Double Complex Salt [Co(NH3)6][Fe(CN)6] Plasma Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining DCS
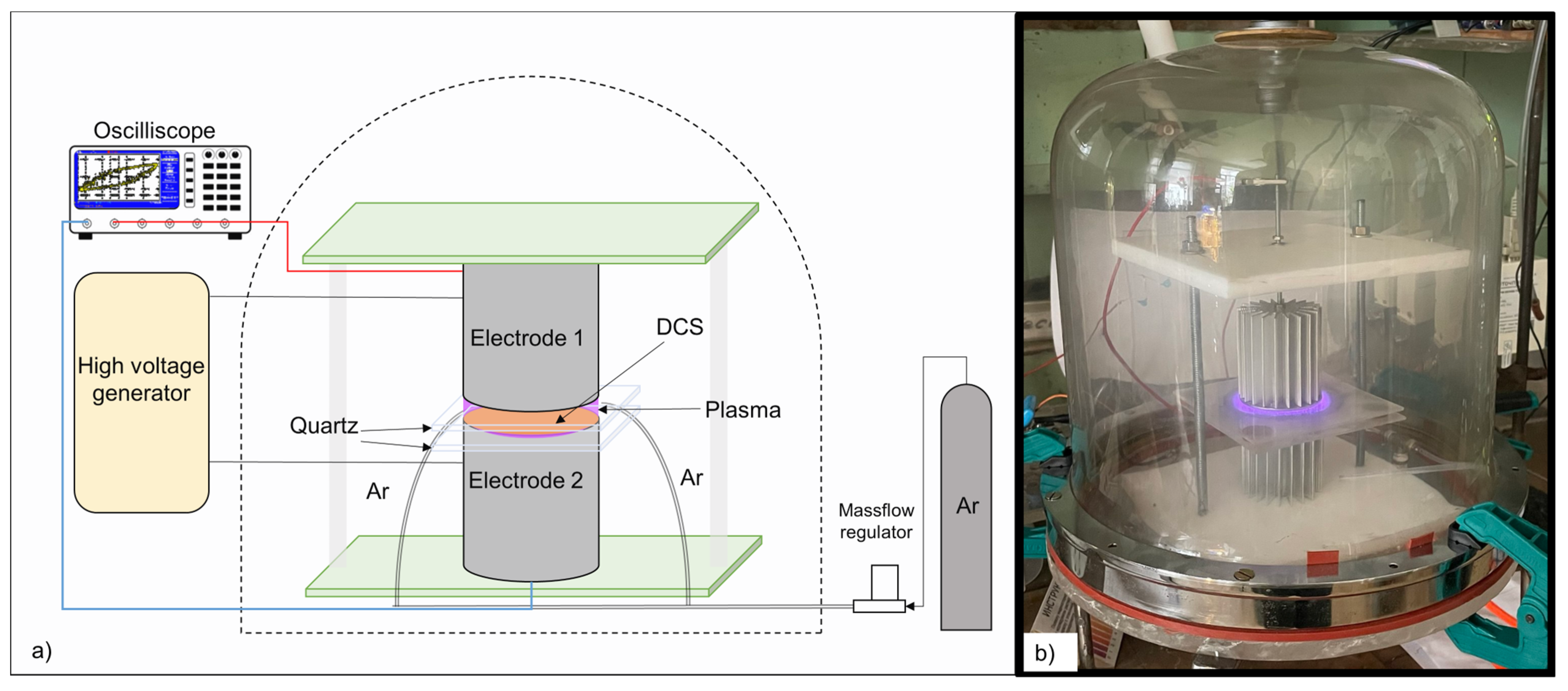
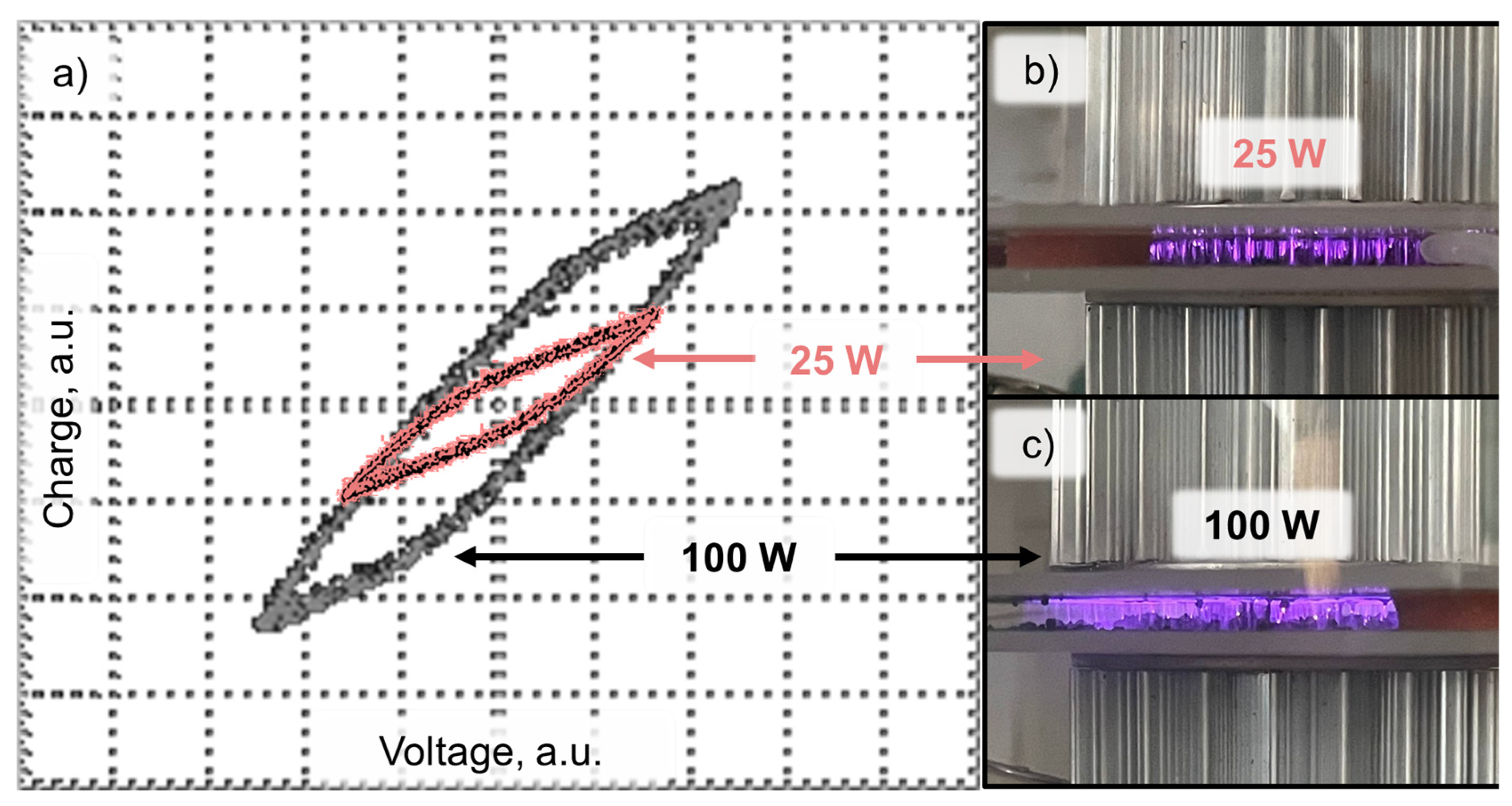
2.2. Treatment of DCS with Plasma
2.3. Research Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCS | Double complex salt |
| STA | Synchronous thermal analysis |
| LD | Linear dichroism |
| TG | Thermogravimetry |
| DSC | Differential scanning calorimetry |
| XRD | X-ray diffraction |
| CSR | Coherent scattering region |
| SEM | Scanning electron microscopy |
| FWHM | Full width at half maximum |
| XPS | X-ray photoelectron spectroscopy |
| NPs | Nanoparticles |
References
- Seitz, K.; Peschel, S.; Babel, D. Über die Kristallstrukturen der Cyanokomplexe [Co(NH3)6][Fe(CN)6], [Co(NH3)6]2[Ni(CN)4]3·2H2O und [Cu(en)2][Ni(CN)4]. Z. Für Anorg. Und Allg. Chem. 2001, 627, 929–934. Available online: https://onlinelibrary.wiley.com/doi/10.1002/1521-3749%28200105%29627%3A5%3C929%3A%3AAID-ZAAC929%3E3.0.CO%3B2-7 (accessed on 17 September 2025). (In German). [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Gosteva, A.N.; Semushina, Y.P.; Shimkin, A.A. Thermal behavior of double complexes [Co(NH3)6][Fe(CN)6] and [CO(en)3][Fe(CN)6]·2H2O. Izv. Vyss. Uchebnykh Zaved. Khimiya I Khimicheskaya Tekhnologiya 2018, 61, 49. (In Russian) [Google Scholar] [CrossRef]
- Potemkin, D.I.; Saparbaev, E.S.; Zadesenets, A.V.; Filatov, E.Y.; Snytnikov, P.V.; Sobyanin, V.A. Preferential CO Oxidation on Bimetallic Pt0.5M0.5 Catalysts (M = Fe, Co, Ni) Prepared from Double Complex Salts. Catal. Ind. 2018, 10, 62–67. [Google Scholar] [CrossRef]
- Zayed, E.M.; Mohamed, G.G.; El Salam, H.A.A. Ni(II), Co(II), Fe(III), and Zn(II) mixed ligand complexes of indoline-dione and naphthalene-dione: Synthesis, characterization, thermal, antimicrobial, and molecular modeling studies. Inorg. Chem. Comm. 2023, 147, 110276. [Google Scholar] [CrossRef]
- Kilic, A.; Alhafez, A.; Aytar, E.; Soylemez, R. The sustainable catalytic conversion of CO2 into value-added chemicals by using cobaloxime-based double complex salts as efficient and solvent-free catalysts. Inorg. Chim. Acta 2023, 554, 121547. [Google Scholar] [CrossRef]
- Afanasenko, E.; Seifullina, I.; Martsinko, E.; Dyakonenko, V.; Shishkina, S.; Gudzenko, O.; Varbanets, L. Supramolecular organization and enzyme-effector properties of double coordination salts with malatostannate/germanate(IV) anions and Fe(II), Co(II), Ni(II), Cu(II) 1,10-phenanthroline cations. J. Molec. Struct. 2023, 1271, 133996. [Google Scholar] [CrossRef]
- Rudneva, Y.V.; Korenev, S.V. Dispersed Metal Alloys: Synthesis Methods and Catalytic Properties (Review). Žurnal Neorganičeskoj Him. 2024, 69, 1181–1200. (In Russian) [Google Scholar] [CrossRef]
- Khalil, L.H.; Mousssa, N.A.; Mikhail, S. Thermal, structural and textural studies on the double complex salt [Co(NH3)6][Fe(CN)6] and on its silica-supported catalysts. J. Mater. Sci. 1992, 27, 557–568. [Google Scholar] [CrossRef]
- Khassin, A.A.; Pechenyuk, S.I.; Domonov, D.P.; Minyukova, T.P.; Chermashentseva, G.K.; Kustova, G.N.; Plysova, L.M. Catalytic Properties of Bimetallic Catalysts Based on Binary Complexes of Transition Metals in Fischer-Tropsch Synthesis. Chem. Sustain. Dev. 2007, 15, 673–683. [Google Scholar]
- Pechenyuk, S.I.; Domonov, D.P.; Rogachev, D.L.; Belyavskii, A.T. Anion Effect on the Thermolysis of Double Complexes [Co(NH3)6][Fe(CN)6] and [Co(NH3)6]4[Fe(CN)6]3. Rus. J. Inorg. Chem. 2007, 52, 1033–1038. [Google Scholar] [CrossRef]
- Gosteva, A.N.; Kulikova, M.V.; Semushina, Y.P.; Chudakova, M.V.; Tsvetov, N.S.; Semushin, V.V. Catalytic Activity of Thermolyzed [Co(NH3)6][Fe(CN)6] in CO Hydrogenation Reaction. Molecules 2021, 26, 3782. [Google Scholar] [CrossRef]
- Gosteva, A.N.; Kulikova, M.V.; Ivantsov, M.I.; Grabchak, A.A.; Semushina, Y.P.; Lapuk, S.E.; Gerasimov, A.V.; Tsvetov, N.S. CO2 Hydrogenation over Fe-Co Bimetallic Catalyst Derived from the Thermolysis of [Co(NH3)6][Fe(CN)6]. Catalysts 2023, 13, 1475. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Semushina, Y.P.; Kadyrova, G.I.; Rogachev, D.L.; Kuz'mich, L.F.; Domonov, D.P.; Kalinnikov, V.T. Synthesis and Properties of Double Complex Salts Containing the Cation [Co(NH3)6]3+. Russ. J Coord. Chem. 2005, 31, 866–871. [Google Scholar] [CrossRef]
- Huang, C.; Li, M.; Wang, P.; Song, S.; Chai, B.; Zhang, M.; Hu, X.; Cai, J.; Wu, S.; He, Q. Tetracycline degradation by persulfate activated with nitrogen magnetic graphene oxide confined Fe/Co dual single-atom catalyst: Performance and degradation mechanism. J. Environ. Chem. Eng. 2023, 11, 109704. [Google Scholar] [CrossRef]
- Zhu, C.; Cun, F.; Fan, Z.; Nie, Y.; Du, Q.; Liu, F.; Yang, W.; Li, A. Heterogeneous Fe-Co dual-atom catalyst outdistances the homogeneous counterpart for peroxymonosulfate-assisted water decontamination: New surface collision oxidation path and diatomic synergy. Water Res. 2023, 241, 120164. [Google Scholar] [CrossRef]
- Wang, J.; You, R.; Zhao, C.; Zhang, W.; Liu, W.; Fu, X.P.; Li, Y.Y.; Zhou, F.Y.; Zheng, X.S.; Xu, Q.; et al. N-Coordinated Dual-Metal Single-Site Catalyst for Low-Temperature CO Oxidation. ACS Catal. 2020, 10, 2754–2761. [Google Scholar] [CrossRef]
- Rybinskaya, A.A.; Plyusnin, P.E.; Bykova, E.A.; Gromilov, S.A.; Shubin, Y.V.; Korenev, S.V. Double complex salts [Pd(NH3)4]3[Rh(NO2)6]2,[Pd(NH3)4]3[Rh(NO2)6]2⋅H2O as promising precursors to prepare Pd–Rh nanoalloys. J. Struct. Chem. 2012, 53, 527–533. [Google Scholar] [CrossRef]
- Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control; Toshima, N., Yang, H., Shiraishi, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; 459p. [Google Scholar] [CrossRef]
- Song, H.T.; Fazeli, A.; Kim, H.D.; Eslami, A.A.; Noh, Y.S.; Saeidabad, N.G.; Moon, D.J. Effect of lanthanum group promoters on Cu/(mixture of ZnO and Zn-Al-spinnel-oxides) catalyst for methanol synthesis by hydrogenation of CO and CO2 mixtures. Fuel 2021, 283, 118987. [Google Scholar] [CrossRef]
- Kang, J.S.; Awate, S.V.; Lee, Y.J.; Kim, S.J.; Park, M.J.; Lee, S.D.; Hong, S.-I.; Moon, D.J. Nano-Sized Cobalt Based Fischer-Tropsch Catalysts for Gas-to-Liquid Process Applications. J. Nanosci. Nanotechnol. 2010, 10, 3700–3704. [Google Scholar] [CrossRef]
- Ilie, I.; Patron, L.; Brezeanu, M.; Segal, E.; Filoti, G. Synthesis of nickel ferrite from polynuclear coordination compounds. J. Mater. Sci. Lett. 1987, 6, 932–935. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Gosteva, A.N. The complexes [Nia(Pn)b]x[Fe(CN)6]y (Pn = 1.3-diaminopropane): Synthesis and thermolysis. Russ. J. Coord. Chem. 2014, 40, 547–557. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalysis: A brief tutorial. Plasma Res. Express 2019, 1, 043001. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.-L.; Liu, C. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, Y.; Wang, S.; Fu, Q.; Guo, X.; Li, Y.; Ren, J.; Cao, Z.; Li, R.; Zhao, L.; et al. Plasma synthesis of K-doped amorphous carbon nitride with passivated trap states for enhanced photocatalytic H2O2 production. J. Alloys Compd. 2023, 947, 169663. [Google Scholar] [CrossRef]
- Heng, Y.; Yu, L.; Chen, Y.; Chen, X.; Wang, W. Plasma-Assisted Material Preparation Strategies and Property Optimization. Phys. Status Solidi A 2024, 222, 2400702. [Google Scholar] [CrossRef]
- Mohan, H.; Mohandoss, S.; Prakash, A.; Balasubramaniyan, N.; Loganathan, S.; Assadi, A.A.; Khacef, A. Cold plasma assisted synthesis of spinel-CoFe2O4 nanoparticle with narrow bandgap and high magnetic activity. Inorg. Chem. Commun. 2024, 167, 112754. [Google Scholar] [CrossRef]
- Witvrouwen, T.; Paulussen, S.; Sels, B. The Use of Non-Equilibrium Plasmas for the Synthesis of Heterogeneous Catalysts. Plasma Process. Polym. 2012, 9, 750–760. [Google Scholar] [CrossRef]
- MhaskeSujit, S.; KakadeSandip, B.; Thorat Sopan, M.; Kalange Ashok, E. Synthesis, Structural and Magnetic Properties of Cobalt Ferrite (CoFe2O4) Nanoparticles by Simple Co-precipitation Technique. Int. Res. J. Sci. Eng. 2023, A12, 11–14. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Moradi, M.; Parvin, N.; Nemati, A.; Rad, A.J.; Sheysi, N.; Abouchenari, A.; Mohammadi, A.; Karbasi, S.; Ahmadi, Z.; et al. Magnetic CoFe2O4 nanoparticles doped with metal ions: A review. Ceram. Inter. 2020, 46, 18391–18412. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Jagadale, A.D.; Shinde, N.M.; Lokhande, C.D. Chemical synthesis of spinel cobalt ferrite (CoFe2O4) nano-flakes for supercapacitor application. Appl. Surf. Sci. 2012, 259, 39–43. [Google Scholar] [CrossRef]
- Sandu, I.; Presmanes, L.; Alphonse, P.; Tailhades, P. Nanostructured cobalt manganese ferrite thin films for gas sensor application. Thin Sol. Films. 2006, 495, 130–133. [Google Scholar] [CrossRef]
- Venturini, J.; Wermuth, T.B.; Machado, M.C.; Arcaro, S.; Alves, A.K.; da Cas Viegas, A.; Bergmann, C.P. The influence of solvent composition in the sol-gel synthesis of cobalt ferrite (CoFe2O4): A route to tuning its magnetic and mechanical properties. J. Eur. Ceram. Soc. 2019, 39, 3442–3449. [Google Scholar] [CrossRef]
- Cote, L.J.; Teja, A.S.; Wilkinson, A.P.; Zhang, Z.J. Continuous hydrothermal synthesis of CoFe2O4 nanoparticles. Fluid Phase Eq. 2003, 210, 307–317. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Yuen, C.W.M.; Jiang, S.-X.; Hu, E. Hydrothermal synthesis of magnetic CoFe2O4/grapheme nanocomposites with improved photocatalytic activity. Appl. Surf. Sci. 2015, 351, 140–147. [Google Scholar] [CrossRef]
- Lavorato, G.; Alzamora, M.; Contreras, C.; Burlandy, G.; Litterst, F.J.; Baggio-Saitovitch, E. Internal Structure and Magnetic Properties in Cobalt Ferrite Nanoparticles: Influence of the Synthesis Method. Part. Part. Syst. Charact. 2019, 36, 1900061. [Google Scholar] [CrossRef]
- Pancotti, A.; Santos, D.P.; Morais, D.O.; de Barros Souza, M.V.; Lima, D.R.; Vulcani, V.A.S.; Martins, A.; Landers, R.; Braoios, A. Synthesis, characterization and in vitro cytotoxicity study of Co and Ni ferrite nanoparticles prepared by sol-gel method. SN Appl. Sci. 2021, 3, 716. [Google Scholar] [CrossRef]
- Sinkó, K.; Manek, E.; Meiszterics, A.; Havancsák, K.; Vainio, U.; Peterlik, H. Liquid-phase syntheses of cobalt ferrite nanoparticles. J. Nanopart. Res. 2012, 14, 894. [Google Scholar] [CrossRef]
- Naik, M.M.; Naik, H.S.B.; Kottam, N.; Vinuth, M.; Nagaraju, G.; Prabhakara, M.C. Multifunctional properties of microwave-assisted bioengineered nickel doped cobalt ferrite nanoparticles. J. Sol-Gel Sci. Technol. 2019, 91, 578–595. [Google Scholar] [CrossRef]
- Vadivel, M.; Ramesh Babu, R.; Ramamurthi, K.; Arivanandhan, M. Effect of PVP concentrations on the structural, morphological, dielectric and magnetic properties of CoFe2O4 magnetic nanoparticles. Nano-Struct. Nano-Obj. 2017, 11, 112–123. [Google Scholar] [CrossRef]
- Houshiar, M.; Zebhi, F.; Jafari Razi, Z.; Alidoust, A.; Askari, Z. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J. Magn. Magn. Mat. 2014, 371, 43–48. [Google Scholar] [CrossRef]
- Al-Yaqoob, K.; Bououdina, M.; Akhter, M.S.; Al-Najar, B.; Vijaya Judith, J. Selectivity and efficient Pb and Cd ions removal by magnetic MFe2O4 (M = Co, Ni, Cu and Zn) nanoparticles. Mater. Chem. Phys. 2019, 232, 254–256. [Google Scholar] [CrossRef]
- Karbasi, M.; Maghazeii, F.; Ghanbari, D. Magnetic investigation of microwave synthesized and thermal stable poly vinyl alcohol-cobalt ferrite nanocomposites. J. Nanostruct. 2019, 9, 365–375. [Google Scholar] [CrossRef]
- Sartori, K.; Choueikani, F.; Gloter, A.; Begin-Colin, S.; Taverna, D.; Pichon, B.P. Room Temperature Blocked Magnetic Nanoparticles Based on Ferrite Promoted by a Three-Step Thermal Decomposition Process. J. Am. Chem. Soc. 2019, 141, 9783–9787. [Google Scholar] [CrossRef]
- Patankar, K.K.; Ghone, D.M.; Mathe, V.L.; Kaushik, S.D. Structural and physical property study of sol–gel synthesized CoFe2−xHoxO4 nano ferrites. J. Magn. Magn. Mat. 2018, 454, 71–77. [Google Scholar] [CrossRef]
- Mullick, S.; Rana, G.; Kumar, A.; Sharma, G.; Naushad, M. “Ferrites”: Synthesis, Structure, Properties and Applications. Mat. Res. Found. 2021, 112, 1–61. [Google Scholar] [CrossRef]
- Travnicek, Z.; Zboril, R.; Matikova-Mal’arova, M.; Drahos, B.; Cernak, J. Thermal decomposition of [Co(en)3][Fe(CN)6]·2H2O: Topotactic dehydration process, valence and spin exchange mechanism elucidation. Chem. Cent. J. 2013, 7, 1–18. [Google Scholar] [CrossRef]
- Semushina, Y.P.; Pechenyuk, S.I.; Ivanov, Y.V.; Plyusnin, P.E.; Shubin, Y.V. Thermal decomposition of [Co(NH3)6][Fe(C2O4)3]·3H2O in inert and reductive atmospheres. Russ. Chem. Bull. 2015, 64, 1963–1966. [Google Scholar] [CrossRef]
- Hashemi, M.; Mohandes, F.; Salavati-Niasari, M. Application of [M(en)3]3[Fe(ox)3]2 (M = Zn, Cd, Ni) complexes as new precursor for the synthesis of ferrite micro/nanostructures. Adv. Pow. Technol. 2016, 27, 388–394. [Google Scholar] [CrossRef]
- Asanova, T.; Asanov, I.; Zadesenets, A.; Filatov, E.; Plyusnin, P.; Gerasimov, E.; Korenev, S. Study on thermal decomposition of double complex salt [Pd(NH3)4][PtCl6]. J. Therm. Anal. Calorim. 2016, 123, 1183–1195. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ma, C.; Zhang, Z.J.; Wang, B.N. Ultrafine gahnite (ZnAl2O4) nanocrystals: Hydrothermal synthesis and photoluminescent properties. Mater. Sci. Eng. B. 2008, 151, 224–230. [Google Scholar] [CrossRef]
- Gilabert, J.; Palacios, M.D.; Sanz, V.; Mestre, S. Characteristics reproducibility of (Fe, Co)(Cr, Al)2O4 pigments obtained by solution combustion synthesis. Ceram. Int. 2016, 42, 12880. [Google Scholar] [CrossRef][Green Version]
- Carp, O.; Patron, L.; Reller, A. Coordination compounds containing urea as precursors for oxides—A new route of obtaining nanosized CoFe2O4. Mater. Chem. Phys. 2007, 101, 142–147. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Brack, W.; Heine, B.; Birkhold, F.; Kruse, M.; Schoch, G.; Tischer, S.; Deutschmann, O. Kinetic modeling of urea decomposition based on systematic thermogravimetric analyses of urea and its most important by-products. Chem. Eng. Sci. 2014, 106, 1–8. [Google Scholar] [CrossRef]
- Biganzoli, I.; Barni, R.; Gurioli, A.; Pertile, R.; Riccardi, C. Experimental Investigation of Lissajous Figure Shapes in Planar and Surface Dielectric Barrier Discharges. J. Phys. Conf. Ser. 2014, 550, 012039. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Shimkin, A.A.; Semushina, Y.P.; Ivanov, Y.V. Thermal Behavior of Binary Complex Compounds Containing the Hexacyanoferrate Anion. Russ. J. General Chem. 2017, 87, 2212–2223. [Google Scholar] [CrossRef]
- Matiková-Mal’arová, M.; Černák, J.; Massa, W.; Varret, F. Three Co(III)–Fe(II) complexes based on hexacyanoferrates: Syntheses, spectroscopic and structural characterizations. Inorg. Chim. Acta 2009, 362, 443–448. [Google Scholar] [CrossRef]
- Filatov, E.Y.; Semushina, Y.P.; Gosteva, A.N. Obtaining and catalytic properties investigation of the products of double-complex salts [Cr(ur)6][M(L)6] thermal oxidation (M = Co, Fe; L = CN−, 1/2C2O2−4). J. Therm. Anal. Calorim. 2018, 134, 355–361. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. X-Ray Photoelectron Spectroscopy and Related Techniques for Surface Analysis. Available online: https://www.thermofisher.com/ru/ru/home/electron-microscopy/products/xps-instruments.html#:~:text=XPS%20is%20extremely%20surface%20sensitive,extremely%20useful%20for%20materials%20analysis (accessed on 10 September 2025).
- Wang, Y.H.; Dai, Z.; Zhang, C.Y.; Sun, G.W.; Lu, Z.W.; Gao, X.P.; Sun, G.Z.; Lan, W.; Zhang, Z.X.; Pan, X.J.; et al. Investigation into performance enhancements of Li–S batteries via oxygen-containing functional groups on activated multi-walled carbon nanotubes using Fourier transform infrared spectroscopy. Current Appl. Phys. 2020, 20, 1049–1057. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Shnitov, V.V.; Brzhezinskaya, M.; Baidakova, M.V.; Stolyarova, D.Y.; Ryzhkov, S.A.; Saveliev, S.D.; Shvidchenko, A.V.; Nefedov, D.Y.; Antonenko, A.O.; et al. Manifesting Epoxide and Hydroxyl Groups in XPS Spectra and Valence Band of Graphene Derivatives. Nanomaterials 2023, 13, 23. [Google Scholar] [CrossRef]
- Shchukarev, A.; Gojkovic, Z.; Funk, C.; Ramstedt, M. Cryo-XPS analysis reveals surface composition of microalgae. Appl. Surf. Sci. 2020, 526, 146538. [Google Scholar] [CrossRef]
- Arman, M.M.; El-Dek, S.I. Structural, Surface, Magnetic Study and Application of Nanoparticles CoFe2O4, ZnO and its Nanocomposite. J. Supercond. Nov. Magn. 2023, 36, 1913–1925. [Google Scholar] [CrossRef]
- Chen, X.; Wu, C.; Guo, Z. Synthesis of Efficient Cu/CoFe2O4 Catalysts for Low Temperature CO Oxidation. Catal. Lett. 2019, 149, 399–409. [Google Scholar] [CrossRef]
- Anjana, P.; Raj, R.S.A.; Jose, R.; Kumari, M.; Sarun, P.M.; Sajan, D.; Joy, L.K. Highly enhanced dielectric permittivity in CoFe2O4 by the Gd substitution in the octahedral sites. J. All. Comp. 2021, 854, 155758. [Google Scholar] [CrossRef]
- Fettkenhauer, C.; Wang, X.; Kailasam, K.; Antoniettia, M.; Dontsova, D. Synthesis of efficient photocatalysts for water oxidation and dye degradation reactions using CoCl2 eutectics. J. Mater. Chem. A 2015, 3, 21227–21232. [Google Scholar] [CrossRef]
- Einert, M.; Waheed, A.; Lauterbach, S.; Melin, M.; Rohnke, M.; Wagner, L.Q.; Chuanmu, T.; Smarsly, B.M.; Jaegermann, W.; Schlaad, H.; et al. Sol-gel-derived Ordered Mesoporous High Entropy Spinel Ferrites and Assessment of their Photoelectrochemical and Electrocatalytic Water Splitting Performance. ChemRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Palatnikov, M.N.; Shcherbina, O.B.; Smirnov, M.V.; Deyneko, D.V.; Masloboeva, S.M. Luminescence and mechanical properties of GdNbO4 ceramic phosphors doped by Eu3+, Sm3+, Tb3+, Er3+. J. All. Comp. 2025, 1037, 182432. [Google Scholar] [CrossRef]

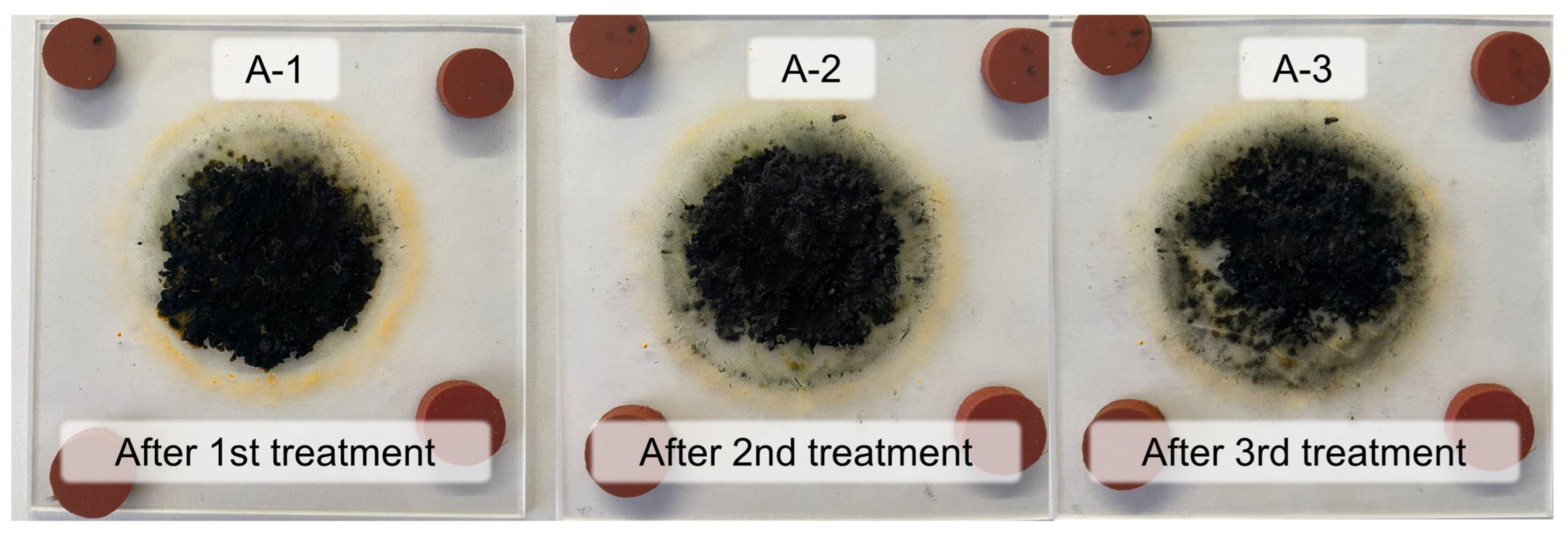



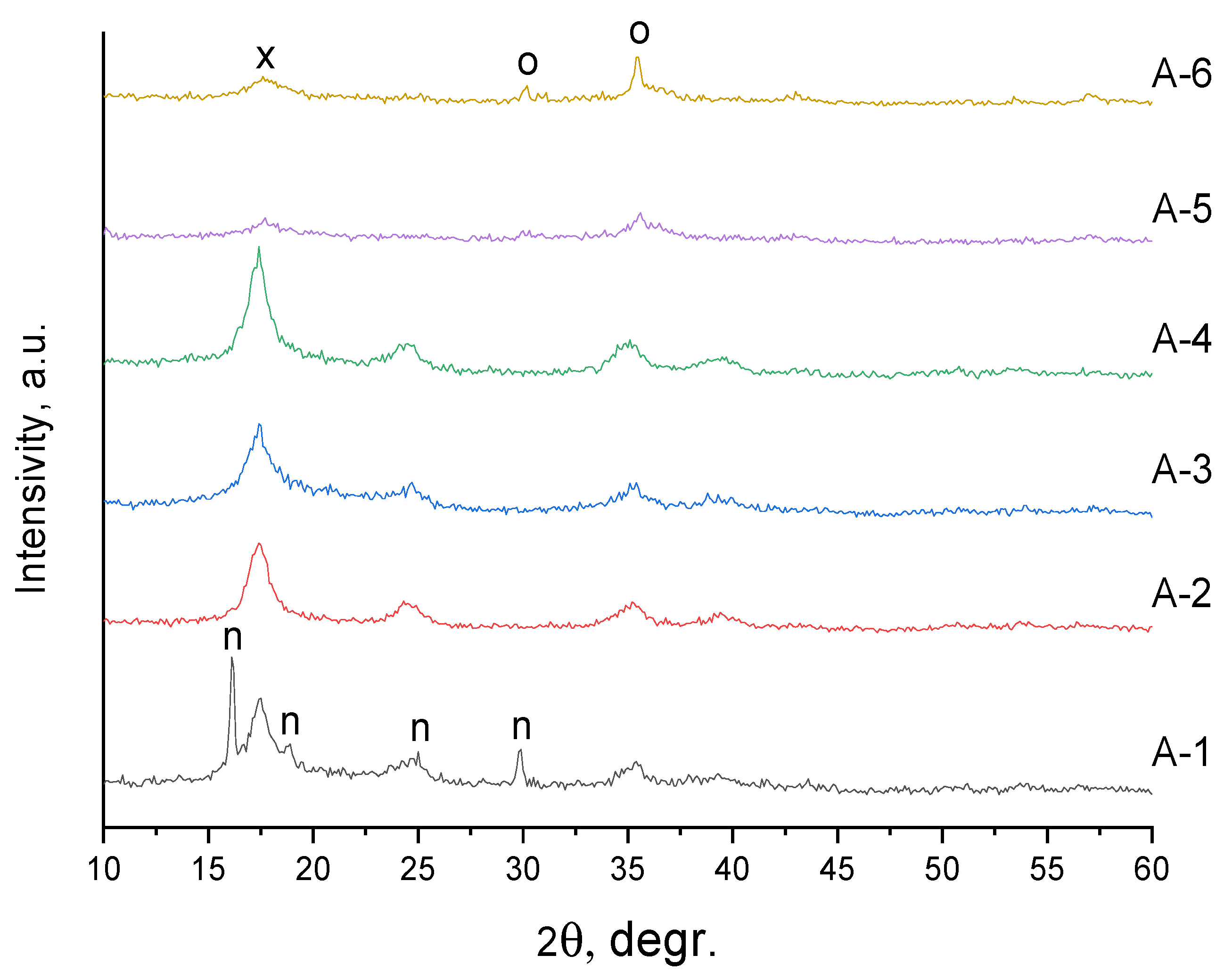
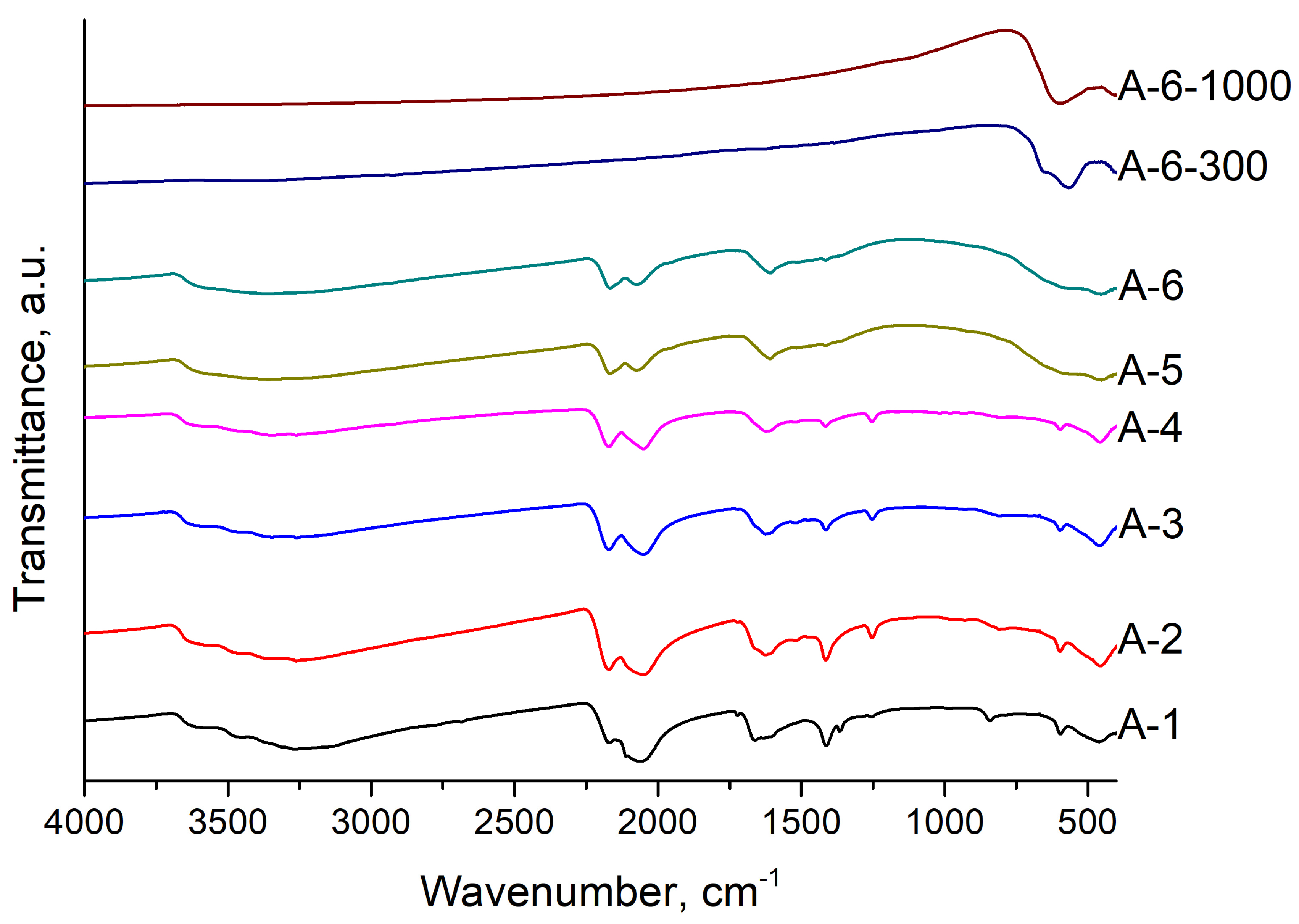
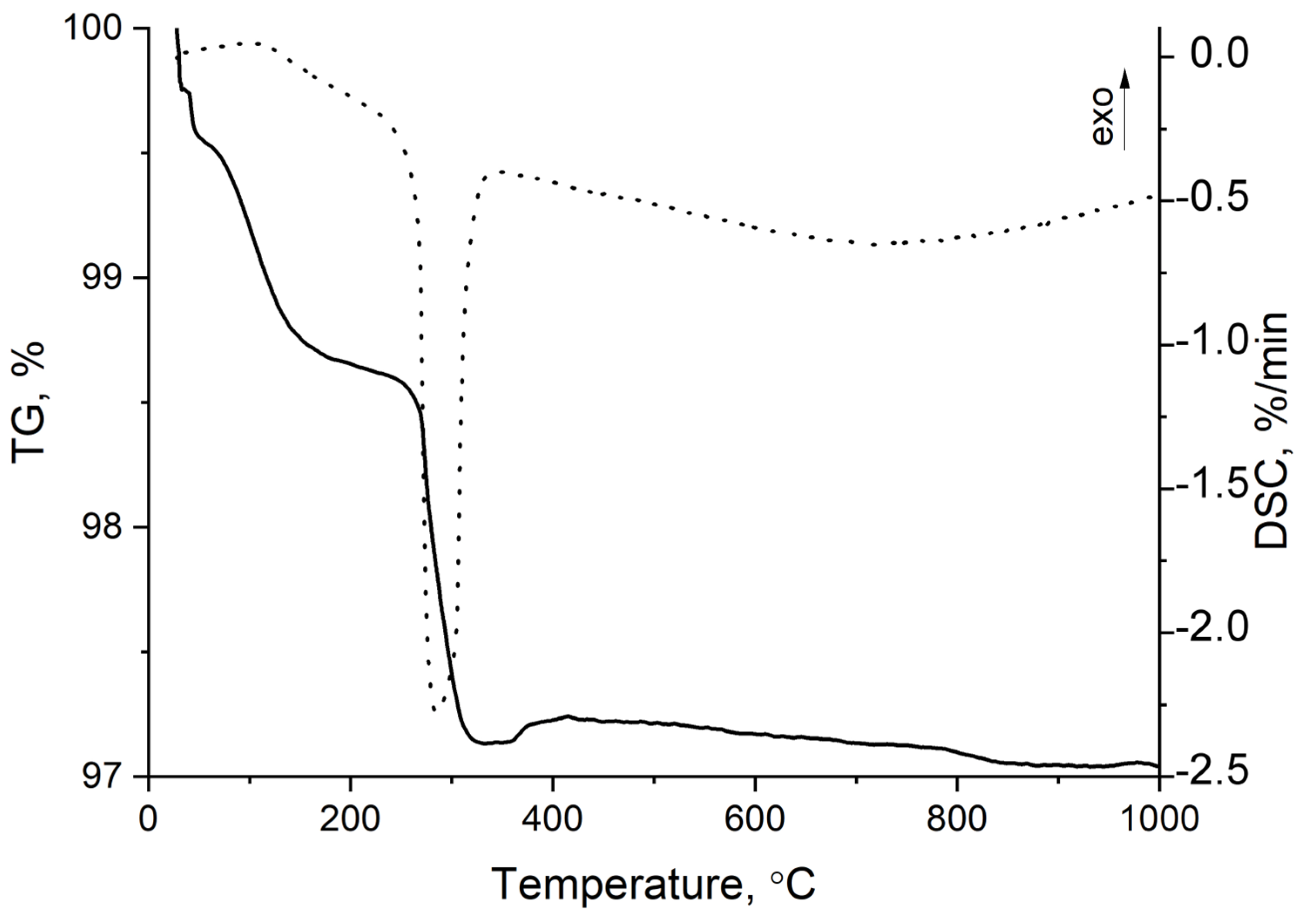
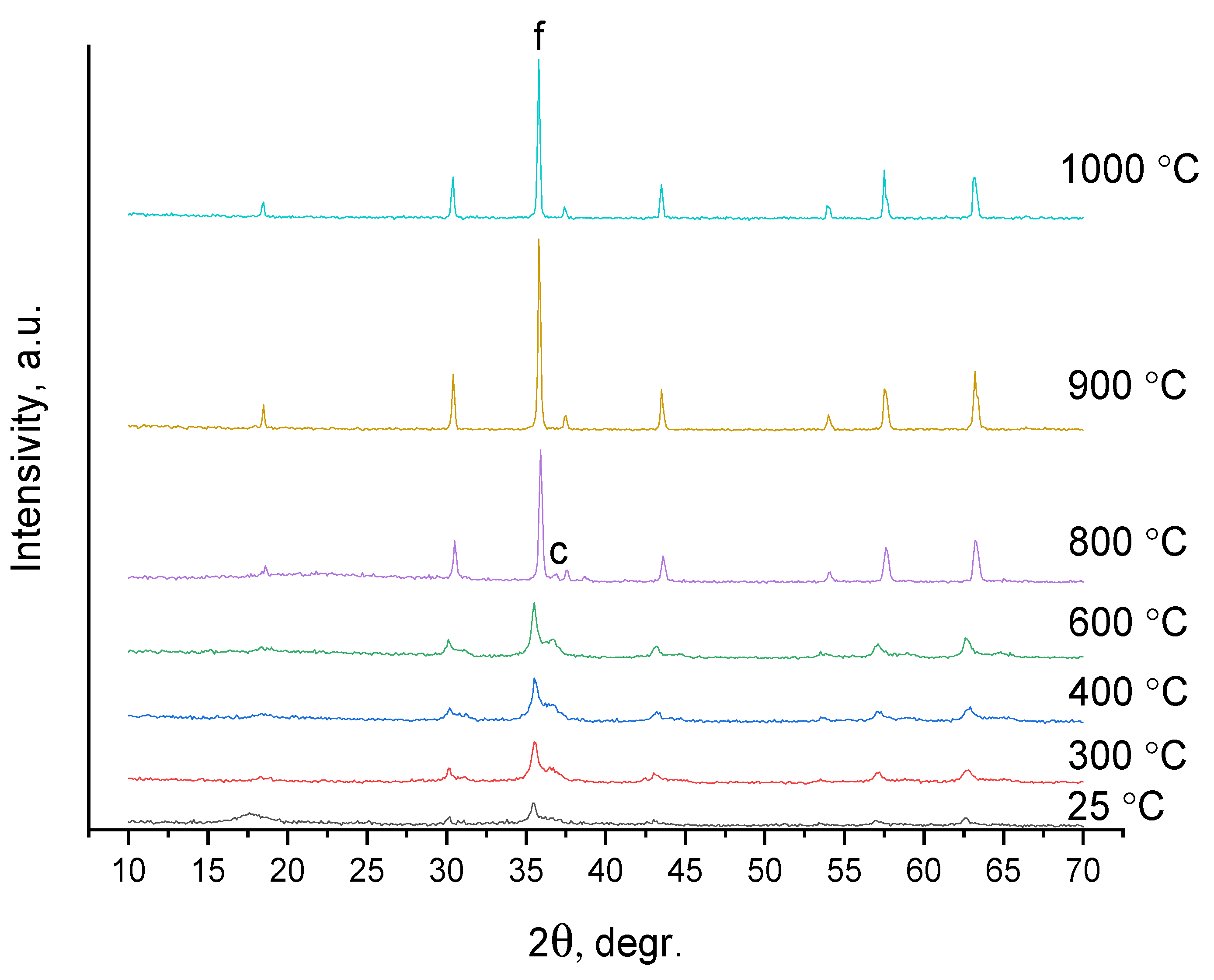
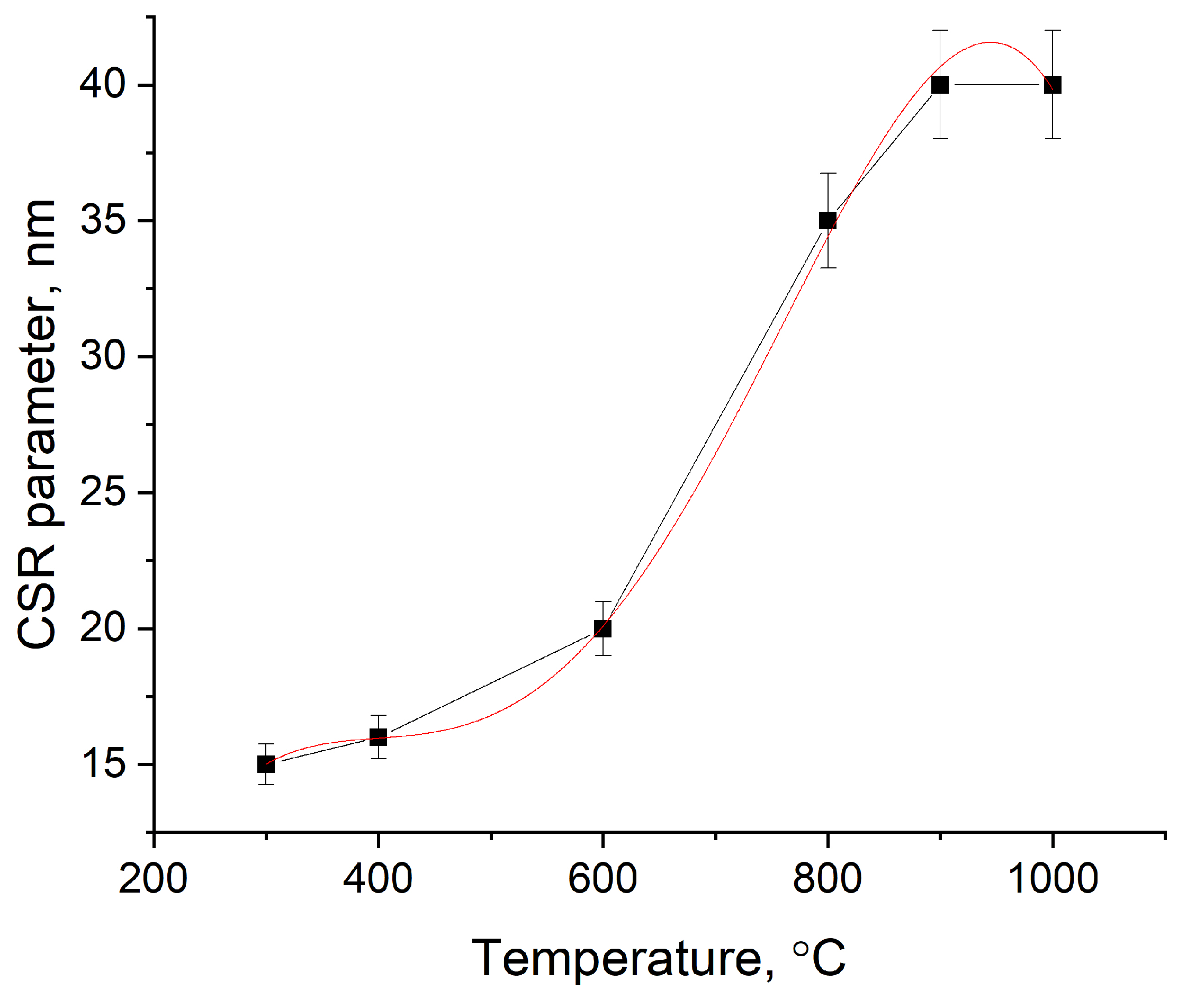
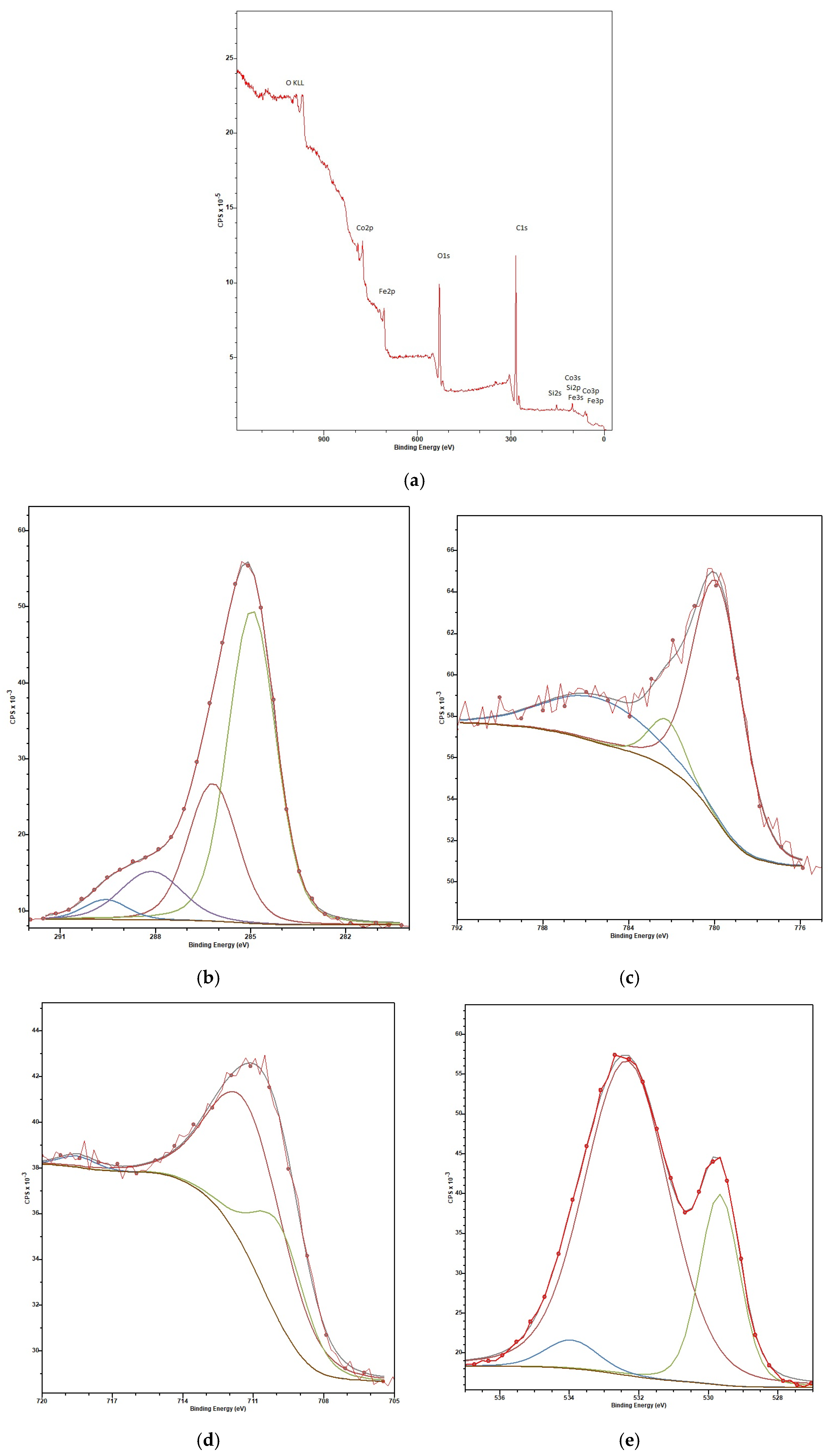
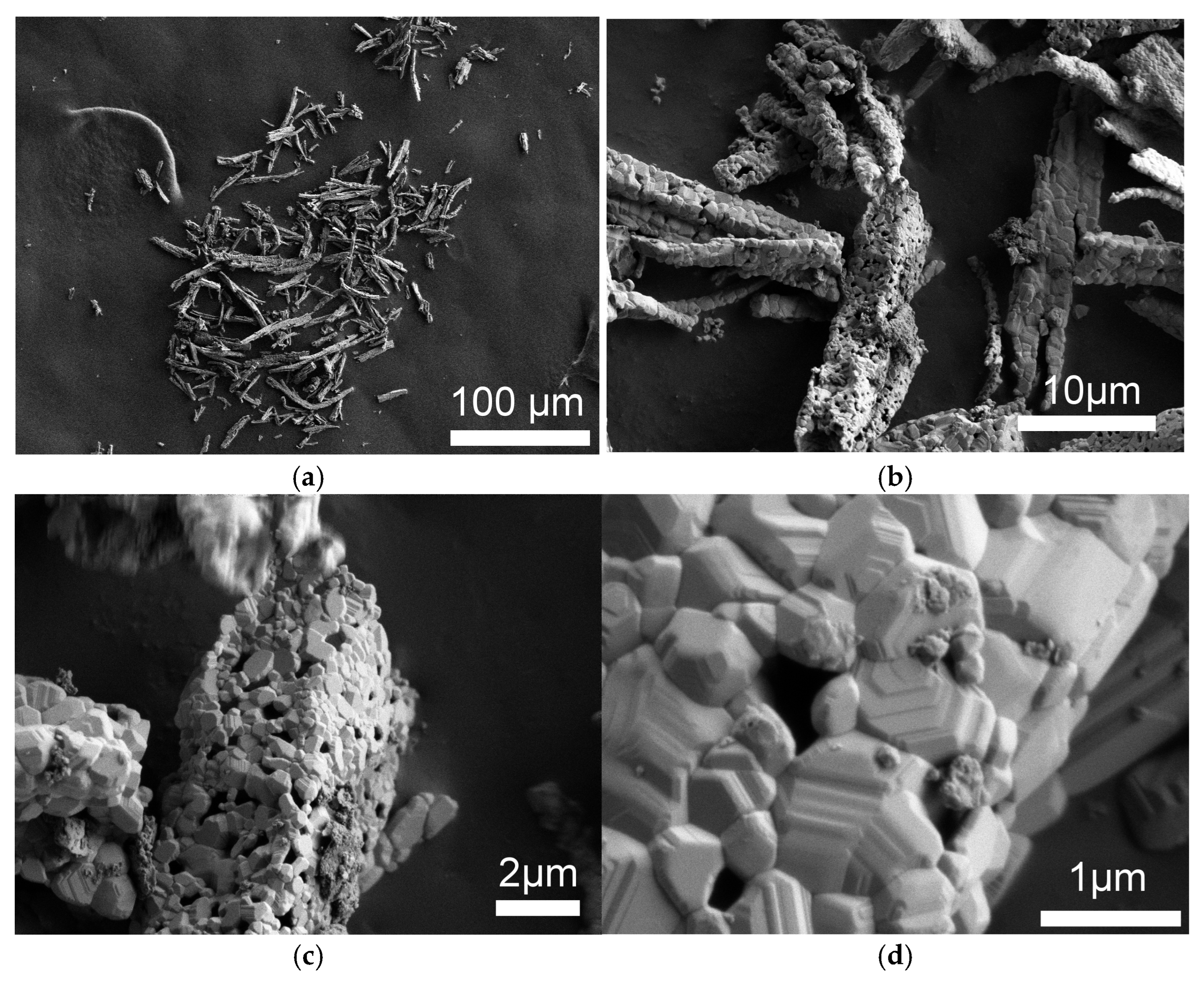
| Sample | Input Power, W | Plasma Treatment Duration | Remaining Mass After Treatment (%) |
|---|---|---|---|
| A-1 | 25 | 1 cycle of 10 min | 67.4 ± 0.2% |
| A-2 | 25 | 2 cycles of 10 min each | 63.2 ± 0.4% |
| A-3 | 25 | 3 cycles of 10 min each | 61.6 ± 0.4% |
| A-4 | 25 | 3 cycles of 15 min each | 62.1 ± 0.5% |
| A-5 | 100 | 1 cycle of 15 min | 57.0 ± 0.4% |
| A-6 | 100 | 2 cycles of 10 min each | 47 ± 0.2% |
| Model | Poly 4 |
|---|---|
| Equation | y = A0 + A1 × x + A2 × x2 + A3 × x3 + A4 × x4 |
| A0 | −43.73 ± 16.54 |
| A1 | 0.49 ± 0.12 |
| A2 | −0.0015 ± 0.0003 |
| A3 | 0.00019 ± 0.00003 |
| A4 | −0.000000082 ± 0.000000001 |
| Reduced Chi-Sqr | 0.24 |
| R2 | 0.999 |
| Adjusted R2 | 0.996 |
| Element | C | O | Si * | Fe | Co |
|---|---|---|---|---|---|
| At % | 70.9 | 23.4 | 4.3 | 0.7 | 0.7 |
| Wt % | 59.7 | 26.3 | 8.4 | 2.7 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosteva, A.; Golubev, O.; Vinogradov, V.; Svidersky, S.; Grabchak, A.; Manukovskaya, D.; Ivantsov, M.; Kulikova, M. Double Complex Salt [Co(NH3)6][Fe(CN)6] Plasma Treatment. Thermo 2025, 5, 36. https://doi.org/10.3390/thermo5030036
Gosteva A, Golubev O, Vinogradov V, Svidersky S, Grabchak A, Manukovskaya D, Ivantsov M, Kulikova M. Double Complex Salt [Co(NH3)6][Fe(CN)6] Plasma Treatment. Thermo. 2025; 5(3):36. https://doi.org/10.3390/thermo5030036
Chicago/Turabian StyleGosteva, Alevtina, Oleg Golubev, Vladimir Vinogradov, Sergei Svidersky, Alena Grabchak, Diana Manukovskaya, Mihail Ivantsov, and Mayya Kulikova. 2025. "Double Complex Salt [Co(NH3)6][Fe(CN)6] Plasma Treatment" Thermo 5, no. 3: 36. https://doi.org/10.3390/thermo5030036
APA StyleGosteva, A., Golubev, O., Vinogradov, V., Svidersky, S., Grabchak, A., Manukovskaya, D., Ivantsov, M., & Kulikova, M. (2025). Double Complex Salt [Co(NH3)6][Fe(CN)6] Plasma Treatment. Thermo, 5(3), 36. https://doi.org/10.3390/thermo5030036









