. The incubation was carried out at 37 ± 1 °C for 24 h under static conditions. Samples were cultured in triplicate. In addition, MRS medium without inoculum was used as a negative control. At the end of the fermentation, each sample was centrifuged at 10,000× g for 5 min. The supernatant was recovered and used to determine LA, AA, and glucose consumption.
For LA and AA analysis, the samples were previously cleaned using Oasis HLB cartridges (Waters, Milford, MA, USA) and then filtered using a 0.45 µm membrane. The determination of LA and AA was performed using a high-performance liquid chromatography (HPLC) system (Shimadzu LC-20AT quaternary pump, Shimadzu SPD-M20A photodiode array detector, Shimadzu CTO-6A column oven, Shimadzu SIL-20A HT autosampler, Agilent Hi-Plex H column, 300 × 7.8 mm, 8 µm) employing isocratic conditions at 0.50 mL/min, which consisted of 0.00225 mol/L H2SO4 in water over 25 min at 60 °C, using an acquisition wavelength of 210 nm and an injection volume of 3 µL. The retention times for LA and AA were 21.9 and 17.9 min, respectively.
For the quantification of residual sugars, the sample was extracted using the method described by the ISO 11868:2007, and the product of this extraction was filtered using a 0.45 µm membrane prior to its HPLC analysis on an Agilent 1260 Infinity HPLC system (G1311B quaternary pump, G1362A refraction index detector, G1316A column oven, G7129A autosampler, Zorbax Carbohydrate column, 150 × 4.6 mm, 5 µm) [19]. The samples were eluted using isocratic conditions at 1.2 mL/min, consisting of 75% acetonitrile in water over 8 min at 30 °C, injecting 5 µL of sample. The retention times for glucose and saccharose were 3.59 and 5.36 min, respectively.
2.4. Metabolic Profile
Each LAB was inoculated on MRS agar plates and incubated for 24 h at 37 ± 1 °C for 24 h. The API® 50 CHL Medium kit (Biomerieux, Madrid, Spain) was used to evaluate the carbohydrate fermentation profile of the nine selected strains. A colony of each bacterium was inoculated in API® 50 CHL MediumTM (Biomerieux). The samples were incubated under aerobic conditions for 48 h, and the results were obtained after reading the color changes. MRS medium was used as a negative control.
2.5. Bioreactor Fermentation
The bacterial suspension of the selected strain was diluted to an initial absorbance of 0.05 at 600 nm (OD600) and subsequently fermented in a 7 L stirred-tank bioreactor (Applikon, Delft, The Netherlands) with an operating volume of 4 L, 0.5 vvm, 200 rpm, and a pH of 6.8 (maintained by the automatic addition of 20% NaOH). The temperature was set at 37 ± 1 °C for 20 h, and 10 mL samples were taken every 2 h. Two different culture media were tested. First, a reference culture medium containing a mixture of 10 g L−1 glucose, fructose, sucrose, and yeast extract (15 g L−1) was employed [20]. Additionally, MW was collected from a local farmer, and a bioreactor fermentation was performed using the selected strain in fresh MW (pH 4.6) supplemented with K2HPO4 (0.5 g L−1) and KH2PO4 buffers (0.5 g L−1), pH 6.5–6.8, and yeast extract (10 g L−1). Before being used, the MW was pasteurized at 75 °C for 5 min in a kettle (Westfalia-GEAS, Bonn, Germany), packed manually using DoyPack bags and stored at −20 °C [19].
The samples taken from each fermentation were centrifuged at 10,000× g for 5 min at room temperature. The supernatant was filtered using a 0.20 µm nylon filter and frozen at −20 °C until analysis. The production of LA, AA, and residual sugars was quantified as described above. LA yield and maximum productivity were estimated in terms of grams of LA per liter of fermentation medium per hour [21].
2.6. Data Analysis
A one-way analysis of variance (ANOVA) and a Tukey test with 95% confidence were performed employing the Minitab software package (version 18) (Pen State, PA, USA). A comparison was made among the averages of absorbance, growth rate, organic acid production, and glucose consumption values. The statistical analysis was performed to determine the strains that presented the highest values of maximum optical density (OD600) and growth rates (h−1).
3. Results
3.1. Growth Kinetics and Bioreactor Fermentation
After 24 h of growth, no significant differences were found for most isolates by OD600 evaluation. However, the species of L. pentosus_70-1 and L. pentosus_19-2 showed the highest OD600 values (1.662 ± 0.011 and 1.563 ± 0.091, respectively), while the minimum OD600 value was reported for the isolate of W. soli_30-2 (0.401 ± 0.226) (Table 1). The growth kinetics (growth rate, h−1) were also determined. The isolates that presented the highest rates were L. pentosus_19-2 (0.267 ± 0.016), followed by W. soli_31 (0.256 ± 0.021). On the other hand, the species that presented the lowest growth rate was W. soli_30-2 (0.048 ± 0.024). In all cases, significant differences were found among the strains under study (p < 0.05). The results of the Tukey test are included in Table 1.
3.2. Growth Kinetics and Microbioreactor Fermentation
A total of nine strains showing the highest growth rates and OD600 combinations were selected for microbioreactor fermentation (OD600 ≥ 1.457). The isolates W. soli_29, L. pentosus_70-1, and L. pentosus_69 presented the highest LA production (g/L) and AA production (g/L). Lower values were observed in the case of LA for L. pentosus_19-2 and in the case of AA for W. soli_31. However, no significant differences were observed for the analyzed samples in the case of LA and AA (Table 2).

Table 2.
Lactic acid (LA) and acetic acid (AA) production, and glucose consumption of selected lactic acid bacteria (LAB) evaluated in a microbioreactor using an DeMan, Rogosa, and Sharpe medium (MRS).
3.3. Sugar Metabolism
The isolates W. soli_29 and L. pentosus_17-2 were able to metabolize most of the sugars from the tested panel (Figure 1). These species are characterized by the transformation of monosaccharides, such as D-arabinose and la D-ribose, as well as D-galactose, D-glucose, and D-fructose, among others; they are also able to reduce some disaccharides, such as D-maltose and D-lactose, and some complex substrates, such as D-raffinose and starch. On the other hand, few isolates (L. pentosus_70-1 and L. paracasei subsp. tolerans_P10) were able to metabolize just one or two sugars from the panel.
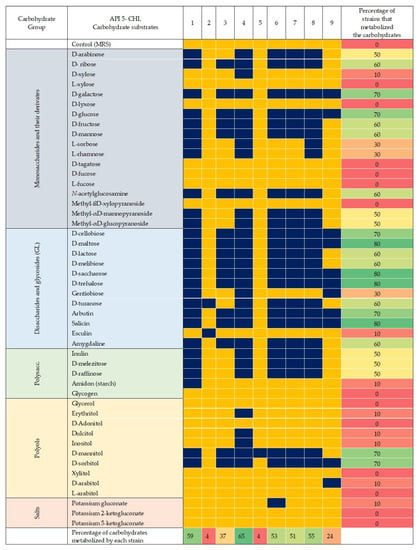
Figure 1.
Heat map with the fermentation profiles of 48 carbohydrates (API 50 CHL) corresponding to nine strains of lactic acid bacteria (LAB). The carbohydrates considered are grouped into monosaccharides and their derivatives: disaccharides and glycosides (GL), polysaccharides, polyols, and salts. Dark blue coloring indicates a positive fermentation, i.e., LAB can metabolize the corresponding carbohydrate. In contrast, yellow coloring indicates failure to conduct to fermentation. The percentage of strains that can metabolize the carbohydrate is shown on the right, while the percentage of carbohydrate that each LAB can metabolize is indicated at the bottom. DeMan, Rogosa, and Sharpe medium (MRS) was used as a control. Gradient scale: 0% (red)—100% (green).
3.4. Biorreactor Fermentation Using Synthetic Medium and Milk Whey
The isolate W. soli_29 was selected for fermentation using standard culture medium and MW. This strain presented some of the highest LA values 20.833 ± 2.731 (g/L) during growth and has the capacity to ferment some sugars commonly present in agro-industrial residuals, such as D-galactose (Figure 1). The fermentation experiments showed that a higher production of LA was obtained with the synthetic medium (approximately 44% higher) when compared to MW, with values of 16.27 ± 2.301 gL−1, and a productivity of 3.214 g/h (Figure 2A). However, when the MW-supplemented medium was used, the LA production was 7.21 ± 1.355 gL−1, and the productivity went down to 0.922 g/h (Figure 2B). Interestingly, the optical density was similar in both cases, with values of 7.076 ± 0.006 in the case of the synthetic medium and 7.012 ± 0.337 for the MW.
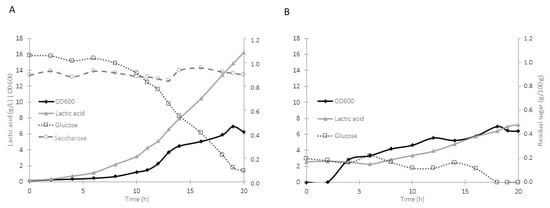
Figure 2.
(A) Growth kinetics of strain W. soli_29 using a synthetic medium with yeast extract and salts. (B) Growth kinetics of strain W. soli_29 using milk whey with yeast extract and salts. A stirred tank batch bioreactor (7 L, with an operating volume of 4 L, 0.5 vvm, a pH of 6.8, and a temperature of 37 °C) was used for both fermentations. Data are expressed as mean values obtained from triplicate experiments.
4. Discussion
It is estimated that the LA market could reach a value of USD 2,640 million since it has potential for the food, cosmetic, and pharmaceutical industries [22]. However, one of the main challenges is to reduce the costs associated with microbial LA production by 50% in order to compete with the petrochemical production of this compound [23]. For this purpose, the isolation and assessment of new bacterial strains with robust characteristics, capable of producing large quantities of LA (>200 g/L) with high performance (>95%) and productivity (>5.0 g/L·h) values, are needed. In addition, this LA production should be based on low-cost raw materials derived from responsible management of agro-industrial residues [22,24]. In this research, 30 bacterial isolates obtained from agro-industrial residues were used. A higher LA production was observed for the genera Lactiplantibacillus and Weissella when tested in a microbioreactor. The isolate W. soli_31 was selected for batch fermentation because it presented one of the highest LA production values, and it was able to ferment different types of sugars. This is not surprising, as some Weissella species have been isolated from fermented foods, such as fruits and vegetables, and meat and fish products, among others; this means that these species are capable of adapting to a variety of environments and substrates [25].
Currently, at least 25 Weissella species (family Leuconostocaceae) have been determined, and they are classified as facultative anaerobic chemoorganotrophs with an obligately fermentative metabolism. This genus has complex nutritional requirements and needs peptides, amino acids, fermentable carbohydrates, nucleic acids, fatty acids, and vitamins for growth [26,27]. This means that, given the proper supplementation, agro-industrial residuals may serve as functional substrates for this group, as shown in this study.
W. soli was able to metabolize LA from supplemented MW. To the best of our knowledge, this is the first time that W. soli has been identified as a bacterium with potential to produce LA and other metabolites. Within their genera, W. cibaria and W. confusa have been reported in fermentation processes [28,29,30], and W. cibaria has been identified as a species with probiotic properties capable of producing exopolysaccharides [30]. Under a batch fermentation procedure, it is possible that Weissella spp. may produce high quantities of LA with good performance as long as the carbon source is consumed entirely. Batch fermentation also presents the advantage of being a closed system where the possibilities of contamination are reduced and a total consumption of substrates can be assured [31]. Additionally, it is worth mentioning that W. soli was better at producing LA when compared with the homofermenters analyzed in this study; control of pH levels may explain this behavior and this could be used as a strategy to improve the performance of heterofermenters.
There is a lack of research in Costa Rica and Central America studying agro-industrial residues as sources of microorganisms of industrial importance and as substrates for producing metabolites such as LA [32]. In this research, MW, a residue from the cheese manufacturing process that can cause environmental problems if not treated properly before its disposal, was used as a substrate to produce LA [33]. The LA production values in MW obtained in this research (7.21 ± 1.355 gL−1) were similar to those reported by Zannini et al. (7.46 gL−1) with W. cibaria MG1 using quinoa flour as a fermentation substrate [30]. However, the current results were lower than those previously reported after fermentation of agro-industrial residues with W. cibaria (44.5 gL−1) [29]. Furthermore, in that study, the LA production values in MW were higher when worm meal and MRS were used as nutrient sources for fermentation with W. confusa (4.79 and 4.33 gL−1, respectively) [34]. These results suggest that agro-industrial residues could help reduce the cost of LA production given the ease of access to these alternative substrates [35]. However, the variety of responses confirms the importance of the study of alternative and novel substrates. In addition, it is necessary to optimize processing conditions (pH, protein content, and nitrogen sources) in order to maximize the productivity and the amount of biomass obtained. Furthermore, W. soli could be applied in combination with other BAL species in order to increase LA yield through synergistic mechanisms [35,36].
Author Contributions
Conceptualization, N.B. and J.A.M.-V.; methodology, J.M.-Z. and S.F.-F.; writing—original draft preparation, J.M.-Z. and N.B.; writing—review and editing, M.R.-S. and B.M.-V.; project administration, N.B. and J.A.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Center for High Technology of Costa Rica (CeNAT-CONARE Scholarship Program 2020), the Center for Research in Tropical Diseases (CIET-UCR), the National Center in Food Science and Technology of University of Costa Rica (CITA-UCR), and the University of Costa Rica (Project 735-B9-457 and 735-C1-512).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Vanny Mora for her technical support during metabolic profile analysis and thanks to Daniela Jaikel for check the spelling and grammar of this document.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazzoli, R.; Bosco, F.; Mizrahi, I.; Bayer, E.A.; Pessione, E. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014, 32, 1216–1236. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chan, S.H.J.; Chen, J.; Solem, C.; Jensen, P.R. Systems biology—A guide for understanding and developing improved strains of lactic acid bacteria. Front. Microbiol. 2019, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-product lactic acid bacteria fermentations: A review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Champagne, C.P. The lactic acid bacteria. Int. Dairy J. 1994, 4, 665–666. [Google Scholar] [CrossRef]
- Sakr, E.A.E.; Massoud, M.I.; Ragaee, S. Food wastes as natural sources of lactic acid bacterial exopolysaccharides for the functional food industry: A review. Int. J. Biol. Macromol. 2021, 189, 232–241. [Google Scholar] [CrossRef]
- Börner, R.A.; Kandasamy, V.; Axelsen, A.M.; Nielsen, A.T.; Bosma, E.F. Genome editing of lactic acid bacteria: Opportunities for food, feed, pharma and biotech. FEMS Microbiol. Lett. 2019, 366, fny291. [Google Scholar] [CrossRef]
- Wen Fang Wu Wu, J.; Redondo-Solano, M.; Uribe, L.; WingChing-Jones, R.; Usaga, J.; Barboza, N. First characterization of the probiotic potential of lactic acid bacteria isolated from Costa Rican pineapple silages. PeerJ 2021, 9, e12437. [Google Scholar] [CrossRef]
- Eş, I.; Mousavi Khaneghah, A.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production—A review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food waste biorefinery: Pathway towards circular bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Inno. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Chen, G.Q.; Leong, T.S.H.; Kentish, S.E.; Ashokkumar, M.; Martin, G.J.O. Membrane separations in the dairy industry. In Separation of Functional Molecules in Food by Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–304. ISBN 978-0-12-815056-6. [Google Scholar]
- León-López, A.; Pérez-Marroquín, X.A.; Campos-Lozada, G.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Characterization of whey-based fermented beverages supplemented with hydrolyzed collagen: Antioxidant activity and bioavailability. Foods 2020, 9, 1106. [Google Scholar] [CrossRef]
- Barba, F.J. An integrated approach for the valorization of cheese whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucl Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.A.; Ibrahim, S.A. Current limitations and challenges with lactic acid bacteria: A review. Food Nutr. Sci. 2013, 4, 73–87. [Google Scholar] [CrossRef]
- Montero-Zamora, J.; Cortés-Muñoz, M.; Esquivel, P.; Mora-Villalobos, J.; Velázquez, C. Growth conditions and survival kinetics during storage of Lactobacillus rhamnosus GG for the design of a sustainable probiotic whey-based beverage containing costa rican guava fruit pulp. J. Food Sci. 2020, 85, 3478–3486. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Venus, J. Batch and continuous lactic acid fermentation based on a multi-substrate approach. Microorganisms 2020, 8, 1084. [Google Scholar] [CrossRef]
- Soares, M.; Christen, P.; Pandey, A.; Soccol, C.R. Fruity flavour production by ceratocystis fimbriata grown on coffee husk in solid-state fermentation. Process Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- Cox, R.; Narisetty, V.; Nagarajan, S.; Agrawal, D.; Ranade, V.V.; Salonitis, K.; Venus, J.; Kumar, V. High-level fermentative production of lactic acid from bread waste under non-sterile conditions with a circular biorefining approach and zero waste discharge. Fuel 2022, 313, 122976. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.; Zhang, Y.; Chen, Y.; Hang, H.; Chu, J.; Zhuang, Y. Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresour. Technol. 2021, 323, 124549. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef]
- Teixeira, C.G.; Fusieger, A.; Milião, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An emerging bacterium with promising health benefits. Probiotics Antimicro. Prot. 2021, 13, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some Leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Micanquer-Carlosama, A.; Cortés-Rodríguez, M.; Serna-Cock, L. Formulation of a fermentation substrate from pineapple and sacha inchi wastes to grow Weissella cibaria. Heliyon 2020, 6, e03790. [Google Scholar] [CrossRef]
- Micanquer-Carlosama, A.; Cortés-Rodríguez, M.; Correa-Londoño, G.; Orozco-Sánchez, F.; Serna-Cock, L. Optimization of the reproduction of Weissella cibaria in a fermentation substrate formulated with agroindustrial waste. Biotechnol. Rep. 2021, 32, e00671. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef]
- Dedenaro, G.; Costa, S.; Rugiero, I.; Pedrini, P.; Tamburini, E. Valorization of agri-food waste via fermentation: Production of L-lactic acid as a building block for the synthesis of biopolymers. Appl. Sci. 2016, 6, 379. [Google Scholar] [CrossRef]
- Araya-Cloutier, C.; Rojas-Garbanzo, C.; Velázquez-Carrillo, C. Effect of initial sugar concentration on the production of L (+) lactic acid by simultaneous enzymatic hydrolysis and fermentation of an agro-industrial waste product of pineapple (Ananas comosus) using Lactobacillus casei subspecies rhamnosus. Int. J. Biotech Well Ind. 2012, 1, 91–100. [Google Scholar] [CrossRef][Green Version]
- Alvarez, M.M.; Aguirre-Ezkauriatza, E.J.; Ramírez-Medrano, A.; Rodríguez-Sánchez, A. Kinetic analysis and mathematical modeling of growth and lactic acid production of Lactobacillus casei var. rhamnosus in milk whey. J. Dairy Sci. 2010, 93, 5552–5560. [Google Scholar] [CrossRef] [PubMed]
- Serna, L.; Rengifo, C.A.; Rojas, M.A. The use of earthworm flour for lactic acid biomass production. Afr. J. Biotechnol. 2013, 12, 5962–5967. [Google Scholar]
- Zhang, Z.; Tsapekos, P.; Alvarado-Morales, M.; Kovalovszki, A.; Yang, X.; Zhu, X.; Angelidaki, I. Improving lactic acid production via bio-augmentation with acid-tolerant isolates from source-sorted organic household waste. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Xiang, W.-L.; Zhang, N.-D.; Lu, Y.; Zhao, Q.-H.; Xu, Q.; Rao, Y.; Liu, L.; Zhang, Q. Effect of Weissella cibaria co-inoculation on the quality of sichuan pickle fermented by Lactobacillus plantarum. LWT 2020, 121, 108975. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).