The Thermochemical Conversion of Municipal Solid Waste by Torrefaction Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
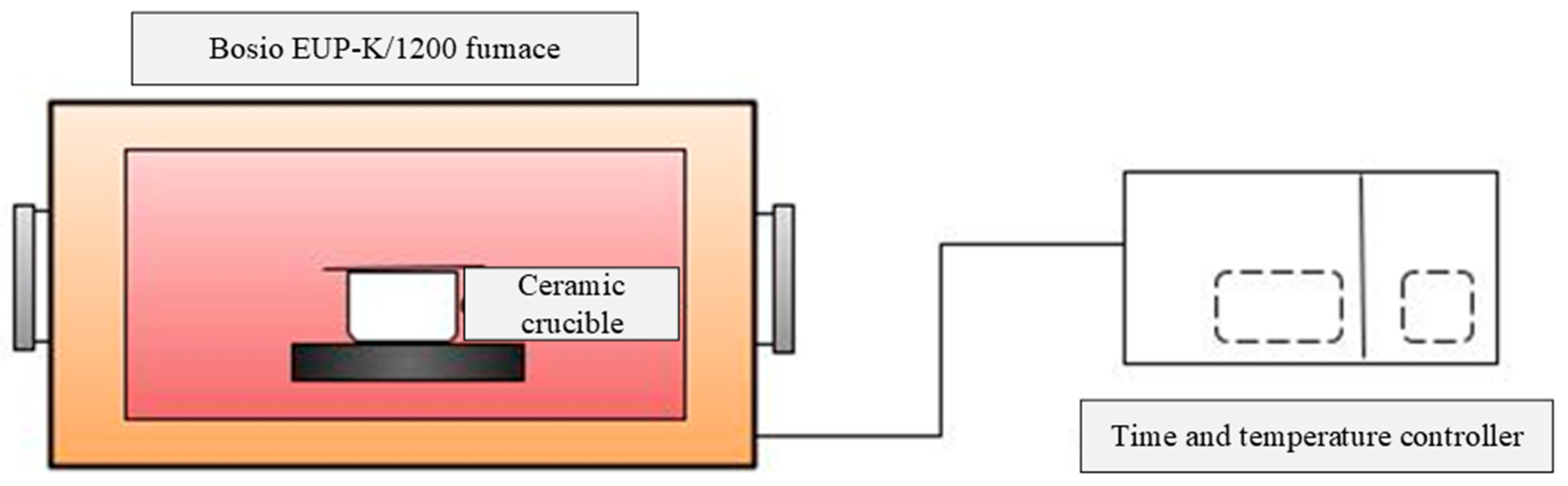
2.2. Torrefaction Experimental Setup and Procedure
2.3. Torrefaction Product Analysis
2.3.1. Elemental Analysis
2.3.2. Proximate Analysis
2.3.3. Heating Value
2.3.4. Thermogravimetric Analysis
3. Results and Discussion
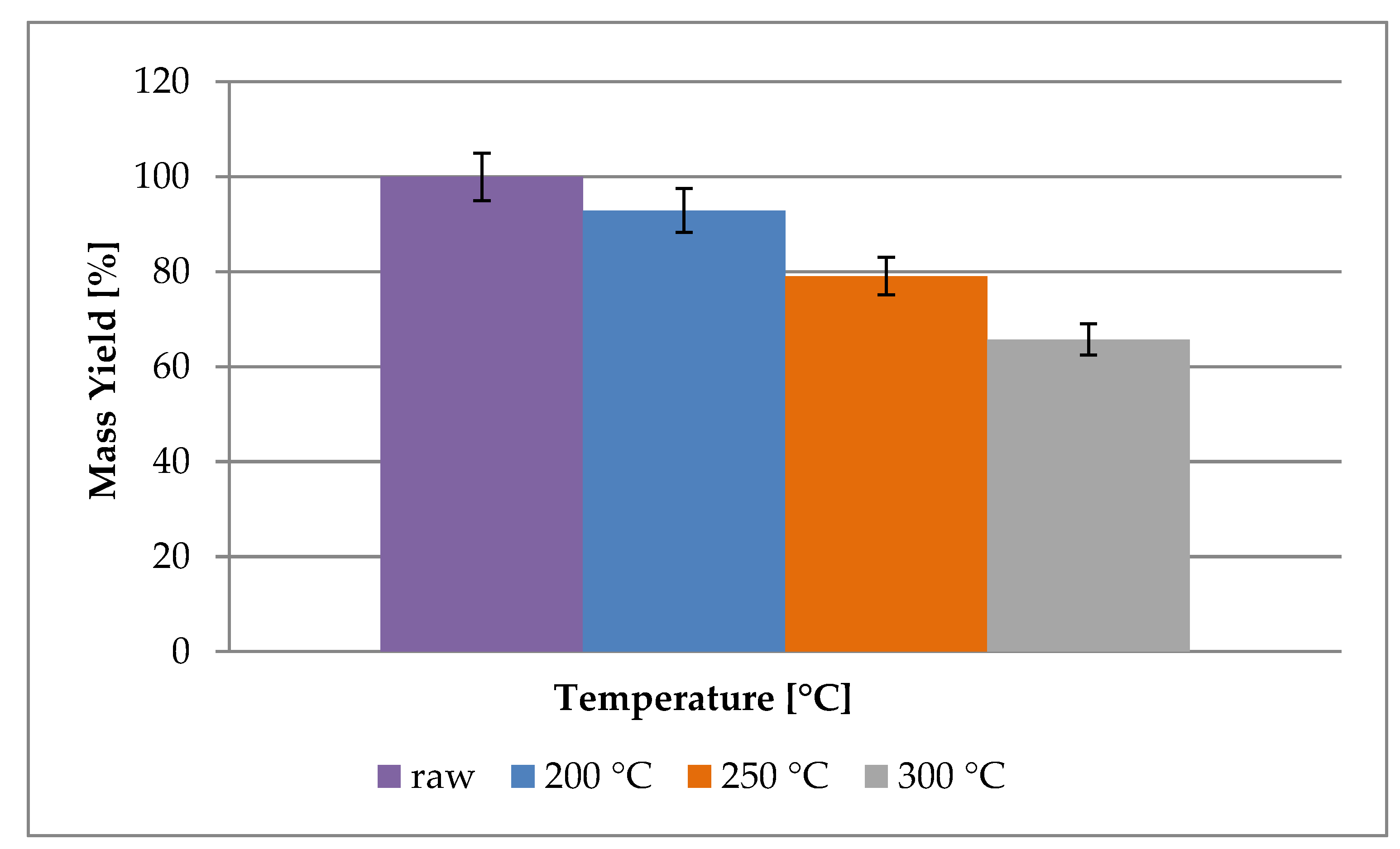
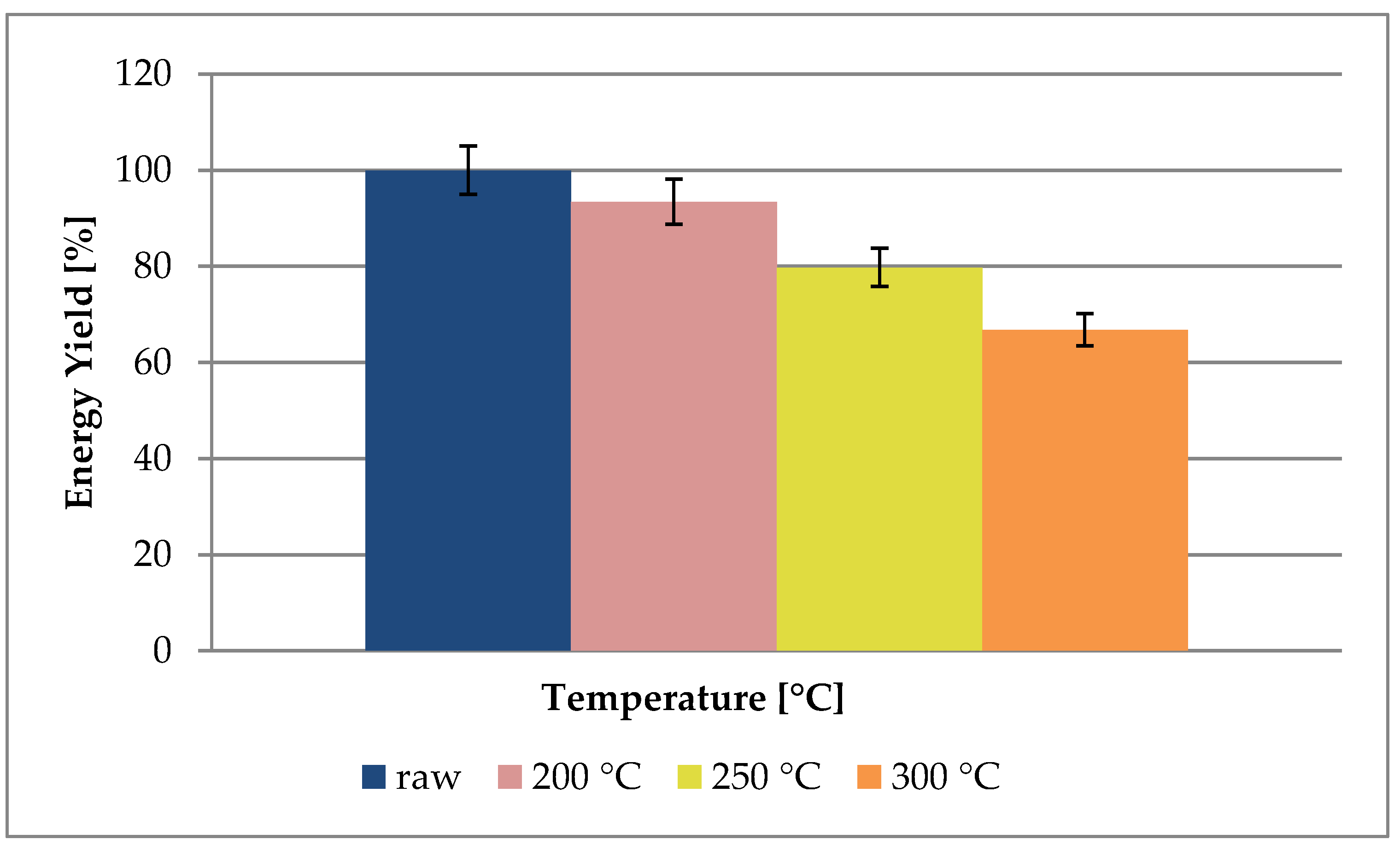
3.1. Mass and Energy Yield
3.2. Ultimate and Proximate Analysis
3.3. Energy Content
3.4. Fuel Ratio
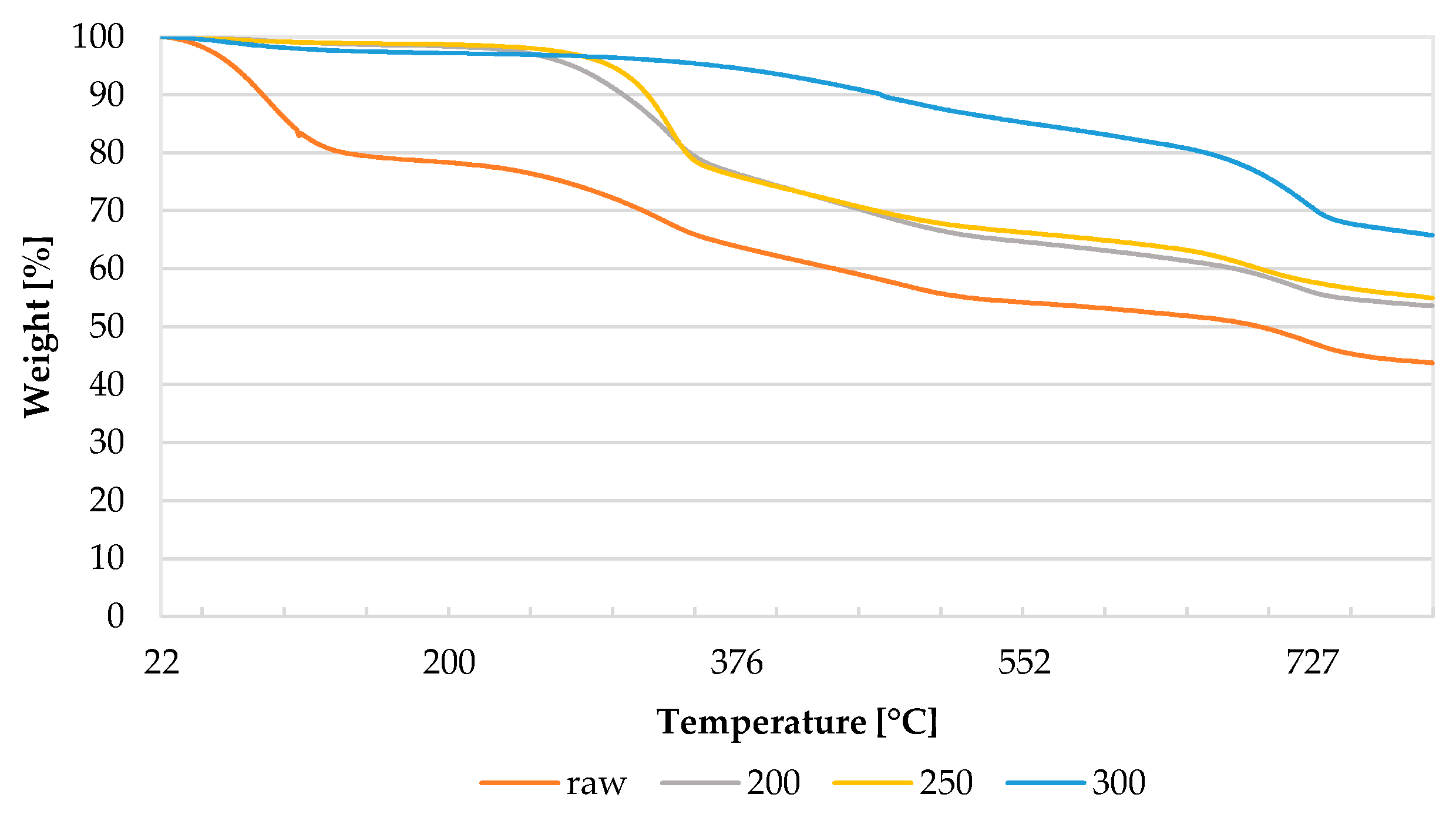
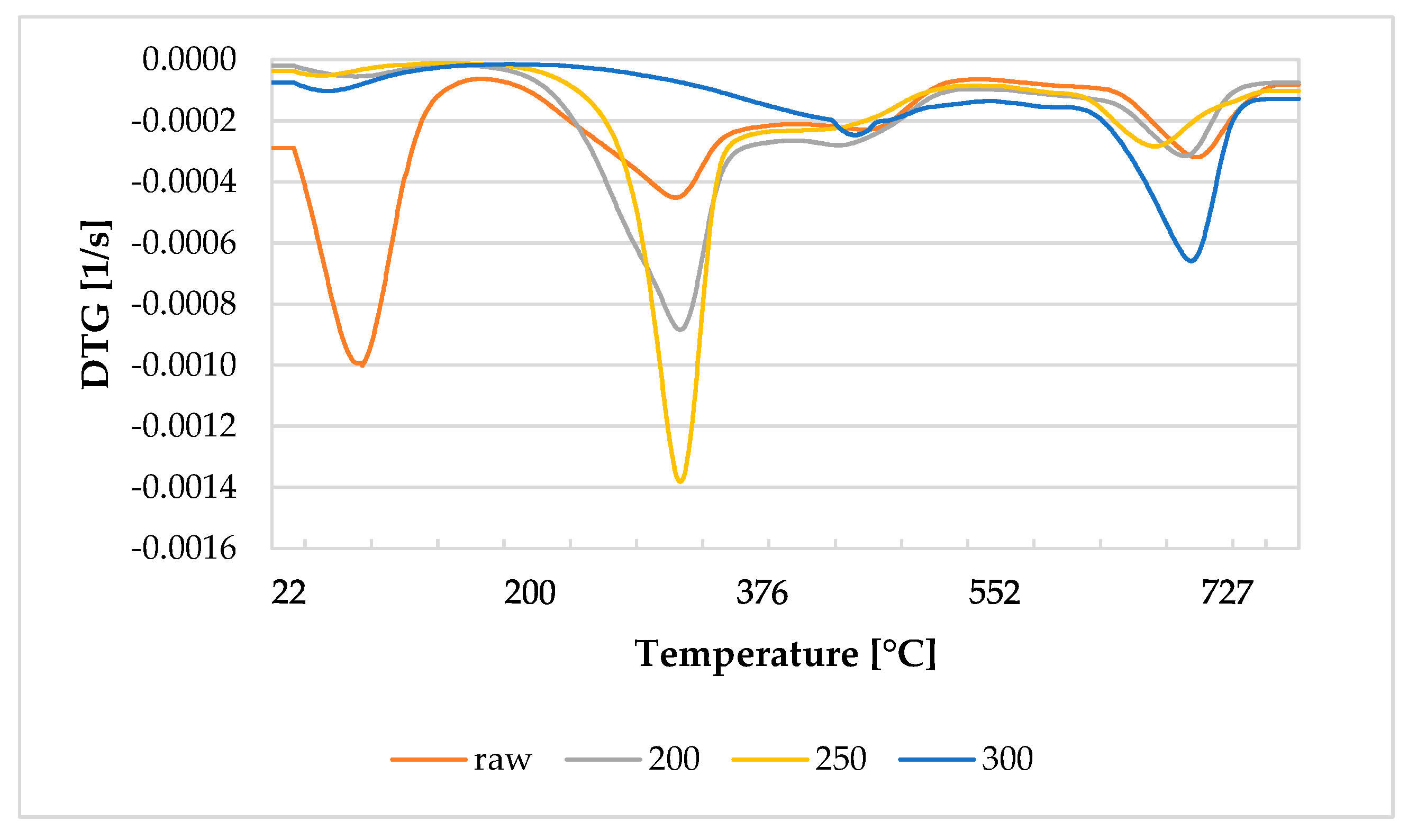
3.5. Thermal Behavior of Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DTG | Derivative thermogravimetric analysis |
| EF | Enhancement factor |
| EU | European Union |
| EY | Energy yield |
| FC | Fixed carbon |
| FR | Fuel ratio |
| GHG | Greenhouse gas emissions |
| HHV | High heating value (MJ kg−1) |
| MC | Moisture content |
| MSW | Municipal solid waste |
| MY | Mass yield |
| RES | Renewable energy sources |
| SURS | Statistical Biro of Slovenia |
| VM | Volatile matter content |
| TGA | Thermogravimetric analysis |
| T | Temperature |
References
- European Commission European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 10 April 2023).
- Climate and Energy Framework. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2030-climate-energy-framework_en (accessed on 10 April 2023).
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A Comprehensive Study of Renewable Energy Sources: Classifications, Challenges and Suggestions. Energy Strateg. Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Bekirsky, N.; Hoicka, C.E.; Brisbois, M.C.; Ramirez Camargo, L. Many Actors amongst Multiple Renewables: A Systematic Review of Actor Involvement in Complementarity of Renewable Energy Sources. Renew. Sustain. Energy Rev. 2022, 161, 112368. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Poudel, J.; Karki, S.; Oh, S.C. Valorization of Waste Wood as a Solid Fuel by Torrefaction. Energies 2018, 11, 1641. [Google Scholar] [CrossRef]
- Novian Cahyanti, M.; Rama Krishna, T.; Doddapaneni, C.; Kikas, T. Biomass Torrefaction: An Overview on Process Parameters, Economic and Environmental Aspects and Recent Advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Z. Cost of Economic Growth: Air Pollution and Health Expenditure. Sci. Total Environ. 2021, 755, 142543. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose Biomass Pyrolysis for Bio-Oil Production: A Review of Biomass Pre-Treatment Methods for Production of Drop-in Fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Das, P.; Chandramohan, V.P.; Mathimani, T.; Pugazhendhi, A. A Comprehensive Review on the Factors Affecting Thermochemical Conversion Efficiency of Algal Biomass to Energy. Sci. Total Environ. 2021, 766, 144213. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.; Ferreira, S. Biomass Waste for Energy Production. Energies 2022, 15, 5943. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction Pretreatment of Lignocellulosic Biomass for Sustainable Solid Biofuel Production. Energy 2021, 240, 122483. [Google Scholar] [CrossRef]
- Gagliano, A.; Nocera, F.; Bruno, M.; Blanco, I. Effectiveness of Thermodynamic Adaptative Equilibrium Models for Modeling the Pyrolysis Process. Sustain. Energy Technol. Assess. 2018, 27, 74–82. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on Torrefaction of Corn Stalk from the Point of View of Deoxygenation and Decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Madissoo, M.; Pärn, L.; Virro, I.; Kikas, T. Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies 2021, 14, 2774. [Google Scholar] [CrossRef]
- Delgado, B.; López González, D.; Godbout, S.; Lagacé, R.; Giroir-Fendler, A.; Avalos Ramirez, A. A Study of Torrefied Cardboard Characterization and Applications: Composition, Oxidation Kinetics and Methane Adsorption. Sci. Total Environ. 2017, 593–594, 406–417. [Google Scholar] [CrossRef]
- Kota, K.B.; Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Ghodke, P.K.; Chen, W.H. Biomass Torrefaction: An Overview of Process and Technology Assessment Based on Global Readiness Level. Fuel 2022, 324, 124663. [Google Scholar] [CrossRef]
- Grams, J. Surface Analysis of Solid Products of Thermal Treatment of Lignocellulosic Biomass. J. Anal. Appl. Pyrolysis 2022, 161, 105429. [Google Scholar] [CrossRef]
- Xu, J.; Huang, M.; Hu, Z.; Zhang, W.; Li, Y.; Yang, Y.; Zhou, Y.; Zhou, S.; Ma, Z. Prediction and Modeling of the Basic Properties of Biomass after Torrefaction Pretreatment. J. Anal. Appl. Pyrolysis 2021, 159, 105287. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, C.L.; Show, P.L.; Ong, H.C. Torrefaction Performance Prediction Approached by Torrefaction Severity Factor. Fuel 2019, 251, 126–135. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of Torrefaction on the Grindability and Fuel Characteristics of Forest Biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Nwabunwanne, N.; Vuyokazi, T.; Olagoke, A.; Mike, O.; Patrick, M.; Anthony, O. Improving the Solid Fuel Properties of Non-Lignocellulose and Lignocellulose Materials through Torrefaction. Materials 2021, 14, 2072. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, Y.; Zhang, S.; Zheng, Y.; Li, W.; Chai, H.; Liu, K. Effect of Temperature Oscillation on Torrefaction and Pyrolysis of Elm. SSRN Electron. J. 2022, 271, 127055. [Google Scholar] [CrossRef]
- Zhang, D.; Han, P.; Zheng, H.; Yan, Z. Torrefaction of Walnut Oil Processing Wastes by Superheated Steam: Effects on Products Characteristics. Sci. Total Environ. 2022, 830, 154649. [Google Scholar] [CrossRef] [PubMed]
- Abdulyekeen, K.A.; Daud, W.M.A.W.; Patah, M.F.A.; Abnisa, F. Torrefaction of Organic Municipal Solid Waste to High Calorific Value Solid Fuel Using Batch Reactor with Helical Screw Induced Rotation. Bioresour. Technol. 2022, 363, 127974. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R. Municipal Solid Waste Management. Routledge Handb. Environ. Policy China 2017, 8, 302–313. [Google Scholar] [CrossRef]
- Babasaheb, M.; Matsagar, K.C.-W.W. Biochar in Agriculture for Achieving Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Pulidori, E.; Gonzalez-Rivera, J.; Pelosi, C.; Ferrari, C.; Bernazzani, L.; Bramanti, E.; Tiné, M.R.; Duce, C. Thermochemical Evaluation of Different Waste Biomasses (Citrus Peels, Aromatic Herbs, and Poultry Feathers) towards Their Use for Energy Production. Thermo 2023, 3, 66–75. [Google Scholar] [CrossRef]
- Knapczyk, A.; Francik, S.; Jewiarz, M.; Zawiślak, A.; Francik, R. Thermal Treatment of Biomass: A Bibliometric Analysis—The Torrefaction Case. Energies 2021, 14, 162. [Google Scholar] [CrossRef]
- Mu’min, G.F.; Prawisudha, P.; Zaini, I.N.; Aziz, M.; Pasek, A.D. Municipal Solid Waste Processing and Separation Employing Wet Torrefaction for Alternative Fuel Production and Aluminum Reclamation. Waste Manag. 2017, 67, 106–120. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. The Solid Waste Management. Available online: https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management (accessed on 10 April 2023).
- Zhu, X.; Li, S.; Zhang, Y.; Li, J.; Zhang, Z.; Sun, Y.; Zhou, S.; Li, N.; Yan, B.; Chen, G. Flue Gas Torrefaction of Municipal Solid Waste: Fuel Properties, Combustion Characterizations, and Nitrogen/Sulfur Emissions. Bioresour. Technol. 2022, 351, 126967. [Google Scholar] [CrossRef] [PubMed]
- Triyono, B.; Prawisudha, P.; Aziz, M.; Mardiyati; Pasek, A.D.; Yoshikawa, K. Utilization of Mixed Organic-Plastic Municipal Solid Waste as Renewable Solid Fuel Employing Wet Torrefaction. Waste Manag. 2019, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Ping, Z.; Xiqiang, Z.; Zhanlong, S.; Wenlong, W.; Jing, S.; Yanpeng, M. Applicability of Municipal Solid Waste Incineration (MSWI) System Integrated with Pre-Drying or Torrefaction for Flue Gas Waste Heat Recovery. Energy 2021, 224, 120157. [Google Scholar] [CrossRef]
- Statistični urad Republike Slovenije. Mešani Komunalni Odpadki. Available online: https://www.stat.si/StatWeb/News/Index/10977 (accessed on 10 April 2023).
- Ivanovski, M.; Petrovic, A.; Ban, I.; Goricanec, D.; Urbancl, D. Determination of the Kinetics and Thermodynamic Parameters of Lignocellulosic Biomass Subjected to the Torrefaction Process. Materials 2021, 14, 7877. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Pärn, L.; Kikas, T. Torrefaction of Pulp Industry Sludge to Enhance Its Fuel Characteristics. Energies 2022, 15, 6175. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Gajek, M.; Zajemska, M.; Jayaraman, K.; Gokalp, I. Combustion and Kinetic Parameters Estimation of Torrefied Pine, Acacia and Miscanthus Giganteus Using Experimental and Modelling Techniques. Bioresour. Technol. 2017, 243, 304–314. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H.; Chu, Y.S.; Skreiberg, Ø. Predictions of Biochar Yield and Elemental Composition during Torrefaction of Forest Residues. Bioresour. Technol. 2016, 215, 239–246. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Li, J.; Zhou, S.; Yan, B.; Sun, Y.; Chen, G. A Component Synergy of Flue Gas Torrefaction of Municipal Solid Waste. Fuel Process. Technol. 2022, 238, 107517. [Google Scholar] [CrossRef]
- Iroba, K.L.; Baik, O.D.; Tabil, L.G. Torrefaction of Biomass from Municipal Solid Waste Fractions I: Temperature Profiles, Moisture Content, Energy Consumption, Mass Yield, and Thermochemical Properties. Biomass Bioenergy 2017, 105, 320–330. [Google Scholar] [CrossRef]
- Rago, Y.P.; Collard, F.X.; Görgens, J.F.; Surroop, D.; Mohee, R. Torrefaction of Biomass and Plastic from Municipal Solid Waste Streams and Their Blends: Evaluation of Interactive Effects. Fuel 2020, 277, 118089. [Google Scholar] [CrossRef]
- Chang, S.S.; Sambeth, S.K.; Abdul Samad, N.A.F.; Saleh, S. Effect of Torrefaction on Thermal Degradation and Functional Group of Oil Palm Solid Waste. Mater. Today Proc. 2022, 57, 1248–1255. [Google Scholar] [CrossRef]
- Ivanovski, M.; Urbancl, D.; Petrovič, A.; Stergar, J.; Goričanec, D.; Simonič, M. Improving Lignocellulosic and Non-Lignocellulosic Biomass Characteristics through Torrefaction Process. Appl. Sci. 2022, 12, 2210. [Google Scholar] [CrossRef]
- Sher, F.; Yaqoob, A.; Saeed, F.; Zhang, S.; Jahan, Z.; Klemeš, J.J. Torrefied Biomass Fuels as a Renewable Alternative to Coal in Co-Firing for Power Generation. Energy 2020, 209, 118444. [Google Scholar] [CrossRef]
- Gajera, B.; Tyagi, U.; Sarma, A.K.; Jha, M.K. A Study of Impact of Torrefaction on Thermal Behavior of Wheat Straw and Groundnut Stalk Biomass from Punjab, India: Kinetic and Thermodynamic Approach. SSRN Electron. J. 2022, 12, 100073. [Google Scholar] [CrossRef]
- Demirbas, A. Potential Applications of Renewable Energy Sources, Biomass Combustion Problems in Boiler Power Systems and Combustion Related Environmental Issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal Solid Waste Management and Waste-to-Energy in the Context of a Circular Economy and Energy Recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Abdulyekeen, K.A.; Umar, A.A.; Patah, M.F.A.; Daud, W.M.A.W. Torrefaction of Biomass: Production of Enhanced Solid Biofuel from Municipal Solid Waste and Other Types of Biomass. Renew. Sustain. Energy Rev. 2021, 150, 111436. [Google Scholar] [CrossRef]
- Fu, J.; Liu, J.; Xu, W.; Chen, Z.; Evrendilek, F.; Sun, S. Torrefaction, Temperature, and Heating Rate Dependencies of Pyrolysis of Coffee Grounds: Its Performances, Bio-Oils, and Emissions. Bioresour. Technol. 2022, 345, 126346. [Google Scholar] [CrossRef]
- Oluoti, K.; Doddapaneni, T.R.K.C.; Richards, T. Investigating the Kinetics and Biofuel Properties of Alstonia Congensis and Ceiba Pentandra via Torrefaction. Energy 2018, 150, 134–141. [Google Scholar] [CrossRef]
- Huang, Y.F.; Cheng, P.H.; Chiueh, P.T.; Lo, S.L. Microwave Torrefaction of Leucaena to Produce Biochar with High Fuel Ratio and Energy Return on Investment. Energy Procedia 2017, 105, 35–40. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Meda, V. Enhancement of Fuel and Physicochemical Properties of Canola Residues via Microwave Torrefaction. Energy Rep. 2021, 7, 6338–6353. [Google Scholar] [CrossRef]
| Elemental Analysis (wt.%, Dry Basis) | |
|---|---|
| C | 43.2 ± 0.9 |
| H | 8.1 ± 0.16 |
| N | 0.78 ± 0.01 |
| O | 47.9 ± 0.9 |
| S | 0.03 ± 0.0 |
| Proximate Analysis (wt.%, dry basis) | |
| Moisture Content (MC) | 44.3 ± 0.9 |
| Volatile Matter (VM) | 54.9 ± 1.09 |
| Fixed Carbon Content (FC) | 6.7 ± 0.1 |
| Ash Content (Ash) | 3.1 ± 0.06 |
| Higher heating value (HHV, MJ/kg) | 24.3 ± 0.5 |
| Theoretical higher heating value (HHVt, MJ/kg) | 19.6 ± 0.4 |
| T (°C) | Elemental Analysis (wt.%, Dry Basis) | MC (wt.%, Dry Basis) | Proximate Analysis (wt.%, Dry Basis) | HHV (MJ/kg) | Theoretical HHV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | FC | VM | Ash | ||||
| 200 | 45.7 ± 0.9 | 7.9 ± 0.2 | 0.79 ± 0.0 | 45.6 ± 0.9 | 0.03 ± 0.00 | 44.6 ± 0.9 | 7.3 ± 0.1 | 44.1 ± 0.9 | 3.9 ± 0.1 | 24.6 ± 0.5 | 20.4 ± 0.4 |
| 250 | 48.1 ± 0.9 | 7.3 ± 0.1 | 0.82 ± 0.0 | 43.6 ± 0.9 | 0.28 ± 0.00 | 41.8 ± 0.8 | 8.3 ± 0.2 | 44.6 ± 0.9 | 5.3 ± 0.1 | 24.6 ± 5 | 20.7 ± 0.4 |
| 300 | 52.9 ± 1.0 | 6.6 ± 0.1 | 0.85 ± 0.0 | 39.5 ± 0.8 | 0.21 ± 0.00 | 38.7 ± 0.7 | 10.1 ± 0.2 | 44.7 ± 0.9 | 6.6 ± 0.1 | 25.3 ± 0.5 | 22.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovski, M.; Goričanec, D.; Urbancl, D. The Thermochemical Conversion of Municipal Solid Waste by Torrefaction Process. Thermo 2023, 3, 277-288. https://doi.org/10.3390/thermo3020017
Ivanovski M, Goričanec D, Urbancl D. The Thermochemical Conversion of Municipal Solid Waste by Torrefaction Process. Thermo. 2023; 3(2):277-288. https://doi.org/10.3390/thermo3020017
Chicago/Turabian StyleIvanovski, Maja, Darko Goričanec, and Danijela Urbancl. 2023. "The Thermochemical Conversion of Municipal Solid Waste by Torrefaction Process" Thermo 3, no. 2: 277-288. https://doi.org/10.3390/thermo3020017
APA StyleIvanovski, M., Goričanec, D., & Urbancl, D. (2023). The Thermochemical Conversion of Municipal Solid Waste by Torrefaction Process. Thermo, 3(2), 277-288. https://doi.org/10.3390/thermo3020017





