The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be
Abstract
1. Introduction
2. Results and Discussion
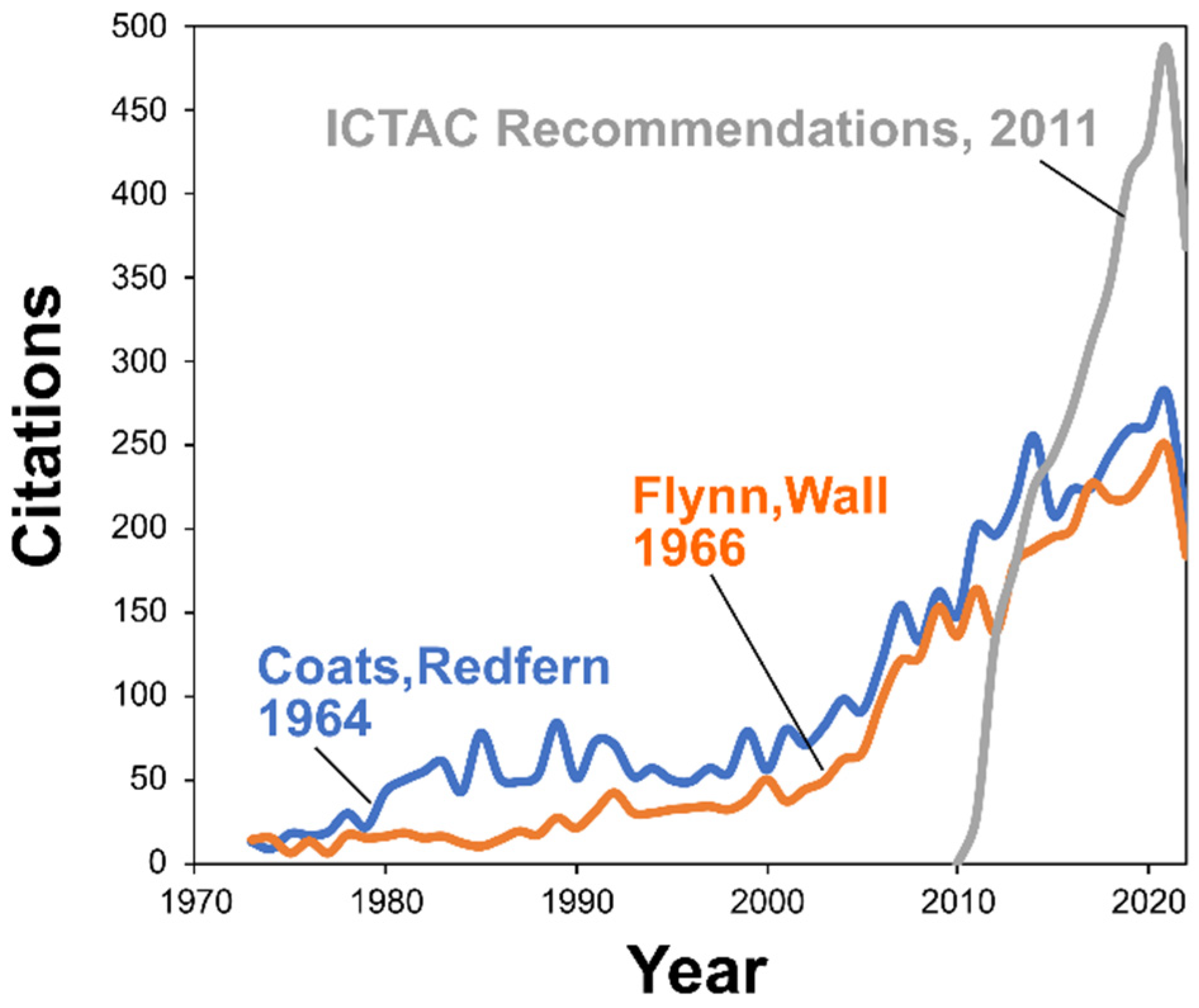
2.1. Overview of Thermokinetic Research Development
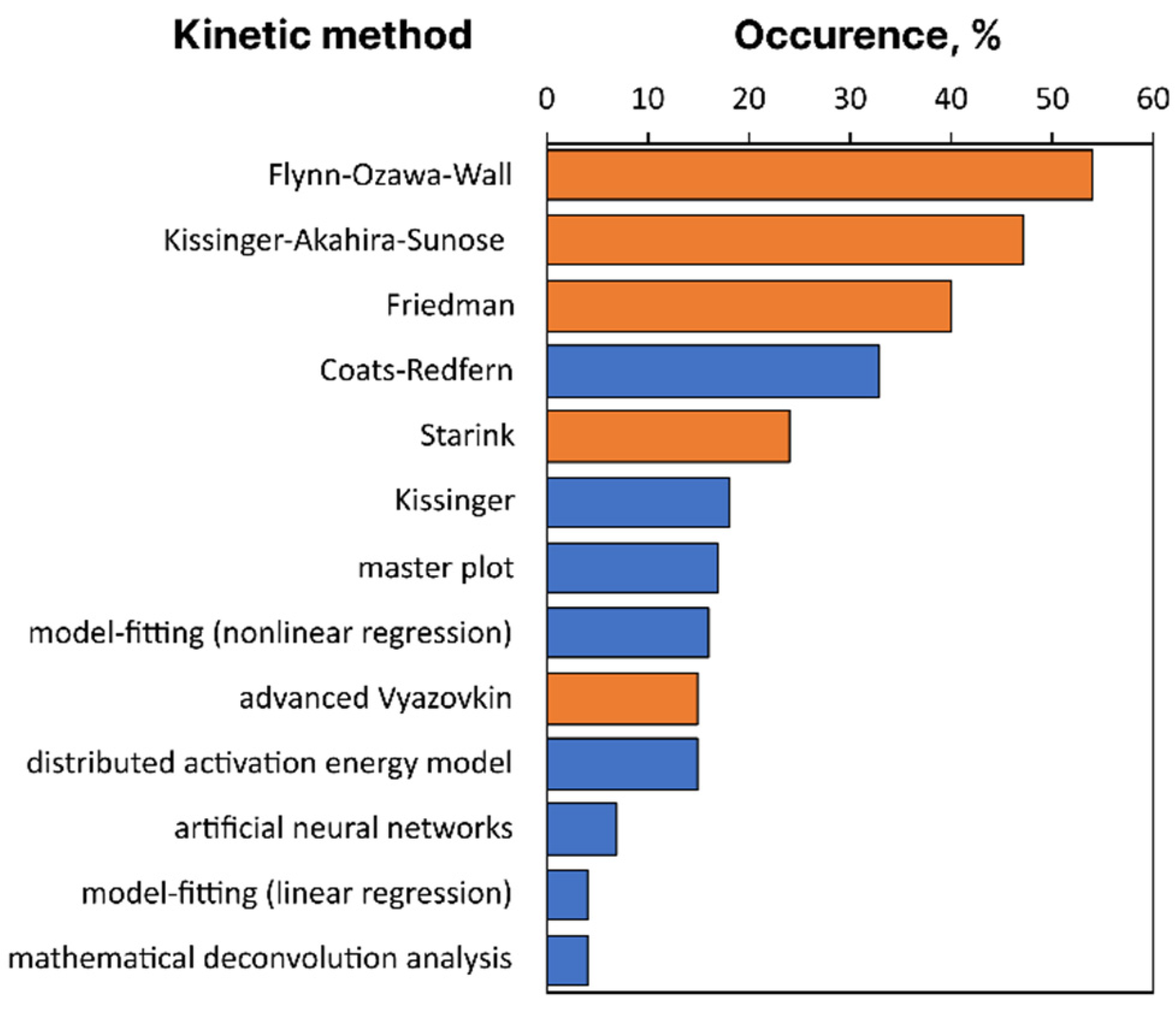
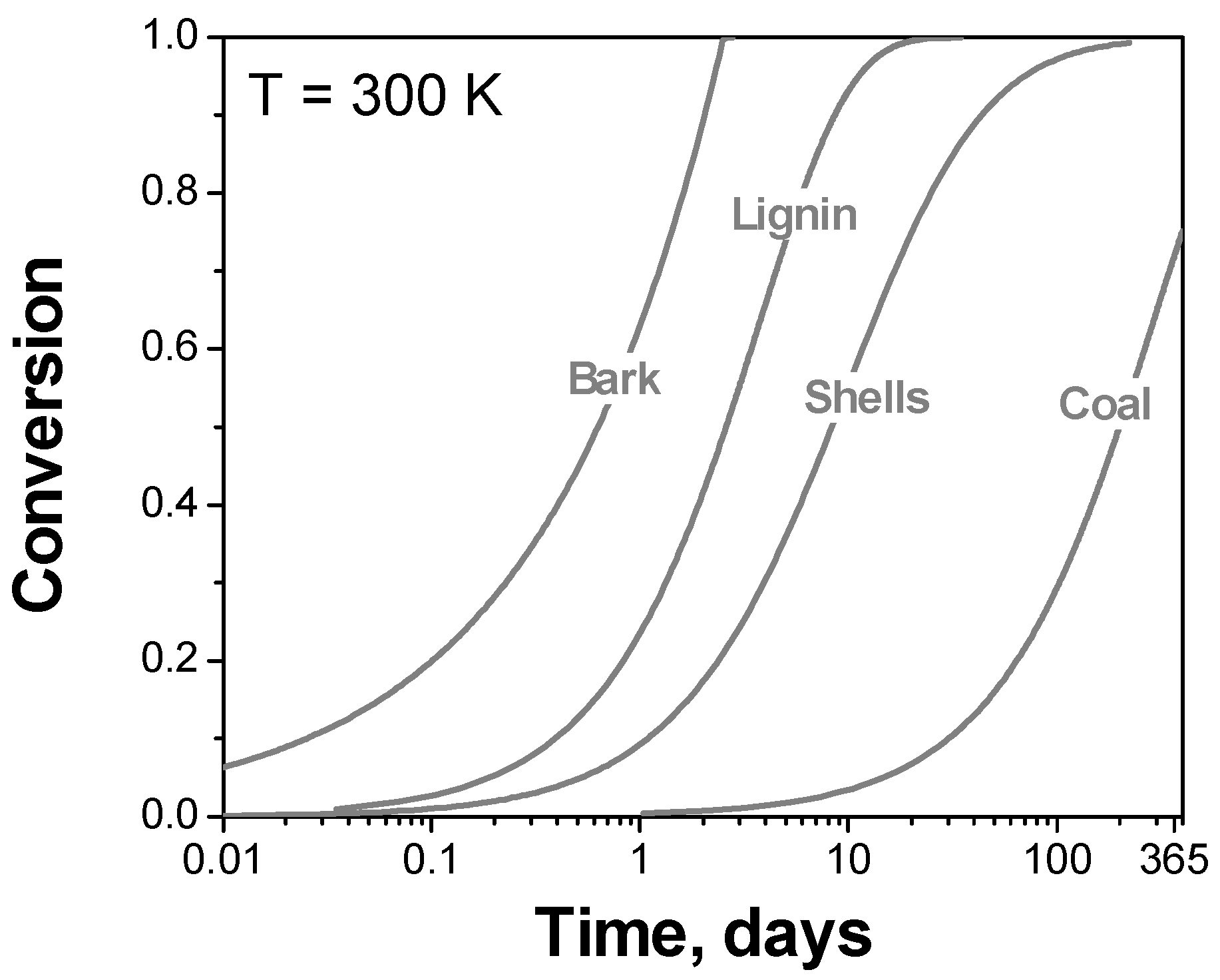
2.2. Typical Problems of Kinetic Computations
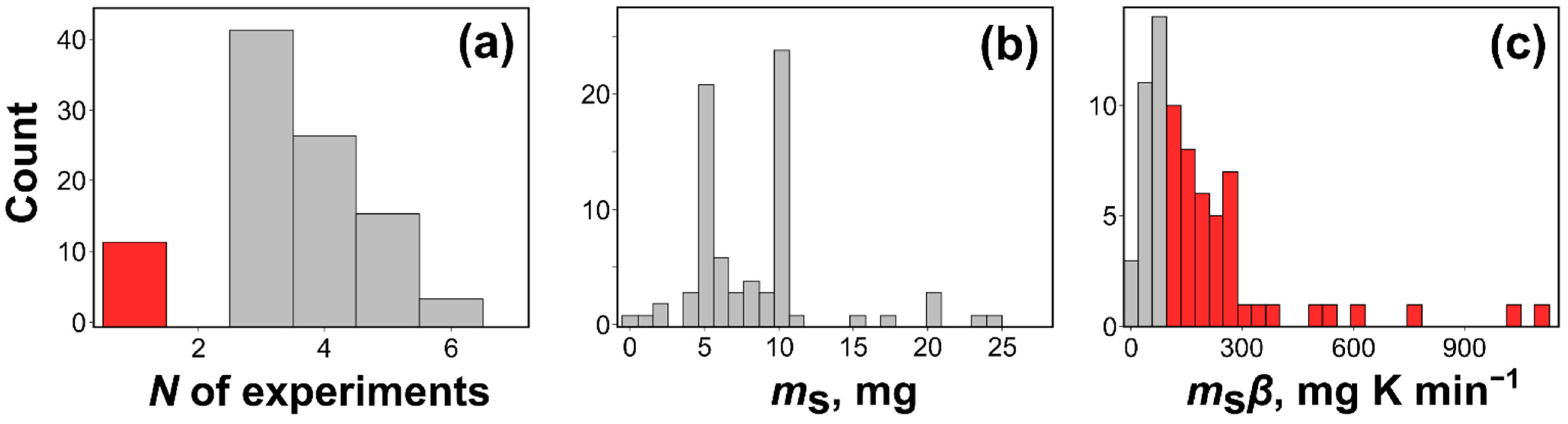
2.3. Typical Problems of Data Collecting and Reporting
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Analysis of Thermal Polymerization Kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Málek, J. Kinetic Analysis of Crystallization Processes in Amorphous Materials. Thermochim. Acta 2000, 355, 239–253. [Google Scholar] [CrossRef]
- Burnham, A.K.; Zhou, X.; Broadbelt, L.J. Critical Review of the Global Chemical Kinetics of Cellulose Thermal Decomposition. Energy Fuels 2015, 29, 2906–2918. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Perez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee Recommendations for Analysis of Thermal Decomposition Kinetics. Thermochim. Acta 2022, 179384, in press. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic Waste Recycling via Pyrolysis: A Bibliometric Survey and Literature Review. J. Anal. Appl. Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass Pyrolysis Kinetics: A Comparative Critical Review with Relevant Agricultural Residue Case Studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Jain, A.; Vinu, R. Kinetic Experiments for Pyrolytic Recycling of Solid Plastic Waste. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2022; p. S0065237722000266. [Google Scholar]
- Dunn, J.G. Recommendations for Reporting Thermal Analysis Data. J. Therm. Anal. 1993, 40, 1431–1436. [Google Scholar] [CrossRef]
- ASTM E698-05; Standard Test Method for Arrhenius Kinetic Constants for Thermally Unstable Materials. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM E2890-21; Standard Test Method for Determination of Kinetic Parameters and Reaction Order for Thermally Unstable Materials by Differential Scanning Calorimetry Using the Kissinger and Farjas Methods. ASTM International: West Conshohocken, PA, USA, 2021.
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Analysis of Multi-Step Kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Hook, D.W.; Porter, S.J.; Herzog, C. Dimensions: Building Context for Search and Evaluation. Front. Res. Metr. Anal. 2018, 3, 23. [Google Scholar] [CrossRef]
- The Final Search Query Was: “(“thermal analysis” OR thermoanalytical OR calorimetry OR calorimetric OR thermogravimetry OR thermogravimetric) AND kinetic NOT “indirect calorimetry” NOT “titration calorimetry” NOT “drug release” NOT “Calorimetry, Indirect” NOT protein NOT antibacterial NOT pharmacological NOT brain NOT pharmacokinetic NOT adsorption NOT “release kinetics” NOT “photocatalytic degradation” NOT “kinetic energy””. Available online: https://app.dimensions.ai/discover/publication?search_mode=content&search_text=(%22thermal%20analysis%22%20OR%20thermoanalytical%20OR%20calorimetry%20OR%20calorimetric%20OR%20thermogravimetry%20OR%20thermogravimetric)%20AND%20kinetic%20NOT%20%22indirect%20calorimetry%22%20NOT%20%22titration%20calorimetry%22%20NOT%20%22drug%20release%22%20NOT%20%22Calorimetry%2C%20Indirect%22%20NOT%20protein%20NOT%20antibacterial%20NOT%20pharmacological%20NOT%20brain%20NOT%20pharmacokinetic%20NOT%20adsorption%20NOT%20%22release%20kinetics%22%20NOT%20%22photocatalytic%20degradation%22%20NOT%20%22kinetic%20energy%22&search_type=kws&search_field=text_search&order=times_cited (accessed on 5 August 2022).
- Visser, M.; van Eck, N.J.; Waltman, L. Large-Scale Comparison of Bibliographic Data Sources: Scopus, Web of Science, Dimensions, Crossref, and Microsoft Academic. Quant. Sci. Stud. 2021, 2, 20–41. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Citation-Based Clustering of Publications Using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef]
- Bornmann, L.; Haunschild, R.; Mutz, R. Growth Rates of Modern Science: A Latent Piecewise Growth Curve Approach to Model Publication Numbers from Established and New Literature Databases. Hum. Soc. Sci. Commun. 2021, 8, 224. [Google Scholar] [CrossRef]
- Chirico, R.D.; Frenkel, M.; Magee, J.W.; Diky, V.; Muzny, C.D.; Kazakov, A.F.; Kroenlein, K.; Abdulagatov, I.; Hardin, G.R.; Acree, W.E.; et al. Improvement of Quality in Publication of Experimental Thermophysical Property Data: Challenges, Assessment Tools, Global Implementation, and Online Support. J. Chem. Eng. Data 2013, 58, 2699–2716. [Google Scholar] [CrossRef]
- Appio, F.P.; Cesaroni, F.; Di Minin, A. Visualizing the Structure and Bridges of the Intellectual Property Management and Strategy Literature: A Document Co-Citation Analysis. Scientometrics 2014, 101, 623–661. [Google Scholar] [CrossRef]
- Stremersch, S.; Verniers, I.; Verhoef, P.C. The Quest for Citations: Drivers of Article Impact. J. Mark. 2007, 71, 171–193. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- Sasaki, H. Introduction of Particle-Size Distribution into Kinetics of Solid-State Reaction. J. Am. Ceram. Soc. 1964, 47, 512–516. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined Kinetic Analysis of Solid-State Reactions: A Powerful Tool for the Simultaneous Determination of Kinetic Parameters and the Kinetic Model without Previous Assumptions on the Reaction Mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef] [PubMed]
- Ozao, R.; Ochiai, M. Fractal Nature and Thermal Analysis of Powders. J. Therm. Anal. 1993, 40, 1331–1340. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of Determining Activation Deterioration Constant of Electrical Insulating Materials. Res. Rep. Chiba Inst. Technol. (Sci. Technol.) 1971, 16, 22–31. [Google Scholar]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Criado, J.M.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Critical Study of the Isoconversional Methods of Kinetic Analysis. J. Therm. Anal. Calorim. 2008, 92, 199–203. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Pivkina, A.N.; Koga, N. Critical Appraisal of Kinetic Calculation Methods Applied to Overlapping Multistep Reactions. Molecules 2019, 24, 2298. [Google Scholar] [CrossRef]
- Burnham, A.K.; Dinh, L.N. A Comparison of Isoconversional and Model-Fitting Approaches to Kinetic Parameter Estimation and Application Predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Isoconversional Analysis of Solid State Transformations: A Critical Review. Part I. Single Step Transformations with Constant Activation Energy. J. Therm. Anal. Calorim. 2011, 105, 757–766. [Google Scholar] [CrossRef]
- Moukhina, E. Determination of Kinetic Mechanisms for Reactions Measured with Thermoanalytical Instruments. J. Therm. Anal. Calorim. 2012, 109, 1203–1214. [Google Scholar] [CrossRef]
- Svoboda, R.; Málek, J. Is the Original Kissinger Equation Obsolete Today? J. Therm. Anal. Calorim. 2014, 115, 1961–1967. [Google Scholar] [CrossRef]
- Burnham, A.K. Global Chemical Kinetics of Fossil Fuels; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-49633-7. [Google Scholar]
- Farjas, J.; Butchosa, N.; Roura, P. A Simple Kinetic Method for the Determination of the Reaction Model from Non-Isothermal Experiments. J. Therm. Anal. Calorim. 2010, 102, 615–625. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and Be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-14174-9. [Google Scholar]
- Vyazovkin, S. A Time to Search: Finding the Meaning of Variable Activation Energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating Isothermal Life from Thermogravimetric Data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- ASTM E1641-18; Standard Test Method for Decomposition Kinetics by Thermogravimetry Using the Ozawa/Flynn/Wall Method. ASTM International: West Conshohocken, PA, USA, 2018.
- Starink, M.J. The Determination of Activation Energy from Linear Heating Rate Experiments: A Comparison of the Accuracy of Isoconversion Methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of Activation Energy of Thermally Stimulated Solid-State Reactions under Arbitrary Variation of Temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the Integral Isoconversional Method to Account for Variation in the Activation Energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Ortega, A. A Simple and Precise Linear Integral Method for Isoconversional Data. Thermochim. Acta 2008, 474, 81–86. [Google Scholar] [CrossRef]
- Vyazovkin, S. Determining Preexponential Factor in Model-Free Kinetic Methods: How and Why? Molecules 2021, 26, 3077. [Google Scholar] [CrossRef] [PubMed]
- Font, R.; García, A.N. Application of the Transition State Theory to the Pyrolysis of Biomass and Tars. J. Anal. Appl. Pyrolysis 1995, 35, 249–258. [Google Scholar] [CrossRef]
- Vyazovkin, S.V.; Lesnikovich, A.I. Estimation of the Pre-Exponential Factor in the Isoconversional Calculation of Effective Kinetic Parameters. Thermochim. Acta 1988, 128, 297–300. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Linert, W. False Isokinetic Relationships Found in the Nonisothermal Decomposition of Solids. Chem. Phys. 1995, 193, 109–118. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of Pre-Exponential Factor and Reaction Mechanism in a Model-Free Way. Thermochim. Acta 2020, 691, 178707. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. Isokinetic Relationship, Isoequilibrium Relationship, and Enthalpy−Entropy Compensation. Chem. Rev. 2001, 101, 673–696. [Google Scholar] [CrossRef]
- Gao, X.; Tan, M.; Jiang, S.; Huang, Z.; Li, C.; Lei, T.; Wang, Z.; Zhu, Y.; Li, H. Pyrolysis of Torrefied Rice Straw from Gas-Pressurized and Oxidative Torrefaction: Pyrolysis Kinetic Analysis and the Properties of Biochars. J. Anal. Appl. Pyrolysis 2021, 157, 105238. [Google Scholar] [CrossRef]
- Ling, P.; Xu, J.; Liu, T.; An, X.; Wang, X.; Mostafa, M.E.; Han, H.; Xu, K.; Jiang, L.; Wang, Y.; et al. Pyrolysis Kinetics and Reaction Mechanisms of Coal Slime for Cleaner Energy. J. Anal. Appl. Pyrolysis 2022, 168, 105718. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis Behaviors of Antibiotic Fermentation Residue and Wastewater Sludge from Penicillin Production: Kinetics, Gaseous Products Distribution, and Nitrogen Transformation. J. Anal. Appl. Pyrolysis 2021, 158, 105208. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Melnikov, I.N.; Monogarov, K.A.; Kuchurov, I.V.; Pivkina, A.N. The Power of Model-Fitting Kinetic Analysis Applied to Complex Thermal Decomposition of Explosives: Reconciling the Kinetics of Bicyclo-HMX Thermolysis in Solid State and Solution. J. Therm. Anal. Calorim. 2022, 147, 3195–3206. [Google Scholar] [CrossRef]
- Kossoy, A.A.; Belokhvostov, V.M.; Koludarova, E.Y. Thermal Decomposition of AIBN: Part D: Verification of Simulation Method for SADT Determination Based on AIBN Benchmark. Thermochim. Acta 2015, 621, 36–43. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M.H. Evaluation of Reliability of Coats-Redfern Method for Kinetic Analysis of Non-Isothermal TGA. Trans. Nonferrous Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-Free and Model-Fitting Approaches to Kinetic Analysis of Isothermal and Nonisothermal Data. Thermochim. Acta 1999, 340–341, 53–68. [Google Scholar] [CrossRef]
- Vyazovkin, S. Two Types of Uncertainty in the Values of Activation Energy. J. Therm. Anal. Calorim. 2001, 64, 829–835. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T.; Zhang, Y.S.; Yao, D.; Yang, H.; Zhou, H. Comparison of Waste Plastics Pyrolysis under Nitrogen and Carbon Dioxide Atmospheres: A Thermogravimetric and Kinetic Study. J. Anal. Appl. Pyrolysis 2021, 156, 105135. [Google Scholar] [CrossRef]
- Park, Y.-K.; Siddiqui, M.Z.; Karagöz, S.; Han, T.U.; Watanabe, A.; Kim, Y.-M. In-Situ Catalytic Co-Pyrolysis of Kukersite Oil Shale with Black Pine Wood over Acid Zeolites. J. Anal. Appl. Pyrolysis 2021, 155, 105050. [Google Scholar] [CrossRef]
- Shi, P.; Chang, G.; Tan, X.; Guo, Q. Enhancement of Bituminous Coal Pyrolysis for BTX Production by Fe2O3/MoSi2-HZSM-5 Catalysts. J. Anal. Appl. Pyrolysis 2020, 150, 104867. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, J.; Ying, Z.; Ji, S.; Feng, Y.; Wang, B.; Dou, B. Thermochemical Conversion of Sewage Sludge-Derived Hydrochars: Volatiles Release and Char Gasification Kinetics. J. Anal. Appl. Pyrolysis 2021, 156, 105138. [Google Scholar] [CrossRef]
- Yang, H.; Han, T.; Yang, W.; Sandström, L.; Jönsson, P.G. Influence of the Porosity and Acidic Properties of Aluminosilicate Catalysts on Coke Formation during the Catalytic Pyrolysis of Lignin. J. Anal. Appl. Pyrolysis 2022, 165, 105536. [Google Scholar] [CrossRef]
- Jang, E.-S.; Song, E.; Zain Siddiqui, M.; Lim, S.J.; Shin, G.H.; Kim, D.; Kim, Y.-M. The Effect of Seawater Aging on the Pyrolysis of Fishing Nets. J. Anal. Appl. Pyrolysis 2021, 156, 105160. [Google Scholar] [CrossRef]
- Chen, P.; Hu, C.; Gu, J.; Lin, X.; Yang, C.; Leu, S.-Y.; Guan, L. Pyrolysis Characteristics of Tea Oil Camellia (Camellia oleifera Abel.) Shells and Their Chemically Pre-Treated Residues: Kinetics, Mechanisms, Product Evaluation and Joint Optimization. J. Anal. Appl. Pyrolysis 2022, 164, 105526. [Google Scholar] [CrossRef]
- Tao, L.; Ma, X.; Ye, L.; Jia, J.; Wang, L.; Ma, P.; Liu, J. Interactions of Lignin and LDPE during Catalytic Co-Pyrolysis: Thermal Behavior and Kinetics Study by TG-FTIR. J. Anal. Appl. Pyrolysis 2021, 158, 105267. [Google Scholar] [CrossRef]
- Ling, Q.; He, S.; Babadi, A.A.; Yuan, C.; Pan, C.; Jiang, D.; Cao, B.; Hu, Y.; Wang, S.; Zheng, A. Preparation of Low-Nitrogen Chlorella Based on Proteolysis Technology and Pyrolysis Characteristics of the Products. J. Anal. Appl. Pyrolysis 2022, 167, 105630. [Google Scholar] [CrossRef]
- Gu, H.; Foong, S.Y.; Lam, S.S.; Yue, X.; Yang, J.; Peng, W. Characterization and Potential Utilization of Extracts and Pyrolyzates from Jasminum Nudiflorum Lindl. Bark. J. Anal. Appl. Pyrolysis 2021, 155, 105092. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, Q.; Yılmaz, M.; Zhang, X. Co-Pyrolysis of Sewage Sludge and Sodium Lignosulfonate: Kinetic Study and Methylene Blue Adsorption Properties of the Biochar. J. Anal. Appl. Pyrolysis 2022, 165, 105586. [Google Scholar] [CrossRef]
- Ding, H.; Ma, Y.; Li, S.; Wang, Q.; Hong, W.; Jiang, H.; Li, H.; Jiang, M. Pyrolysis Characteristics of Oil Shale Semi-Coke and Its Extracted Bitumen. J. Anal. Appl. Pyrolysis 2021, 156, 105120. [Google Scholar] [CrossRef]
- Meng, T.-T.; Zhang, H.; Lü, F.; Shao, L.-M.; He, P.-J. Comparing the Effects of Different Metal Oxides on Low Temperature Decomposition of PVC. J. Anal. Appl. Pyrolysis 2021, 159, 105312. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, G.; Yin, H.; Zhou, C.; Wu, D.; Tan, F. A Comparative Study on Thermal Behavior of Functional Groups in Coals with Different Ranks during Low Temperature Pyrolysis. J. Anal. Appl. Pyrolysis 2021, 158, 105258. [Google Scholar] [CrossRef]
- Yao, Q.; Ma, M.; Liu, Y.; He, L.; Sun, M.; Ma, X. Pyrolysis Characteristics of Metal Ion-Exchanged Shendong Coal and Its Char Gasification Performance. J. Anal. Appl. Pyrolysis 2021, 155, 105087. [Google Scholar] [CrossRef]
- Mo, W.; Wang, Y.; Ma, Y.; Peng, Y.; Fan, X.; Wu, Y.; Wei, X. Direct Liquefaction Performance of Sub-Bituminous Coal from Hefeng by Solid Super Acids and Pyrolysis Kinetic Analysis of the Corresponding Residue. J. Anal. Appl. Pyrolysis 2021, 159, 105181. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, X.; Ma, Z.; Guo, X.; Wang, J.; Luan, P.; He, S.; Yan, B.; Chen, G. Studies on Synergistic Effects in Co-Pyrolysis of Sargassum and Poplar: Thermal Behavior and Kinetics. J. Anal. Appl. Pyrolysis 2022, 167, 105660. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Muravyev, N.V.; Luciano, G.; Ornaghi, H.L.; Svoboda, R.; Vyazovkin, S. Artificial Neural Networks for Pyrolysis, Thermal Analysis, and Thermokinetic Studies: The Status Quo. Molecules 2021, 26, 3727. [Google Scholar] [CrossRef]
- Conesa, J.A.; Caballero, J.A.; Reyes-Labarta, J.A. Artificial Neural Network for Modelling Thermal Decompositions. J. Anal. Appl. Pyrol. 2004, 71, 343–352. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Pivkina, A.N. New Concept of Thermokinetic Analysis with Artificial Neural Networks. Thermochim. Acta 2016, 637, 69–73. [Google Scholar] [CrossRef]
- Liland, K.H.; Svoboda, R.; Luciano, G.; Muravyev, N. Neural Networks Applied in Kinetic Analysis of Complex Nucleation-Growth Processes: Outstanding Solution for Fully Overlapping Reaction Mechanisms. J. Non-Cryst. Solids 2022, 588, 121640. [Google Scholar] [CrossRef]
- Perejón, A.; Sánchez-Jiménez, P.E.; Criado, J.M.; Pérez-Maqueda, L.A. Kinetic Analysis of Complex Solid-State Reactions. A New Deconvolution Procedure. J. Phys. Chem. B 2011, 115, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, R.; Málek, J. Applicability of Fraser–Suzuki Function in Kinetic Analysis of Complex Crystallization Processes. J. Therm. Anal. Calorim. 2013, 111, 1045–1056. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A Quick, Direct Method for the Determination of Activation Energy from Thermogravimetric Data. J. Polym. Sci. B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Monogarov, K.A.; Asachenko, A.F.; Nechaev, M.S.; Ananyev, I.V.; Fomenkov, I.V.; Kiselev, V.G.; Pivkina, A.N. Pursuing Reliable Thermal Analysis Techniques for Energetic Materials: Decomposition Kinetics and Thermal Stability of Dihydroxylammonium 5,5′-Bistetrazole-1,1′-Diolate (TKX-50). Phys. Chem. Chem. Phys. 2017, 19, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Burnham, A.K.; Stanford, V.L.; Vyazovkin, S.; Kahl, E.M. Effect of Pressure on TATB and LX-17 Thermal Decomposition. Thermochim. Acta 2021, 699, 178908. [Google Scholar] [CrossRef]
- Kossoy, A. Effect of Thermal Inertia-Induced Distortions of DSC Data on the Correctness of the Kinetics Evaluated. J. Therm. Anal. Calorim. 2021, 143, 599–608. [Google Scholar] [CrossRef]
- Vyazovkin, S. How Much Is the Accuracy of Activation Energy Affected by Ignoring Thermal Inertia? Int. J. Chem. Kinet. 2020, 52, 23–28. [Google Scholar] [CrossRef]
- Lyon, R.E.; Safronava, N.; Senese, J.; Stoliarov, S.I. Thermokinetic Model of Sample Response in Nonisothermal Analysis. Thermochim. Acta 2012, 545, 82–89. [Google Scholar] [CrossRef]
- Weathington, B.L.; Cunningham, C.J.L.; Pittenger, D.J. Appendix B. Statistical Tables. In Understanding Business Research; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-1-118-13426-9. [Google Scholar]
- Critical Values for Pearson’s Correlation Coefficient. Available online: http://commres.net/wiki/_media/correlationtable.pdf (accessed on 17 August 2022).
- Burnham, A.K.; Weese, R.K. Kinetics of Thermal Degradation of Explosive Binders Viton A, Estane, and Kel-F. Thermochim. Acta 2005, 426, 85–92. [Google Scholar] [CrossRef]
- McAdie, H.G. Recommendations for Reporting Thermal Analysis Data. Anal. Chem. 1967, 39, 543. [Google Scholar] [CrossRef]
- Perkel, J.M. A Toolkit for Data Transparency Takes Shape. Nature 2018, 560, 513–515. [Google Scholar] [CrossRef] [PubMed]
- ThermV—Comprehensive Thermal Analysis Software; Blagojevic, V., Source Forge: San Diego, CA, USA. 2016. Available online: https://sourceforge.net/projects/thermv/ (accessed on 17 August 2022).
- THINKS—Free Open-Source Thermokinetic Software; Muravyev, N.V. 2016. Available online: https://chemphys.space/thinks (accessed on 17 August 2022).
- Luciano, G.; Liland, K.H.; Marsotto, M.; Svoboda, R.; Berretta, S. Takos: An R Package for Thermal Analysis Calculations. SoftwareX 2020, 12, 100637. [Google Scholar] [CrossRef]
- Drozin, D.; Sozykin, S.; Ivanova, N.; Olenchikova, T.; Krupnova, T.; Krupina, N.; Avdin, V. Kinetic Calculation: Software Tool for Determining the Kinetic Parameters of the Thermal Decomposition Process Using the Vyazovkin Method. SoftwareX 2020, 11, 100359. [Google Scholar] [CrossRef]
- Windecker, S.M.; Vesk, P.A.; Trevathan-Tackett, S.M.; Golding, N. Mixchar: An R Package for the Deconvolution of Thermal Decay Curves. J. Open Res. Softw. 2021, 9, 27. [Google Scholar] [CrossRef]
- Ramírez, E.; Hernández-López, S.; Torres-Garcia, E.; Reyes-Morales, K.; Balmaseda, J. PICNIK: A Python Package with Isoconversional Computations for Non-Isothermal Kinetics. Comput. Phys. Commun. 2022, 278, 108416. [Google Scholar] [CrossRef]
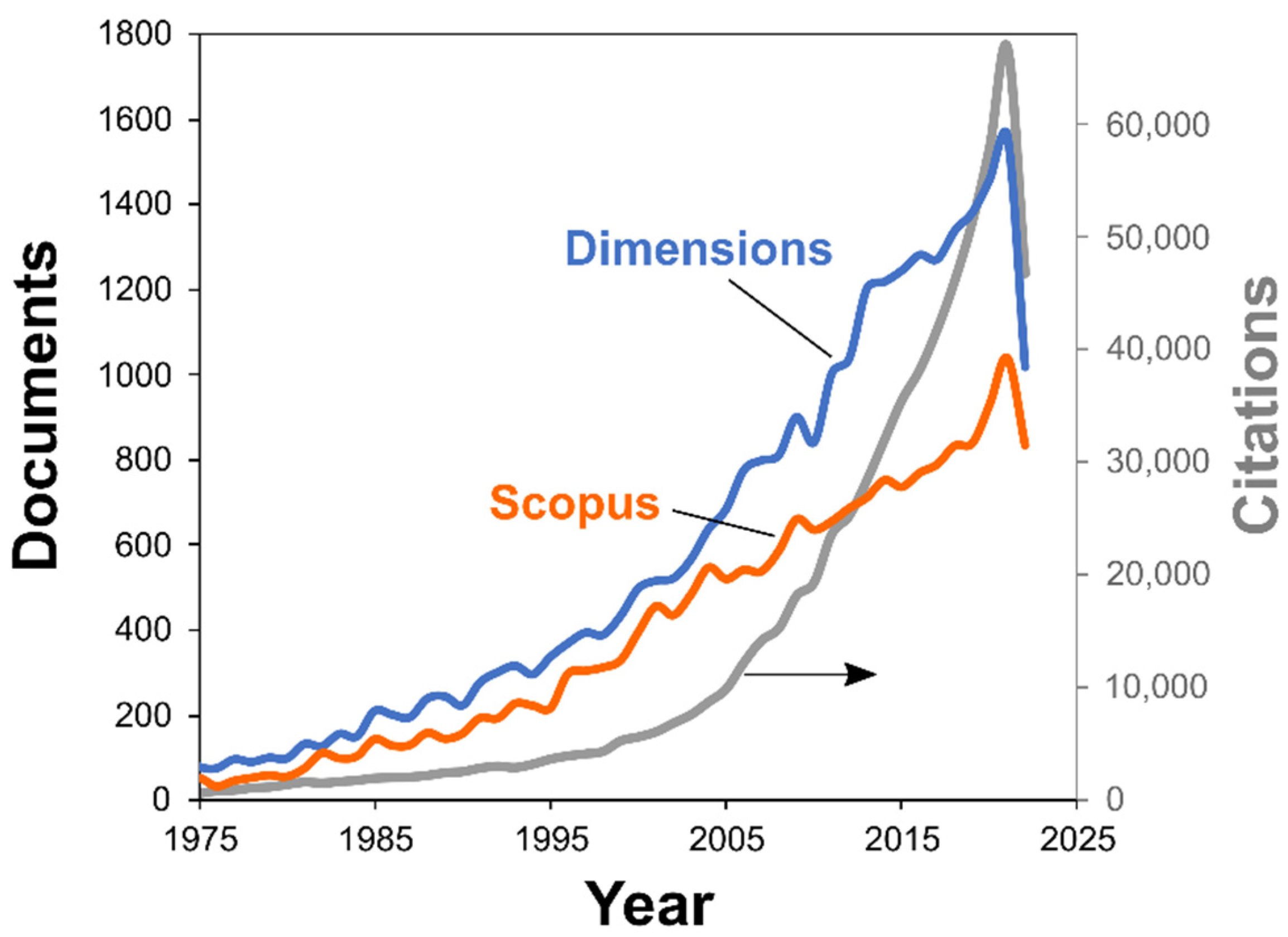
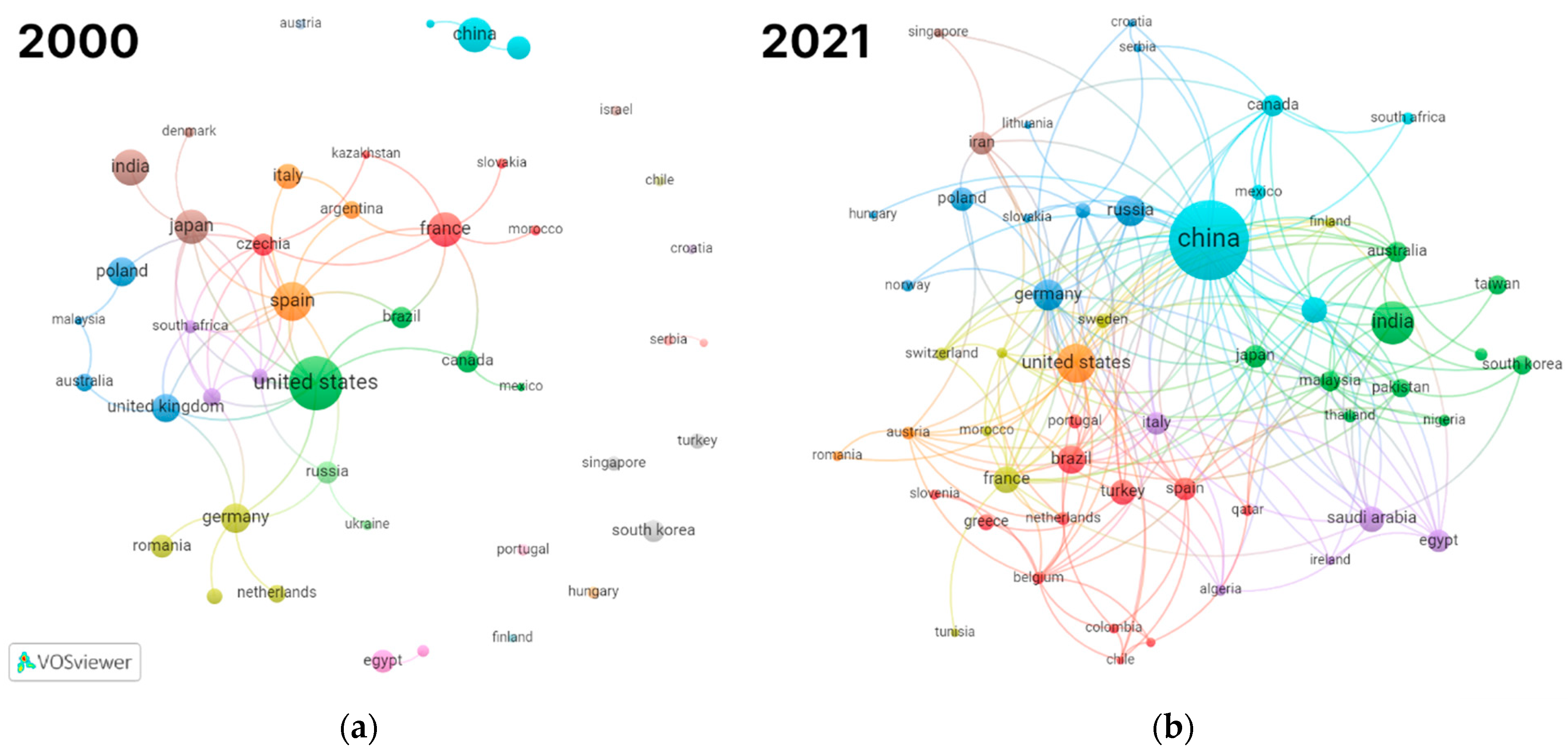
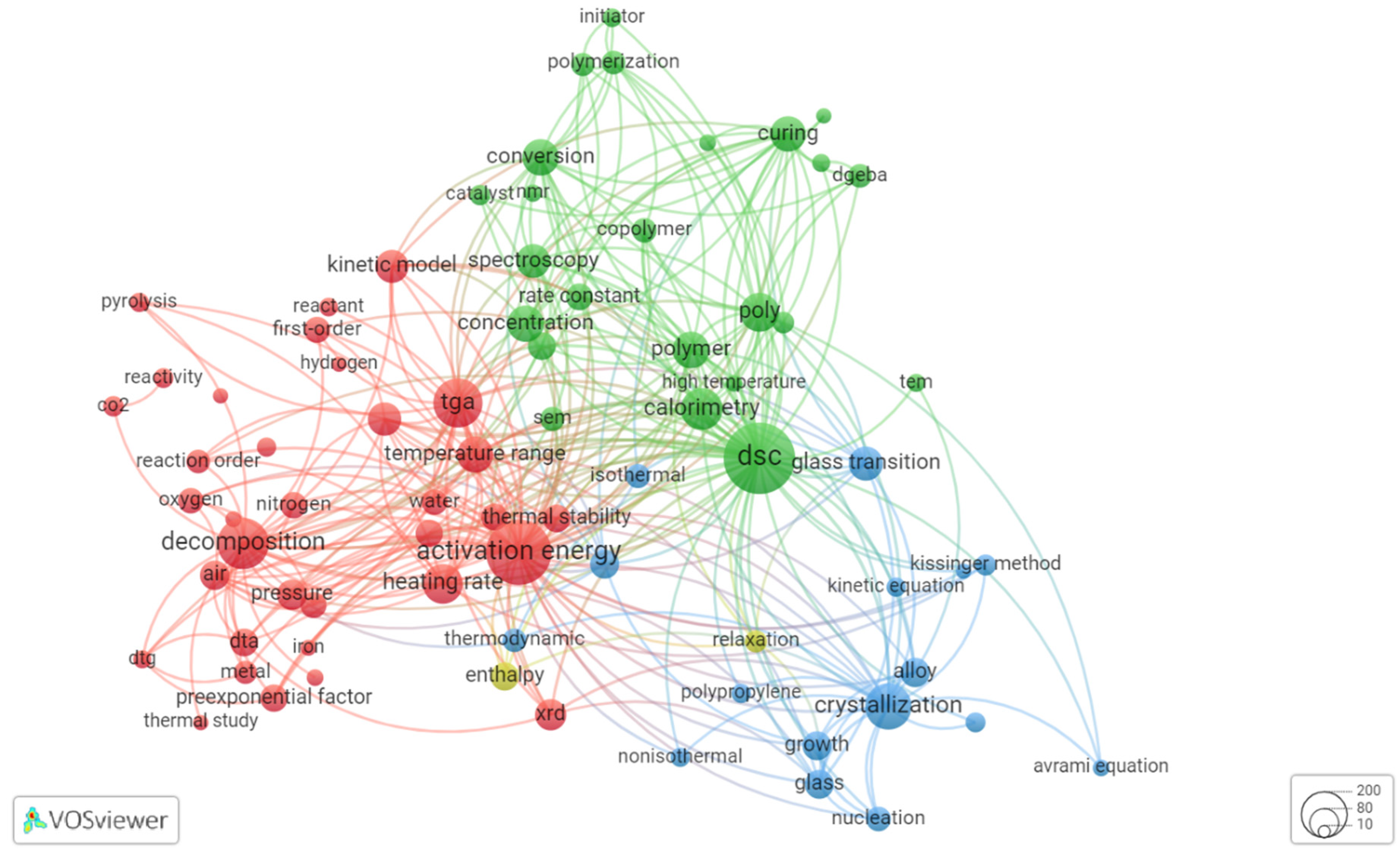
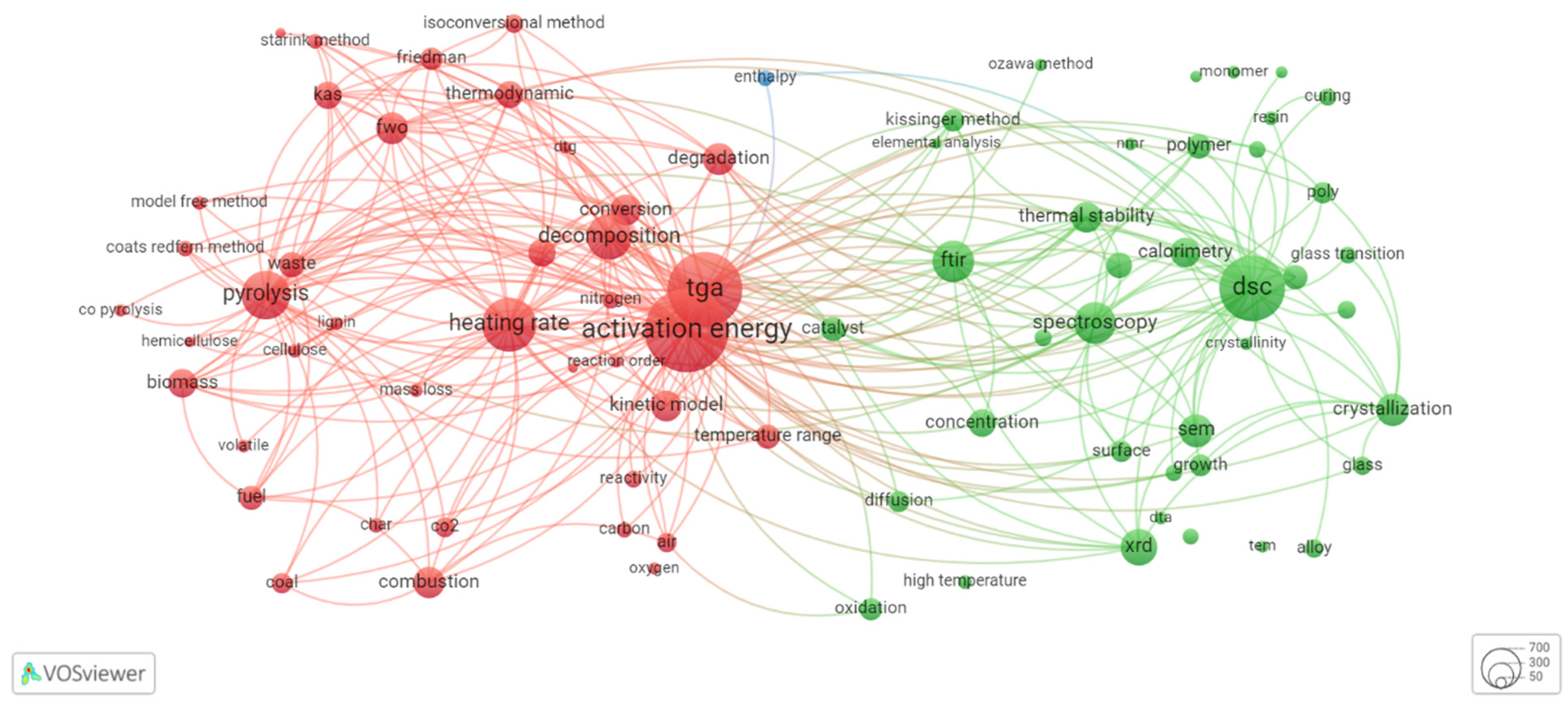
| 2000 | 2021 | ||||
|---|---|---|---|---|---|
| Source Title | Documents | Citations | Source Title | Documents | Citations |
| Journal of Thermal Analysis and Calorimetry | 114 | 2072 | Journal of Thermal Analysis and Calorimetry | 94 | 182 |
| Thermochimica Acta | 68 | 3957 | Thermochimica Acta | 66 | 213 |
| Journal of Applied Polymer Science | 31 | 818 | Polymers | 44 | 147 |
| Polymer | 26 | 1307 | Fuel | 41 | 367 |
| Polymer Degradation and Stability | 10 | 668 | Materials | 31 | 62 |
| Journal of Polymer Science Part B Polymer Physics | 10 | 349 | ACS Omega | 28 | 52 |
| European Polymer Journal | 8 | 189 | Biomass Conversion and Biorefinery | 26 | 98 |
| Polymer Engineering & Science | 8 | 158 | Bioresource Technology | 21 | 185 |
| Macromolecules | 7 | 473 | Journal of Analytical and Applied Pyrolysis | 19 | 125 |
| Materials Science and Engineering A | 7 | 301 | Journal of Applied Polymer Science | 19 | 35 |
| Total (2000) | 597 | 20163 | Total (2021) | 1630 | 5509 |
| Specific Aspect of Thermal Analysis Measurement | Percent of Studies Where It Is Reported |
|---|---|
| Sample mass | 79 |
| Crucible type | 8 |
| Software used for kinetic analysis | 12 |
| Gaseous atmosphere (gas type) | 95 |
| Calibration info | 8 |
| Purge gas flow rate | 86 |
| Repeatability of measurements | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muravyev, N.V.; Vyazovkin, S. The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be. Thermo 2022, 2, 435-452. https://doi.org/10.3390/thermo2040029
Muravyev NV, Vyazovkin S. The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be. Thermo. 2022; 2(4):435-452. https://doi.org/10.3390/thermo2040029
Chicago/Turabian StyleMuravyev, Nikita V., and Sergey Vyazovkin. 2022. "The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be" Thermo 2, no. 4: 435-452. https://doi.org/10.3390/thermo2040029
APA StyleMuravyev, N. V., & Vyazovkin, S. (2022). The Status of Pyrolysis Kinetics Studies by Thermal Analysis: Quality Is Not as Good as It Should and Can Readily Be. Thermo, 2(4), 435-452. https://doi.org/10.3390/thermo2040029







