Abstract
While dietary supplements can have beneficial effects on the health of the intestine, these effects can come with unresolved issues in terms of therapeutic efficacy and mechanisms of action. In this study, the model probiotic Lacticaseibacillus rhamnosus GG ATCC 53103 and the anciently used dietary supplement Limnospira indica strain PCC 8005 were compared for their effects on murine intestinal ecology. Healthy male mice received either saline or suspensions of living cells of L. indica PCC 8005 or L. rhamnosus GG daily along a two-week intervention period, followed by a two-week washout period. Both bacteria-based solutions appeared able to transiently shift the microbial community, which were characterized by a higher relative abundance of members of the butyrate producing Lachnospiraceae and Porphyromonadaceae families.
1. Introduction
The gastrointestinal tract harbors trillions of microbes and can collectively be considered as a complex yet functional organ mediating the health status of the host. Herein, the gut microbial community is evaluated in terms of its distribution, diversity and functionality. The composition of gut microbiota is frequently challenged by factors including diet, environment and host genetics. As a consequence, the term dysbiosis was introduced to define the qualitative and quantitative compositional alteration in intestinal microbiota, which favors emergence and outbreak of pathogens and has a cascading impact on the immune system [1]. Therefore, a dysbiotic community might be associated with the pathogenesis of many diseases, of which cancer and inflammatory bowel disease are well-known examples [2,3].
To sustain or restore the intestinal ecology, different microbial therapies have been investigated, including the dietary supplementation of (living) microbial products, transplantation of fecal microbiota or bacterial consortium, as well as bacteriocins and bacteriophages-based treatments. While transplantation of fecal microbiota or bacterial consortium, as well as bacteriocins and bacteriophages-based treatments, opt for a therapeutic approach, dietary supplements have shown to be more effective in preventing intestinal diseases [4]. Microbial dietary supplements have been reported to prevent and/or ameliorate intestinal diseases by influencing the growth of beneficial/pathogenic microorganisms, as well as immunomodulatory and/or barrier protective effects [4]. Nevertheless, (pre)clinical studies have shown inter-individual variability in microbial therapy responsiveness. These inconsistent results are likely explained by the lack of an evidence-based consensus in terms of dosing, formulation and route of administration, as well as the large knowledge gap concerning their mechanism of action and the functional relationship between microbial therapies, the commensal microbiota, the host and the claimed health effects. As such, no health claims for probiotics have yet been approved by the European Food Safety Authority. Although incredibly challenging, a reliable translation of mechanistic insights into measurable clinical effects will help us to deliver the appropriate dietary supplement needed to increase (pre)clinical successes [5].
One of the best-documented probiotic strains Lacticaseibacillus rhamnosus GG (before the recent taxonomic reclassification of the Lactobacillus genus complex known as Lactobacillus rhamnosus GG [6]), used as living lyophilized microbial product, was reported to exert intestinal radioprotection [7,8,9], and to attenuate diarrhea [10,11], colon cancer [12,13] and inflammatory bowel disease [14,15]. Still, important aspects concerning its efficacy and mechanism(s) of action remain to be defined.
Apart from traditional dietary supplements, we introduce anciently used cyanobacteria Limnospira indica PCC 8005 (also known as Arthrospira sp. or its generic product name Spirulina), since research has shown the beneficial effects of dried biomass and/or isolated bioactive compounds on the intestine’s antioxidant status, immune system and/or bacterial communities [16,17,18,19,20,21]. Typically, Limnospira spp. are used as dried and inactivated whole biomass products or extracts thereof. However, different processing and preservation steps were reported to impact the nutritional and antioxidant values of resulting Spirulina products [22]. To the best of our knowledge, the impact of unprocessed Limnospira spp. on the gut bacterial ecosystem has not yet been investigated using extensive 16S microbial profiling. Thus, we set out a comparative study, in which healthy mice were administered fresh, in-house prepared biomass of either L. indica PCC 8005 or L. rhamnosus GG ATCC 53103, or saline on a daily basis along a two-week intervention period, followed by a two-week washout period.
2. Materials and Methods
2.1. Mice
All animal experiments were approved by SCK CEN’s animal welfare committee and carried out in compliance with the Ethical Committee Animal Studies of Medanex Clinic (EC MxCl 2018-093), the Belgian laboratory animal legislation and the European Communities Council Directive of 22 September 2010 (2010/63/EU).
Five week-old, male C57Bl/6JRj mice were purchased from Janvier (Bio Services, Uden, The Netherlands). Upon arrival, they were housed individually in ventilated cages under standard laboratory conditions (12 h light/dark cycle) with ad libitum access to regular chow and water, and mice were acclimatized for two weeks.
Confounding factors including sex, age and housing conditions were minimized across experimental cohorts [23]. Potentially other confounding factors including caretaker(s), order of handling and differences in weight were assessed and excluded for their impact on the microbiome. Finally, to minimize differences between created groups, mice were randomly assigned to a pre-specified number of groups using the minDiff package in RStudio (v.3.5.0, RStudio, Boston, MA, USA).
2.2. Bacterial Strains and Growth Conditions
In this study, L. indica PCC 8005 substrain P1 possessing helically coiled trichomes was taken from the continuous SCK CEN culture collection, of which the mother strain was originally obtained from the Pasteur Culture collection of Cyanobacteria (PCC) (Institut Pasteur, Paris, France). L. indica PCC 8005 cultures were grown axenically in Zarrouk medium at pH ~9.8 on a Heidolph Unimax 2010 rotary orbital shaker (Analis SA, Suarlée, Belgium) at 120 rpm and a constant temperature of 30 °C in a Binder KBW400 growth chamber (Analis SA, Suarlée, Belgium) [24]. Cells were illuminated with a continuous photon flux density of 45 μmol photonsm−2 s−1 produced by Osram Daylight tubes (Osram, Zellik, Belgium).
In parallel, L. rhamnosus GG ATCC 53103, a strain originally isolated from human fecal samples [25,26], was obtained from Professor Sarah Lebeer (University of Antwerp, Antwerp, Belgium). This strain was grown statically in the dark at 37 °C in de Man, Rogosa and Sharpe (MRS) medium (BD, Olen, Belgium) [27].
To monitor cell growth, the optical density (OD) of L. indica PCC 8005 and L. rhamnosus GG cultures was measured at 750 nm and 600 nm, respectively, using a Thermo Spectronic Unicam Aquamate Helios Spectrophotometer (Thermofisher Scientific, Merelbeke, Belgium).
To prepare fresh bacterial biomass for supplementation, L. indica PCC 8005 cultures were grown to reach OD750 nm~1. Likewise, L. rhamnosus GG cultures were grown to reach OD600 nm~0.25. Briefly, bacterial cultures were centrifuged at 7500× g for 10 min at 4 °C. The pellet was washed three times using sterile saline solution and eventually dissolved in saline to reach the desired amount of bacteria per 200 μL for murine gavage.
2.3. Supplementation Protocol
After two weeks of acclimatization, mice were randomly distributed into three different supplementation groups (n = 10 per group); mice daily receiving (1) saline (200 μL/mouse) to rule out effects induced by repeated oral gavage; (2) L. rhamnosus GG (7 × 108 cells/mouse) and (3) L. indica PCC 8005 (3 × 107 cells/mouse of 20 g) solutions (Figure 1). Suspensions were administered daily by oral gavage in a maximum volume of 10 μL per grams body weight, in accordance with ethical recommendations. The supplementation dose used for L. rhamnosus GG was associated with probiotic effects [28,29,30]. The amount of L. indica PCC 8005 administered to mice corresponds to a dosing regimen of 800 mg/kg, previously reported to have antioxidative effects in vivo [31,32,33,34,35]. After the two-week intervention period, a two-week washout period was incorporated to assess the stability of microbial changes introduced by both interventions (Figure 1). Over the entire experimental setup, overall health was monitored, and body weight was recorded every other day.
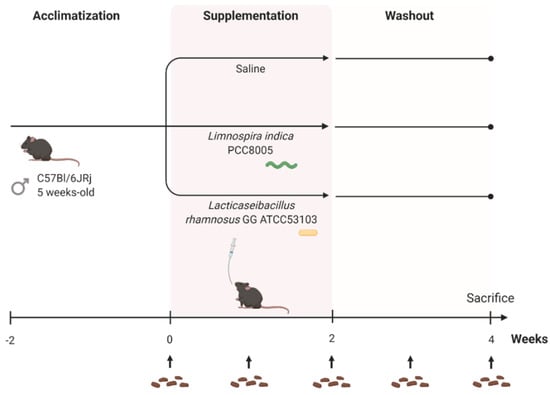
Figure 1.
Experimental setup of healthy mice supplementation. Created with BioRender.com.
2.4. Fecal DNA Extraction and 16S rRNA Gene Sequencing
To assess the impact of unprocessed L. indica PCC 8005 and L. rhamnosus GG on the gut bacterial ecosystem, fecal samples were longitudinally collected every other day. Total fecal DNA was extracted and high throughput amplicon sequencing was performed as previously described [36].
2.5. Sequencing Data Processing and Analyses
16S rRNA gene sequencing data were processed and analyzed as previously described [36].
2.6. Statistical Analysis
Data were processed, analyzed and visualized using RStudio software packages ggplot2 and ggsci. Outliers defined by the Tukey’s fences criteria were excluded from further statistical analyses. Statistical significance (p < 0.05) was determined using linear (mixed) models using the lme4 package in R studio, unless otherwise mentioned.
3. Results
3.1. L. indica PCC 8005 and L. rhamnosus GG Supplementation Does Not Affect Body Weight
First, daily monitoring of the mice’s body weight showed that they initially had a comparable body weight (Figure 2, Table 1). During the entire supplementation course, L. rhamnosus GG administered mice gained more body weight in comparison to mice given saline or L. indica PCC 8005. However, this gain did not impact absolute body weights observed at the end of supplementation. Then, during and after washout, no differences among different supplementation groups were observed in terms of gained body weight or absolute body weights (Figure 2, Table 1).
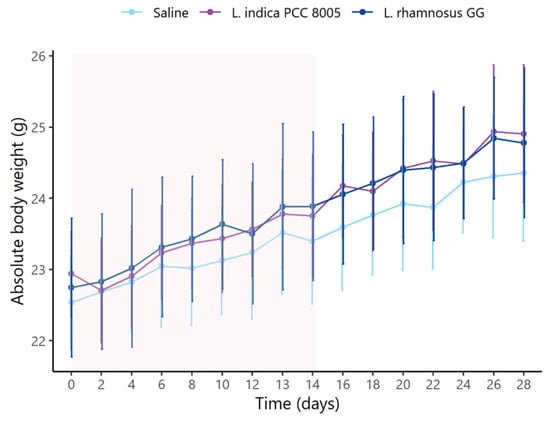
Figure 2.
L. indica PCC 8005 and L. rhamnosus GG supplementation do not affect absolute body weights of mice. The window of supplementation is highlighted. Data (in grams) are presented as mean ± standard deviation, n = 10 per group. Time independent differences were assessed by linear modelling.

Table 1.
L. indica PCC 8005 and L. rhamnosus GG supplementation do not affect absolute body weights of mice. Data (in grams) are presented as means ± standard deviation, n = 10 per group.
3.2. The Intestinal Microbial Community Temporarily Changes following L. indica PCC 8005 and L. rhamnosus GG Supplementation
Next, to assess the impact of fresh L. indica PCC 8005 and L. rhamnosus GG on the gut bacterial ecosystem, fecal samples were longitudinally collected for 16S microbial profiling. First, in this sequencing data set, rarefication was performed to a depth of ≥10,229 reads in accordance with the smallest sample depth (Figure 3A) [37]. The average of estimated coverages was 99.62 ± 0.12% for all samples, suggesting that the 16S rRNA results from each library represented an adequate level of sequencing to identify most diversity in the samples (Figure 3B) [37,38]. Following rarefaction, the obtained reads corresponded to a total of 1740 OTUs (242 ± 21 OTUs on average per sample).
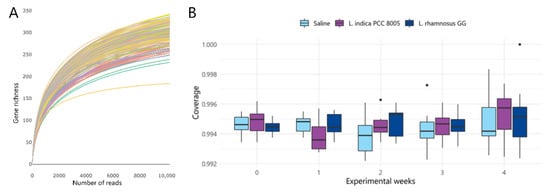
Figure 3.
An adequate depth of sequencing was reached to identify most diversity in the samples. (A) Rarefaction curve displaying gene richness as a function of the number of reads for each sample separately. (B) Good’s estimator of coverage as a measure of sample completeness. Data are presented in boxplots and outliers are depicted by dots, n = 10 per group.
To assess structural alterations in the microbial communities introduced by the different supplementation strategies, microbial alpha diversity (i.e., diversity within a sample, taking into account richness and/or evenness) and beta diversity indices (i.e., diversity between samples, with and without considering abundances) were calculated. Firstly, over the entire 4-week experimental course, a gradual increase in alpha diversity was noted in all mice, irrespective of the administered agent (Shannon index; Figure 4). This was mainly due to an increase in evenness rather than richness (Shannon even and Chao richness indices; Figure 5 and Figure 6, respectively). Fecal samples taken over time during saline intervention were considered to account for the natural variation in the gut ecosystem and thus set the baseline for the expected bacterial profiles in our mouse population. Hence, comparisons of the saline group with the bacterial intervention groups were performed for each time point to reveal possible shifts in microbiota composition induced by the tested dietary supplements. Overall, no significant changes in alpha diversity indices were observed (Figure 4, Figure 5 and Figure 6). However, following the 2-week washout (at t4), a significantly increased evenness was noted in L. rhamnosus GG administered mice (Shannon even; Figure 5), as compared to L. indica PCC 8005 administered mice.
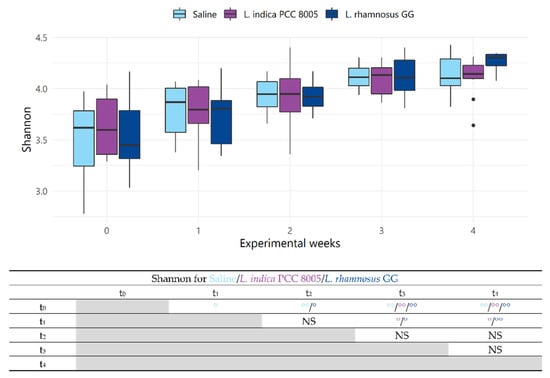
Figure 4.
Changes in alpha diversity index Shannon, considering both richness and evenness, of saline, L. indica PCC 8005 and L. rhamnosus GG supplemented mice over the entire experimental course. Data are presented in boxplots and outliers are depicted by dots, n = 10 per group. Supporting table summarizes time-series, pairwise comparisons using ° p < 0.05 and °° p < 0.01 by linear mixed effects modelling. NS = not significant.
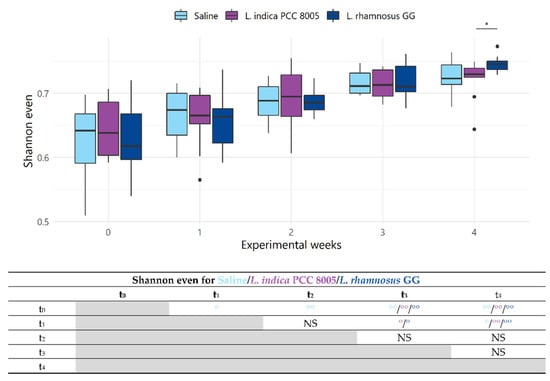
Figure 5.
Changes in alpha diversity index Shannon even, considering solely evenness, of saline, L. indica PCC 8005 and L. rhamnosus GG supplemented mice over the entire experimental course. Data are presented in boxplots and outliers are depicted by dots, n = 10 per group. * p < 0.05 by linear modelling for time independent comparisons. Supporting table summarizes time-series, pairwise comparisons using ° p <0.05 and °° p < 0.01 by linear mixed effects modelling. NS = not significant.
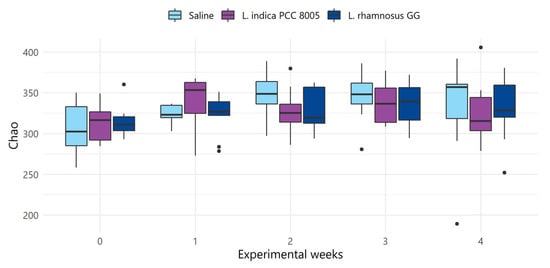
Figure 6.
Changes in alpha diversity index Chao, considering solely richness, of saline, L. indica PCC 8005 and L. rhamnosus GG supplemented mice over the entire experimental course. Data are presented in boxplots and outliers are depicted by dots, n = 10 per group. Time independent and–dependent differences were assessed by linear and linear mixed effects modelling, respectively.
The microbial community was further compared by a distance matrix based on weighted (considering relative microbial abundance) and unweighted (considering microbial membership) UniFrac beta diversity indices with 1000 permutations (Supplementary Figure S1 and Figure S2, respectively). First, paired analysis showed that most temporal differences could be explained by the natural variation in the gut ecosystem, as indicated by UniFrac distance analyses (Supplementary Figures S1 and S2). However, to exclude the dominant effects of natural variation, analyses were performed in a time-independent manner, focusing on the microbiota at a certain time point and investigating the effect of dedicated supplements relative to saline treated mice. When comparing microbial profiles among the different supplementation groups before the start of supplementation (t0), baseline variations could be excluded (Figure 7A and Figure 8A). Further analysis using weighted UniFrac distances could not depict statistical differences at any of the investigated time points (Figure 7). However, unweighted UniFrac analyses revealed a significant shift in beta diversity appearing at t2, when comparing both supplemented microbial communities to each other (L. indica PCC 8005 vs. L. rhamnosus GG p = 0.033 by AMOVA) (Figure 8C). Of interest, when comparing each supplemented microbial community to the saline treated microbiome, a trend towards a shift in beta diversity was observed for L. indica PCC 8005 supplemented mice (L. indica PCC 8005 vs. saline p = 0.059 by AMOVA), while this could not be observed for L. rhamnosus GG supplementation (L. rhamnosus GG vs. saline p = 0.198 by AMOVA) (Figure 8C). Hereafter, during and after washout, differences were no longer observed among the different groups (Figure 8D,E). These results thus show a temporal change in microbial communities, rather than changes in its relative abundances. These temporal changes appeared to be induced to a larger extent by L. indica PCC 8005 when compared to L. rhamnosus GG supplementation.
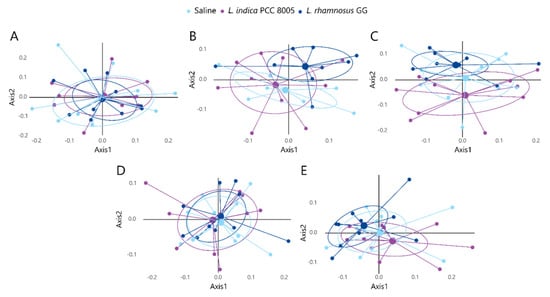
Figure 7.
Weighted UniFrac NMDS plots displaying inter−sample diversity among the different treatment groups at (A) baseline (t0), (B) during (t1) and (C) after (t2) supplementation, and (D) during (t3) and (E) after (t4) washout. Individual samples are depicted by dots, n = 10 per group.
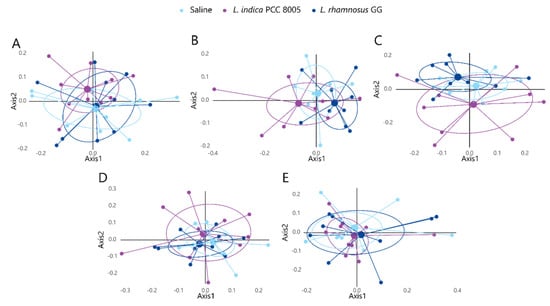
Figure 8.
Unweighted UniFrac NMDS plots displaying inter−sample diversity among the different treatment groups at (A) baseline (t0), (B) during (t1) and (C) after (t2) supplementation, and (D) during (t3) and (E) after (t4) washout. Individual samples are depicted by dots, n = 10 per group.
3.3. L. indica PCC 8005 and L. rhamnosus GG Supplementation Affect Members Belonging to the Lachnospiraceae and Porphyromonadaceae Families
Next, we compared the microbial composition at various taxonomic levels between mice among the different sampling points representing baseline (t0), during (t1) and after (t2) supplementation, as well as during (t3) and after (t4) washout periods (Supplementary Figures S3–S7). In particular, to identify specific OTUs associated with the observed significant differences in alpha and beta diversity at t4 and t2, respectively, the composition of fecal microbiota was further investigated using ANCOM, which allows for pair-wise comparisons over time (Table 2 and Table 3) [36,39]. Only 6 and 9 OTUs, mainly belonging to the Lachnospiraceae and Porphyromonadaceae families, appeared to be significantly affected by both supplementation strategies L. indica PCC 8005 and L. rhamnosus GG, respectively (Table 2 and Table 3). These were predominantly present in relative abundances < 1% (Figure 9 and Figure 10). Oligotyping demonstrated that each of these OTUs constitutes a single taxonomic unit.

Table 2.
List of taxa with significant changes in relative abundance associated with the temporal change in microbiota following L. indica PCC 8005 supplementation (at t2 and t4, in respect to t0).

Table 3.
List of taxa with significant changes in relative abundance associated with the temporal change in microbiota following L. rhamnosus GG supplementation (at t2 and t4, in respect to t0).
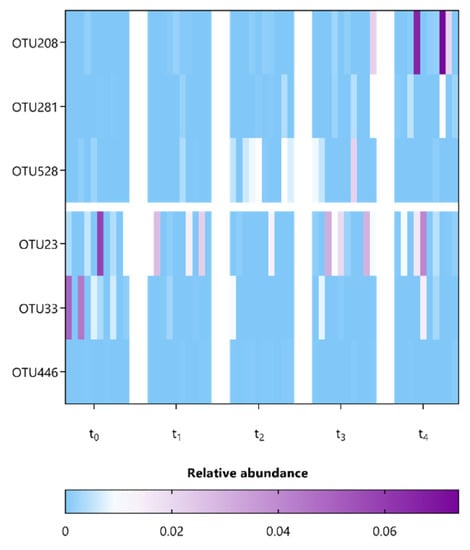
Figure 9.
Heatmap representing the relative abundance of differential OTUs detected in mice following L. indica PCC 8005 supplementation (in respect to t0) as listed in Table 2, n = 10 per time point.
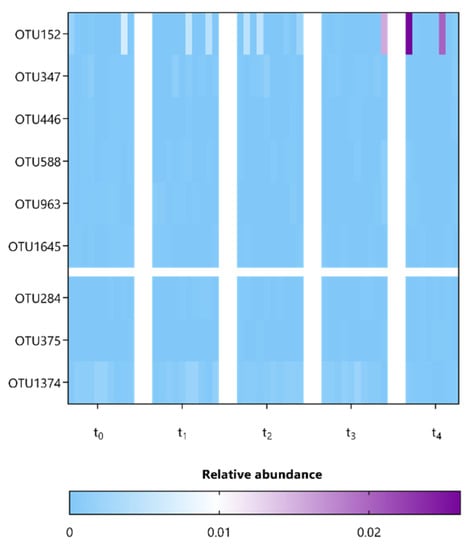
Figure 10.
Heatmap representing the relative abundance of differential OTUs detected in mice following L. rhamnosus GG supplementation (in respect to t0) as listed in Table 3, n = 10 per time point.
4. Discussion
Research on dietary supplement-based interventions has been increasing in popularity because, likely by reshaping the gut microbiome, they might provide an alternative treatment for multiple intestine-associated diseases, including intestinal radiotoxicity, as reviewed earlier [4]. Here, we present the first comparative study in which healthy mice were administered fresh, in-house prepared biomass of either Limnospira indica PCC 8005 or Lacticaseibacillus rhamnosus GG ATCC 53103 to investigate the impact on the gut bacterial ecosystem of healthy mice.
Animal wellbeing is generally considered to be minimally hampered by repeated oral gavage when carried out by an experienced scientist [40]. Moreover, mice subjected to this procedure did not experience significant changes in absolute body weight in respect to saline administered mice, consistent with previous studies giving Limnospira sp. [41] or Lacticaseibacillus sp. [42,43] to healthy rodents.
Although little impact was shown on general animal wellbeing over the experimental course, we reported a dominant effect of time on the composition of gut microbiota, which may be evoked by physiological stress responses to daily handlings, as was discussed earlier [44,45].
Although richness is proposed to be a major marker for gut health because high bacterial richness and diversity often reflect ecosystem stability and resilience [46], we could not report an equivalent change in alpha diversity metrics during or after two-week supplementation. This might be explained by the initial health condition of the mice that were supplemented, as was also described in studies using higher doses of Limnospira sp. [19] or equivalent doses of Lacticaseibacillus sp. [47]. Yet, supporting our hypothesis of a supplementation-induced shift in microbial beta diversity, a temporal shift in unweighted UniFrac beta diversity was observed, implying a change in microbial membership rather than relative abundances of particular taxa. For this, supporting evidence was found in a two-week intervention study applying high doses (1.5 g/kg and 3.0 g/kg) of Limnospira sp. [19]. In this perspective, relevant OTUs significantly affected by either of the applied supplementation regimens, yet present in relative abundances below 1%, pointed towards Lachnospiraceae and Porphyromonadaceae members, two families with potentially health-promoting properties based on their butyrate producing capacities [48,49,50,51,52,53,54]. In particular, within the group of Lachnospiraceae, L. indica PCC 8005 supplementation was associated with an increased relative abundance of Kineothrix alysoides, a recently identified saccharolytic butyrate producer belonging to the Clostridium XIVa cluster and found in healthy human subjects [55,56]. This observation might be explained by metabolic cross-feeding, a well-described feature for lactate-producing lactobacilli steering butyrate conversion by lactate-utilizing, butyrate-producing colon bacteria [57]. In view of Limnospira spp. supplementation, it is thought to deliver a range of prebiotics including carbohydrates, polyphenols, and polyunsaturated fatty acids, which may stimulate the growth of beneficial bacteria, exerting secondary health benefits [19]. To assess the consequences hereof, future research including randomized controlled trials, metabolic modelling (e.g., using CarveMe [58]) and the use of gut-on-a-chip models for co-cultivation with selected bacteria [59] would need to investigate fecal metabolites to validate the mechanisms of cross-feeding between these taxa, as well as the potential health-promoting attributes of these butyrate producers.
Intriguingly, neither L. indica PCC 8005 nor L. rhamnosus GG-related sequences could be recovered from our sequencing dataset. For Limnospira spp., no research could yet confirm its survival following exposure to chemical and mechanical stresses to be conquered along the intestinal transit. In contrast, L. rhamnosus GG was described to be very resistant to gastric acidity and the action of gastric juice by Lebeer et al. [60]. Yet, they showed a short transition time of 6–48 h following single oral administration, which likely explains their absence or non-detectability in our study, where fecal samples were collected 24 h post-administration. To confirm this rapid clearance or show intestinal adherence, fecal samples collected at earlier time points following oral gavage should be analyzed.
Of interest, no particular effects persisted after supplementation was stopped, highlighting the transient character of dietary supplements introduced in the gastrointestinal tract, which is commonly observed [61]. Nevertheless, multiple studies could still show significant health benefits, even after a single administration, suggesting that the colonization of probiotic strains is not necessary to exert health benefits [60,62]. Still, a durable impact on the gut microbiome and health outcomes following dietary supplement intake is preferred. Unfortunately, one of the greatest challenges in this research area concerns the high variability in individual response, which contributes to conflicting outcomes. Improvements herein may be reached upon stratification of study participants into responders and non-responders based on their baseline microbial profile. This way, personalized therapeutic strategies can be explored during long-term intervention studies including long-term post-intervention follow-ups, which offer more insights.
In conclusion, our comparative study described a transient effect of both Limnospira indica PCC 8005 and Lacticaseibacillus rhamnosus GG ATCC 53103 on the gut microbial beta diversity, characterized by members of the butyrate producing Lachnospiraceae and Porphyromonadaceae families, respectively. Consequently, these selected strains are of great interest for future investigations as a dietary supplement, or as ingredients in the development of food products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol2030049/s1, Figure S1: Weighted UniFrac NMDS plots of (A) Saline, (B) L. indica PCC 8005 and (C) L. rhamnosus GG administered mice showing beta diversity with consideration of relative abundances; Figure S2: Unweighted UniFrac NMDS plots of (A) Saline, (B) L. indica PCC 8005 and (C) L. rhamnosus GG administered mice showing beta diversity without consideration of relative abundances; Figure S3. Stacked bar plots representing the taxonomic profile evolution over the entire experimental course; Figure S4. Stacked bar plots representing the taxonomic profile evolution over the entire experimental course; Figure S5. Stacked bar plots representing the taxonomic profile evolution over the entire experimental course; Figure S6. Stacked bar plots representing the taxonomic profile evolution over the entire experimental course; Figure S7. Stacked bar plots representing the taxonomic profile evolution over the entire experimental course.
Author Contributions
Execution and design of experiments, data acquisition and interpretation, manuscript preparation, C.S.; 16s rRNA profiling and interpretation, M.M.; execution of experiments, A.C.; supervision, financial support and lab management, S.B.; supervision, financial support, microbiome expertise input and lab management, N.L. and S.L.; original concept, design of experiments, data interpretation and manuscript management, F.M. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
C.S. was supported by the Belgian Nuclear Research Centre, SCK CEN, through the 2017 PhD Grant program, in collaboration with the University of Antwerp.
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Committee Animal Studies of Medanex Clinic (EC MxCl 2018-093) and by the SCK CEN animal welfare committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the members of the Interdisciplinary Biosciences group of SCK CEN, in particular M. Neefs, L. Daenen and B. Proesmans for their help with animal maintenance and assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; MacHado, J.C.; Figueiredo, C. Gastric Microbial Community Profiling Reveals a Dysbiotic Cancer-Associated Microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.B.; Rostami, E.; Sephay, A.A.; Shahrokh, S.; Balaii, H.; Aghdaei, H.A.; Zali, M.R. Alterations of the Human Gut Methanobrevibacter Smithii as a Biomarker for Inflammatory Bowel Diseases. Microb. Pathog. 2018, 117, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Segers, C.; Verslegers, M.; Baatout, S.; Leys, N.; Lebeer, S.; Mastroleo, F. Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy. Microorganisms 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Binda, S.; Bron, P.A.; Gross, G.; Hill, C.; van Hylckama Vlieg, J.E.; Lebeer, S.; Satokari, R.; Ouwehand, A.C. Understanding Mode of Action Can Drive the Translational Pipeline towards More Reliable Health Benefits for Probiotics. Curr. Opin. Biotechnol. 2019, 56, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wittouck, S.; Wuyts, S.; Meehan, C.J.; van Noort, V.; Lebeer, S. A Genome-Based Species Taxonomy of the Lactobacillus Genus Complex. mSystems 2019, 4, e00264-19. [Google Scholar] [CrossRef]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus Probiotic Protects Intestinal Epithelium from Radiation Injury in a TLR-2/Cyclo-Oxygenase-2-Dependent Manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Riehl, T.E.; Alvarado, D.; Ee, X.; Zuckerman, A.; Foster, L.; Kapoor, V.; Thotala, D.; Ciorba, M.A.; Stenson, W.F. Lactobacillus rhamnosus GG Protects the Intestinal Epithelium from Radiation Injury through Release of Lipoteichoic Acid, Macrophage Activation and the Migration of Mesenchymal Stem Cells. Gut 2018, 68, 1003–1013. [Google Scholar] [CrossRef]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus Supplementation for Diarrhoea Related to Chemotherapy of Colorectal Cancer: A Randomised Study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef]
- Baù, M.; Moretti, A.; Bertoni, E.; Vazzoler, V.; Luini, C.; Agosti, M.; Salvatore, S. Risk and Protective Factors for Gastrointestinal Symptoms Associated with Antibiotic Treatment in Children: A Population Study. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 35–48. [Google Scholar] [CrossRef]
- Esposito, C.; Roberti, A.; Turrà, F.; Cerulo, M.; Severino, G.; Settimi, A.; Escolino, M. Frequency of Antibiotic-Associated Diarrhea and Related Complications in Pediatric Patients Who Underwent Hypospadias Repair: A Comparative Study Using Probiotics vs Placebo. Probiotics Antimicrob. Proteins 2018, 10, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus Induced Epithelial Cell Apoptosis, Ameliorates Inflammation and Prevents Colon Cancer Development in an Animal Model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus Casei and Lactobacillus rhamnosus GG Decrease Colon Cancer Cell Invasion in Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-Fermented Milk Prevents DSS-Induced Colitis and Regulates Intestinal Epithelial Homeostasis through Activation of Epidermal Growth Factor Receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Claes, I.J.J.; Lebeer, S.; Shen, C.; Verhoeven, T.L.A.; Dilissen, E.; De Hertogh, G.; Bullens, D.M.A.; Ceuppens, J.L.; Van Assche, G.; Vermeire, S.; et al. Impact of Lipoteichoic Acid Modification on the Performance of the Probiotic Lactobacillus rhamnosus GG in Experimental Colitis. Clin. Exp. Immunol. 2010, 162, 306–314. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-Inflammatory and Immunomodulatory Effects of Spirulina platensis in Comparison to Dunaliella salina in Acetic Acid-Induced Rat Experimental Colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef]
- Morsy, M.A.; Gupta, S.; Nair, A.B.; Venugopala, K.N.; Greish, K.; El-Daly, M. Protective Effect of Spirulina platensis Extract against Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Rats. Nutrients 2019, 11, 2309. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina platensis Alleviates Chronic Inflammation with Modulation of Gut Microbiota and Intestinal Permeability in Rats Fed a High-Fat Diet. J. Cell. Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose Effects of Orally Administered Spirulina Suspension on Colonic Microbiota in Healthy Mice. Front. Cell. Infect. Microbiol. 2019, 9, 243. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Alwaleed, E.A.; El-Sheekh, M.; Abdel-Daim, M.M.; Saber, H. Effects of Spirulina platensis and Amphora coffeaeformis as Dietary Supplements on Blood Biochemical Parameters, Intestinal Microbial Population, and Productive Performance in Broiler Chickens. Environ. Sci. Pollut. Res. 2020, 28, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Leone, V.; Nobutani, K.; Musch, M.W.; Martinez-Guryn, K.; Wang, Y.; Miyoshi, S.; Bobe, A.M.; Murat Eren, A.; Chang, E.B. Minimizing Confounders and Increasing Data Quality in Murine Models for Studies of the Gut Microbiome. PeerJ 2018, 6, e5166. [Google Scholar] [CrossRef] [PubMed]
- Cogne, G.; Lehmann, B.; Dussap, C.-G.; Gros, J.-B. Uptake of Macrominerals and Trace Elements by the Cyanobacterium Spirulina platensis (Arthrospira platensis PCC 8005) under Photoautotrophic Conditions: Culture Medium Optimization. Biotechnol. Bioeng. 2003, 81, 588–593. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R.; Gorbach, S.L. Lactobacillus GG: Bacteriology and Clinical Applications. Gastroenterol. Clin. N. Am. 2005, 34, 483–498. [Google Scholar] [CrossRef]
- Gorbach, S.L. The Discovery of Lactobacillus GG. Nutr. Today 1996, 31, 2S–4S. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Gamallat, Y.; Ren, X.; Walana, W.; Meyiah, A.; Xinxiu, R.; Zhu, Y.; Li, M.; Song, S.; Xie, L.; Jamalat, Y.; et al. Probiotic Lactobacillus rhamnosus Modulates the Gut Microbiome Composition Attenuates Preneoplastic Colorectal Aberrant Crypt Foci. J. Funct. Foods 2019, 53, 146–156. [Google Scholar] [CrossRef]
- Ki, Y.; Kim, W.; Cho, H.; Ahn, K.; Choi, Y.; Kim, D. The Effect of Probiotics for Preventing Radiation-Induced Morphological Changes in Intestinal Mucosa of Rats. J. Korean Med. Sci. 2014, 29, 1372–1378. [Google Scholar] [CrossRef]
- Li, X.; Hu, D.; Tian, Y.; Song, Y.; Hou, Y.; Sun, L.; Zhang, Y.; Man, C.; Zhang, W.; Jiang, Y. Protective Effects of a Novel Lactobacillus rhamnosus Strain with Probiotic Characteristics against Lipopolysaccharide-Induced Intestinal Inflammation In Vitro and In Vivo. Food Funct. 2020, 11, 5799–5814. [Google Scholar] [CrossRef]
- Mittal, A.; Suresh Kumar, P.V.; Banerjee, S.; Rao, A.R.; Kumar, A. Modulatory Potential of Spirulina fusiformis on Carcinogen Metabolizing Enzymes in Swiss Albino Mice. Phyther. Res. 1999, 13, 111–114. [Google Scholar] [CrossRef]
- Chamorro-Cevallos, G.; Garduño-Siciliano, L.; Barrón, B.L.; Madrigal-Bujaidar, E.; Cruz-Vega, D.E.; Pages, N. Chemoprotective Effect of Spirulina (Arthrospira) against Cyclophosphamide-Induced Mutagenicity in Mice. Food Chem. Toxicol. 2008, 46, 567–574. [Google Scholar] [CrossRef]
- El Bialy, B.E.; El-Boraey, N.G.; Hamouda, R.A.; Abdel-Daim, M.M. Comparative Protective Effects of Spirulina and Spirulina Supplemented with Thiamine against Sub-Acute Carbon Tetrachloride Toxicity in Rats. Biomed. Pharmacol. J. 2019, 12, 511–525. [Google Scholar] [CrossRef]
- Sharma, M.K.; Sharma, A.; Kumar, A.; Kumar, M. Spirulina fusiformis Provides Protection against Mercuric Chloride Induced Oxidative Stress in Swiss Albino Mice. Food Chem. Toxicol. 2007, 45, 2412–2419. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rebolledo, G.A.; Galar-Martínez, M.; García-Rodríguez, R.V.; Chamorro-Cevallos, G.A.; Hernández-Reyes, A.G.; Martínez-Galero, E. Antioxidant Effect of Spirulina (Arthrospira) Maxima on Chronic Inflammation Induced by Freund’s Complete Adjuvant in Rats. J. Med. Food 2015, 18, 865–871. [Google Scholar] [CrossRef]
- Segers, C.; Mysara, M.; Claesen, J.; Baatout, S.; Leys, N.; Lebeer, S.; Verslegers, M.; Mastroleo, F. Intestinal Mucositis Precedes Dysbiosis in a Mouse Model for Pelvic Irradiation. ISME Commun. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather than Size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef]
- Arantes-Rodrigues, R.; Henriques, A.; Pinto-Leite, R.; Faustino-Rocha, A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Eixas, F.; Gama, A.; Colaço, B.; Colaço, A. The Effects of Repeated Oral Gavage on the Health of Male CD-1 Mice. Nature 2012, 41, 129–134. [Google Scholar] [CrossRef]
- Hutadilok-Towatana, N.; Reanmongkol, W.; Satitit, S.; Panichayupakaranant, P.; Ritthisunthorn, P. A Subchronic Toxicity Study of Spirulina platensis. Food Sci. Technol. Res. 2008, 14, 351–358. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, K.Y.; Kim, B.; Kim, E.; Hyun, C.K. Lactobacillus rhamnosus GG Improves Insulin Sensitivity and Reduces Adiposity in High-Fat Diet-Fed Mice through Enhancement of Adiponectin Production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Akkermans, L.; Soderholm, J. The Role of Microbiota and Probiotics in Stress-Induced Gastrointestinal Damage. Curr. Mol. Med. 2008, 8, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Karl, P.J.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool Consistency Is Strongly Associated with Gut Microbiota Richness and Composition, Enterotypes and Bacterial Growth Rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.W.; Cheng, M.Y.; Yang, X.; Lu, Y.Y.; Yin, H.D.; Zeng, Y.; Wang, R.Y.; Jiang, Y.L.; Yang, W.T.; Wang, J.Z.; et al. Probiotic Lactobacillus rhamnosus GG Promotes Mouse Gut Microbiota Diversity and T Cell Differentiation. Front. Microbiol. 2020, 11, 607735. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Fracasso, P.L.; Casale, V.; Villotti, G.; Marcheggiano, A.; Stigliano, V.; Pinnaro, P.; Bagnardi, V.; Caprilli, R. Topical Butyrate for Acute Radiation Proctitis: Randomised, Crossover Trial. Lancet 2000, 356, 1232–1235. [Google Scholar] [CrossRef]
- Maggio, A.; Magli, A.; Rancati, T.; Fiorino, C.; Valvo, F.; Fellin, G.; Ricardi, U.; Munoz, F.; Cosentino, D.; Cazzaniga, L.F.; et al. Daily Sodium Butyrate Enema for the Prevention of Radiation Proctitis in Prostate Cancer Patients Undergoing Radical Radiation Therapy: Results of a Multicenter Randomized Placebo-Controlled Dose-Finding Phase 2 Study. Int. J. Radiat. Oncol. 2018, 89, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, Gen. Nov., Sp. Nov., a Saccharolytic Butyrate-Producer within the Family Lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Zhou, F.; Zhang, B.; Wu, J.; Zhang, X.; Xu, H.; Ren, J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020, 11, 383. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef]
- Machado, D.; Andrejev, S.; Tramontano, M.; Patil, K.R. Fast Automated Reconstruction of Genome-Scale Metabolic Models for Microbial Species and Communities. Nucleic Acids Res. 2018, 46, 7542–7553. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.-J.; Yoon, J.Y.; Kemmitt, J.; Wright, C.; Schneider, K.; Sphabmixay, P.; Hernandez-Gordillo, V.; Holcomb, S.J.; Bhushan, B.; et al. Primary Human Colonic Mucosal Barrier Crosstalk with Super Oxygen-Sensitive Faecalibacterium Prausnitzii in Continuous Culture. Med 2021, 2, 74–98.e9. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.J.J.; Verhoeven, T.L.A.; Shen, C.; Lambrichts, I.; Ceuppens, J.L.; Vanderleyden, J.; De Keersmaecker, S.C.J. Impact of LuxS and Suppressor Mutations on the Gastrointestinal Transit of Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2008, 74, 4711–4718. [Google Scholar] [CrossRef] [Green Version]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Roy, C.I.L. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Meance, S.; Cayuela, C.; Raimondi, A.; Turchet, P.; Lucas, C.; Antoine, J.M. Recent Advances in the Use of Functional Foods: Effects of the Commercial Fermented Milk with Bifidobacterium Animalis Strain DN-173 010 and Yoghurt Strains on Gut Transit Time in the Elderly. Microb. Ecol. Health Dis. 2003, 15, 15–22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).