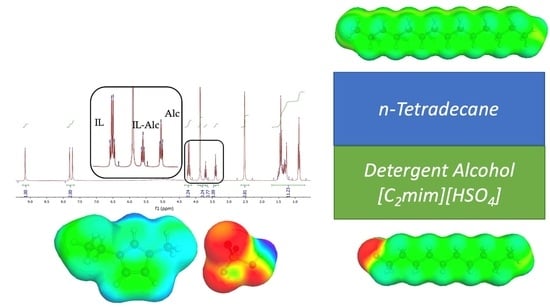
Separation of Alcohols from n-Tetradecane Using 1-Ethyl-3-methylimidazolium Hydrogensulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of 1-ethyl-3-methylimidazolium Hydrogensulfate
2.3. Experimental Procedure
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falbe, J.; Barhmann, H.; Lipps, W.; Mayer, D.; Frey, G.D. Alcohol, Aliphatic. In Ullmann’s Ecyclopedia of Industrial Chemistry, 1st ed.; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2013; pp. 1–26. [Google Scholar]
- Sinuhaji, T.; Manurung, R. Introduction of Fatty Alcohol Sulfates: A Short Review. Engrxiv 2021. [Google Scholar] [CrossRef]
- Crause, J. Production of Detergent Range Alcohols. U.S. Patent 7,652,173 B2, 26 January 2010. [Google Scholar]
- Wu, Z.; Liu, C.; Cheng, H.; Chen, L.; Qi, Z. Tuned Extraction and Regeneration Process for Separation of Hydrophobic Compounds by Aqueous Ionic Liquids. J. Mol. Liq. 2020, 308, 113032. [Google Scholar] [CrossRef]
- Lee, F.M.; Brown, R.E. Separation of Alkenes from Close Boiling Alkanes. U.S. Patent 5,100,515, 26 October 1990. [Google Scholar]
- Zaretskii, M.I.; Rusak, V.V.; Chartov, E.M. Sulfolane in Liquid Extraction: A Review. Coke Chem. 2011, 54, 211–214. [Google Scholar] [CrossRef]
- Dantas, C.E.S.; Ceriani, R. γ-Valerolactone as a Green Solvent for Extracting Carboxylic Acids and Alcohols from n-Tetradecane: Equilibrium Data for Model Systems at 298.15 K. J. Chem. Eng. Data 2022, 67, 1460–1473. [Google Scholar] [CrossRef]
- Du Rand, M.; Nieuwoudti, I. Fluids Measurement of Phase Equilibria of Supercritical Ethane and Paraffins. J. Supercrit. Fluids 2001, 21, 181–193. [Google Scholar] [CrossRef]
- Nieuwoudti, I.; Du Rand, M. Measurement of Phase Equilibria of Supercritical Carbon Dioxide and Paraffins. J. Supercrit. Fluids 2002, 22, 185–199. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Knoetze, J.H. Phase Equilibria of High Molecular Mass 1-Alcohols in Supercritical Propane. Fluid Phase Equilibria 2007, 258, 51–57. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Knoetze, J.H. Separation of Alkanes and Alcohols with Supercritical Fluids: Part. I: Phase Equilibria and Viaility. J. Supercrit. Fluids 2014, 57, 101–111. [Google Scholar]
- Manjare, S.D.; Dhingra, K. Supercritical Fluids in Separation and Purification A Review. Mater. Sci. Ener. Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Hansmeier, A.R.; De Haan, A.B. Ionic Liquids for Aromatics Extraction. Present Status and Future Outlook. Ind. Eng. Chem. Res. 2010, 49, 7530–7540. [Google Scholar] [CrossRef]
- Sas, O.G.; Dominguez, A. Liquid-Liquid Extraction of Phenolic Compounds from Water using Ionic Liquids: Literature Review and New Experimental Data Using [C2mim]FSI. J. Environ. Manag. 2018, 15, 475–842. [Google Scholar] [CrossRef]
- González, B.; Corderí, S. Capacity of Two 1-Butyl-1-Methylpyrrolidinium-based Ionic Liquids for the Extraction of Ethanol from its Mixtures with Heptane and Hexane. Fluid Phase Equilibria 2013, 354, 89–94. [Google Scholar] [CrossRef]
- González, B.; Corderí, S.; Santamaría, A.G. Application of 1-Alkyl-3-Methylpyridinium Bis(trifluoromethylsulfonyl)imide Ionic Liquids for the Ethanol Removal from its Mixtures with Alkanes. J. Chem. Thermodyn. 2013, 60, 9–14. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Rodríguez, A. A Study on the Liquid–Liquid Equilibria of 1-Alkyl-3-Methylimidazolium Hexafluorophosphate with Ethanol and Alkanes. Fluid Phase Equilibria 2008, 270, 23–29. [Google Scholar] [CrossRef]
- Marciniak, A.; Królikowski, M. Ternary Liquid–Liquid Equilibria of Bis(trifluoromethylsulfonyl)-amide Based Ionic Liquids + Methanol + Heptanes. Fluid Phase Equilibria 2012, 318, 56–60. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Araújo, J.M.M.; Esperança, J.M.S.S.; Marrucho, I.M.; Rebelo, L.P.N. Ionic liquids in Separations of Azeotropic Systems—A Review. J. Chem. Thermodyn. 2012, 46, 2–28. [Google Scholar] [CrossRef]
- Cai, F.; Xiao, G. Liquid + Liquid Extraction of Methanol from Alkanes using Dialkylphosphate-based Ionic Liquids as Solvents. J. Chem. Thermodyn. 2015, 87, 110–116. [Google Scholar] [CrossRef]
- García, S.; Larriba, M.; García, J.; Torrecilla, J.S.; Rodríguez, F. Liquid–Liquid Extraction of Toluene from n-heptane using Binary Mixtures of N-butylpyridinium Tetrafluoroborate and N-Butylpyridinium Bis(trifluoromethylsulfonyl) Imide Ionic Liquids. Chem. Eng. J. 2012, 180, 210–215. [Google Scholar] [CrossRef]
- Gomez, E.; González, B.; Calvar, N.; Tojo, E.; Dominguez, A. Physical Properties of Pure 1-ethyl-3-methylimidaolium Ethylsufate and its Binary Mixtures ith Ethanol and Water at Several Temperatures. J. Chem. Eng. Data 2006, 51, 2096–2102. [Google Scholar] [CrossRef]
- Himmler, S.; Hörmann, S.; van Hal, R.; Schulz, P.; Wassercheid, P. Transesterification of Methylsulfate and Ethylsulfate Ionic Liquids—An Environmentally Benign Way to Synthesize Long-chain and Functionalized Alkylsulfate Ionic Liquids. Green Chem. 2006, 8, 887–894. [Google Scholar] [CrossRef]
- Jacquemin, J.; Goodrich, P.; Jiang, W.; Rooney, D.W.; Hardacre, C. Are Alkyl Sulfate-Based Protic and Aprotic Ionic Liquids Stable with Water and Alcohols? A Thermodynamic Approach. J. Phys. Chem. B 2013, 117, 1938–1949. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; de Barros, R.L.F.; Sintra, T.; Soares, C.M.F.; Lima, Á.S.; Coutinho, J.A.P. Simple Screening Method to Identify Toxic/Non-Toxic Ionic Liquids: Agar Diffusion Test Adaptation. Ecotoxicol. Environ. Saf. 2012, 83, 55–62. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-Active Ionic Liquids: A Review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Crosswaithe, J.M.; Aki, S.N.V.K.; Magin, E.J.; Brennecke, J.F. Liquid Phase Behavior of Imidazolium-Based Ionic Liquids with Alcohols. J. Phys. Chem. B 2004, 108, 5113–5119. [Google Scholar] [CrossRef]
- He, H.; Chen, H.; Zheng, Y.; Zhang, X.; Yao, X.; Yu, Z.; Zhang, S. The Hydrogen-Bonding Interactions between 1-Ethyl-3-Methylimidazolium Lactate Ionic Liquid and Methanol. Aust. J. Chem. 2013, 66, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Domańska, U.; Pobudkowska, A.; Eckert, F. Liquid-Liquid Equilibria in the Binary Systems (1,3-dimethylimidazolium or 1-Butyl-3-Methylimidazolium methylsulfate + hydrocarbons). Green Chem. 2006, 8, 268–276. [Google Scholar] [CrossRef]
- Federova, I.V.; Safonova, L.P. Ion Pair Structures and Hydrogen Bonding in RnNH4-n Alkylammonium Ionic Liquids with Hydrogen sulphate an Mesylate Anions by DFT Computations. J. Phys. Chem. A 2020, 124, 3170–3179. [Google Scholar] [CrossRef]
- Schwerdtfeger, S.; Köhler, F.; Pottel, R.; Kaatze, U. Dielectric Relaxation of Hydrogen Bonded Liquids: Mixtures of Monohydric Alcohols with n-Alkanes. J. Chem. Phys. 2001, 115, 4186. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, U.; Wiśniewska, A. Solubility and Excess Molar Properties of 1,3-Dimethylimidazolium Methylsulfate, or 1-Butyl-3-Methylimidazolium Methylsulfate or 1-Butyl-3-Methylimidazolium Octylsulfate Ionic Liquids with n-Alkanes and Alcohols: Analysis in Terms of the PFP and FBT Models. J. Solut. Chem. 2006, 35, 311–334. [Google Scholar] [CrossRef]

| Alcohol | IL System * | Sulfolane System | ||||
|---|---|---|---|---|---|---|
| IL Phase | Sulfolane Phase | Alcohol Phase | ||||
| xIL | xAlc. | xTMS | xAlc. | xTMS | xAlc. | |
| 1-butanol (C4) | 0.631 | 0.369 | ∞ | ∞ | ∞ | ∞ |
| 1-hexanol (C6) | 0.956 | 0.044 | 0.901 | 0.099 | 0.045 | 0.955 |
| 1-octanol (C8) | 0.968 | 0.032 | 0.926 | 0.074 | 0.203 | 0.797 |
| 1-decanol (C10) | 0.977 | 0.023 | 0.943 | 0.057 | 0.242 | 0.758 |
| 1-dodecanol (C12) | 0.987 | 0.013 | 0.980 | 0.020 | 0.174 | 0.826 |
| Extractant | Alcohols | Extractant Phase (Lower Layer) | n-Tetradecane Phase (Upper Layer) | Dist. | Select. | ||||
|---|---|---|---|---|---|---|---|---|---|
| CnOH | x1 II | x2 II | x3 II | x1 I | x2 I | x3 I | D | S | |
| Sulfolane | C8 | 0.025 | 0.020 | 0.955 | 0.537 | 0.463 | 0 | 0.043 | 0.928 |
| C10 | 0.006 | 0.013 | 0.981 | 0.508 | 0.492 | 0 | 0.026 | 2.237 | |
| C12 | 0.004 | 0.023 | 0.973 | 0.510 | 0.490 | 0 | 0.047 | 5.985 | |
| [C2mim] [HSO4] | C8 | 0.062 | 0.109 | 0.829 | 0.537 | 0.463 | 0 | 0.235 | 2.039 |
| C10 | 0.051 | 0.099 | 0.850 | 0.523 | 0.477 | 0 | 0.207 | 2.120 | |
| C12 | 0.056 | 0.087 | 0.857 | 0.509 | 0.491 | 0 | 0.177 | 3.387 | |
| [C2mim] [HSO4] Solvent | IL Rich Phase (Lower Layer) | Tetradecane-Rich Phase (Upper Layer) | Distribution | Selectivity | |||||
|---|---|---|---|---|---|---|---|---|---|
| T | x1 II | x2 II | x3 II | x4 II | x1 I | x2 I | x3 I | D | S |
| C4OH | |||||||||
| 323 K | 0.014 | 0.262 | 0.185 | 0.539 | 0.899 | 0.101 | 0 | 0.388 | 7.674 |
| 393 K | 0.051 | 0.308 | 0.228 | 0.413 | 0.854 | 0.146 | 0 | 2.110 | 35.325 |
| C6OH | |||||||||
| 323 K | 0.071 | 0.291 | 0 | 0.638 | 0.533 | 0.467 | 0 | 0.301 | 3.130 |
| 393 K | 0.066 | 0.373 | 0.295 | 0.266 | 0.728 | 0.272 | 0 | 1.371 | 15.126 |
| C8OH | |||||||||
| 323 K | 0.108 | 0.175 | 0 | 0.717 | 0.524 | 0.476 | 0 | 0.642 | 7.001 |
| 393 K | 0.420 | 0.319 | 0.090 | 0.171 | 0.488 | 0.373 | 0.139 | 0.855 | 0.994 |
| C10OH | |||||||||
| 323 K | 0.087 | 0.194 | 0 | 0.719 | 0.522 | 0.478 | 0 | 0.890 | 5.556 |
| 393 K | 0.153 | 0.249 | 0.075 | 0.523 | 0.567 | 0.362 | 0.071 | 0.688 | 2.549 |
| C12OH | |||||||||
| 323 K | 0.036 | 0.273 | 0 | 0.691 | 0.529 | 0.471 | 0 | 0.568 | 8.182 |
| 393 K | 0.130 | 0.442 | 0.185 | 0.243 | 0.611 | 0.325 | 0.064 | 1.360 | 6.915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ah-Lung, G.; Besnard, C.; Ivol, F.; Maaliki, C.; Hughes, T.-L.; Goodrich, P.; Jacquemin, J. Separation of Alcohols from n-Tetradecane Using 1-Ethyl-3-methylimidazolium Hydrogensulfate. Thermo 2022, 2, 200-208. https://doi.org/10.3390/thermo2030015
Ah-Lung G, Besnard C, Ivol F, Maaliki C, Hughes T-L, Goodrich P, Jacquemin J. Separation of Alcohols from n-Tetradecane Using 1-Ethyl-3-methylimidazolium Hydrogensulfate. Thermo. 2022; 2(3):200-208. https://doi.org/10.3390/thermo2030015
Chicago/Turabian StyleAh-Lung, Guillaume, Claire Besnard, Flavien Ivol, Carine Maaliki, Terri-Louise Hughes, Peter Goodrich, and Johan Jacquemin. 2022. "Separation of Alcohols from n-Tetradecane Using 1-Ethyl-3-methylimidazolium Hydrogensulfate" Thermo 2, no. 3: 200-208. https://doi.org/10.3390/thermo2030015
APA StyleAh-Lung, G., Besnard, C., Ivol, F., Maaliki, C., Hughes, T.-L., Goodrich, P., & Jacquemin, J. (2022). Separation of Alcohols from n-Tetradecane Using 1-Ethyl-3-methylimidazolium Hydrogensulfate. Thermo, 2(3), 200-208. https://doi.org/10.3390/thermo2030015