The Influence of Plant Extract on the Phase Equilibrium of Structure I Gas Hydrate in a Simulated Offshore Environment
Abstract
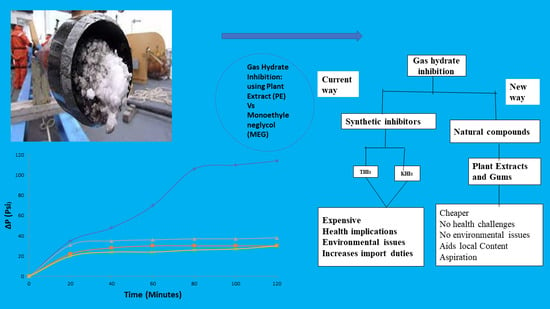
:1. Introduction
2. Materials and Methods
2.1. Assumption/Limitations of Research Work
- The Mini flow loop used operates within a Loop pressure of 3500 psi and temperature between 0 and 50 °C.
- The maximum allowable pressure for the loop was 150 psi because above this pressure, the screw pump failed because the pump used was for single-phase liquid flow so the quantity of pumped gas had a limit.
- The system operates as a constant volume batch process therefore the amount of gas used up is reflected in the pressure of the system at the end of the experiment.
- Rapid temperature increases and excessive decreases in pressure were an indication of hydrate formation in the system. This is so because hydrate formation is an exothermic reaction indicated by temperature increase. The pressure decrease is due to a reduction in the number of gas molecules in the system
- The system is made of 316 stainless steel pipes that are insulated inside a 4-inch PVC pipe with cold water circulated constantly to cool the stainless-steel pipe, mimicking the offshore environment.
- The system studies gas hydrate formation in a gas-dominated two-phase flow system and predominantly studies how pressure affects gas hydrate formation in a gas-dominated 2-phase system.
- About 1 m of the 0.5-inch internal diameter pipe is spiraled and exposed inside the refrigerator (cooling unit) to increase the retention time of the hydrate-forming fluid in the coldest part where gas hydrate is likely to form.
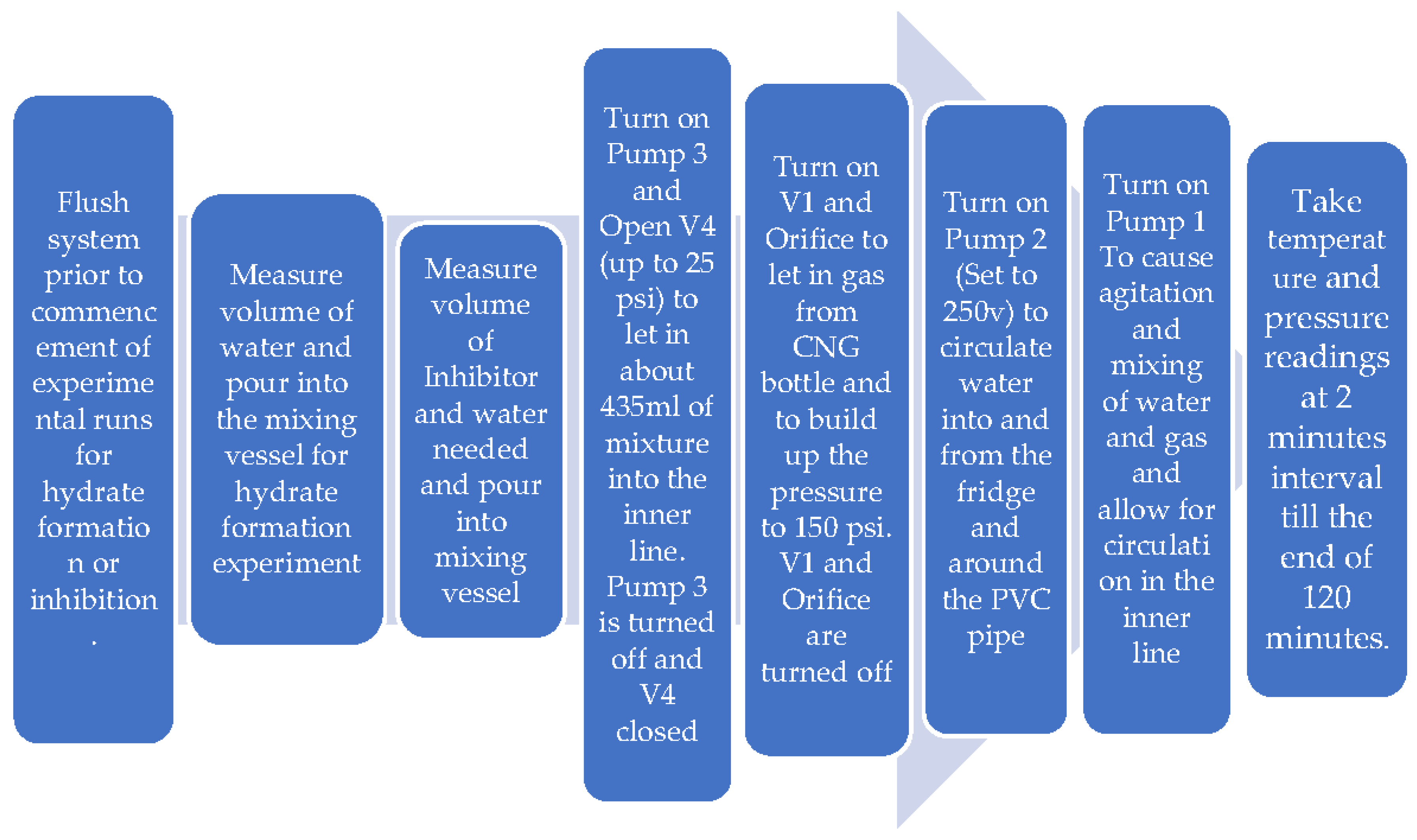
2.2. Procedure
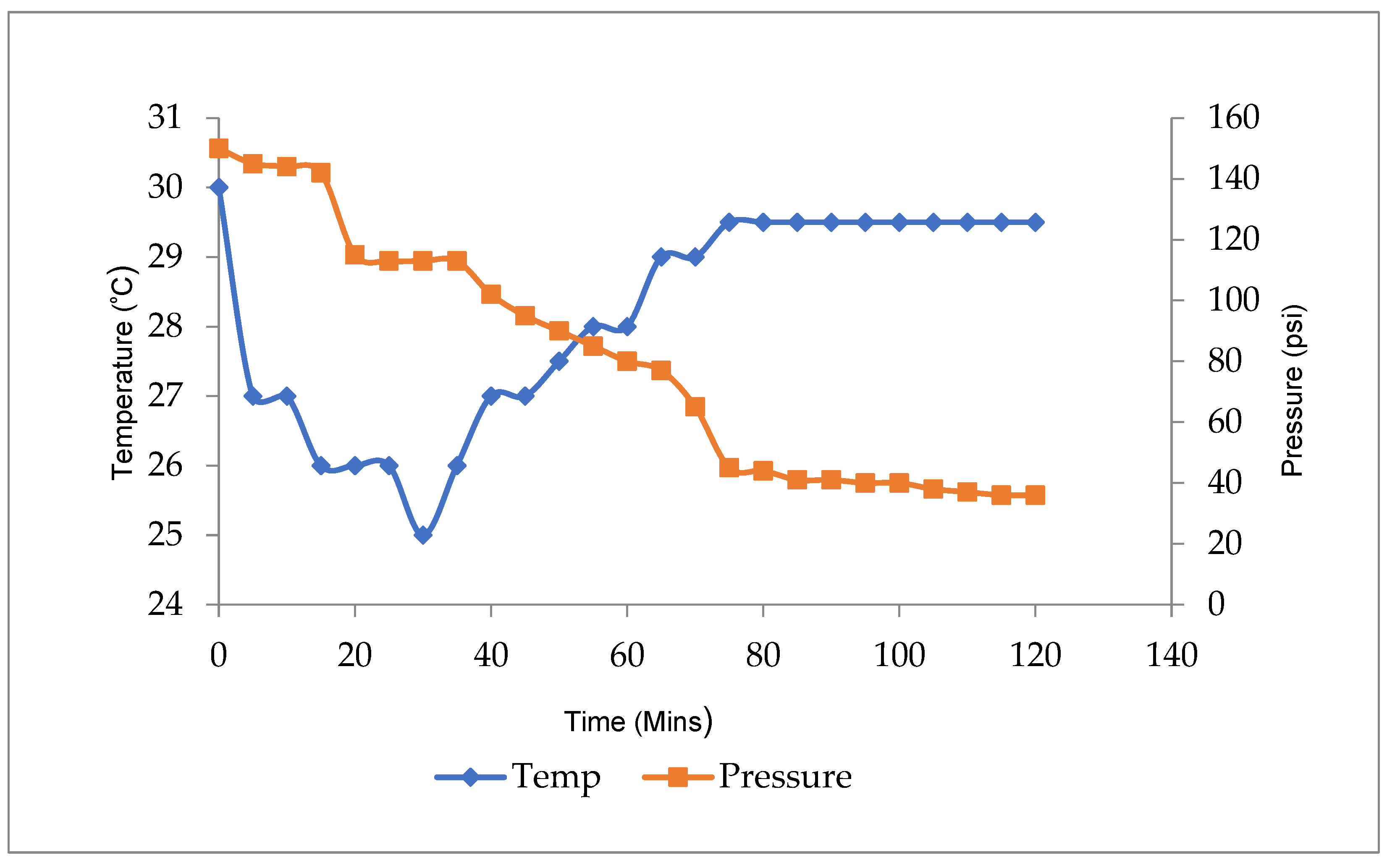
3. Results and Discussion
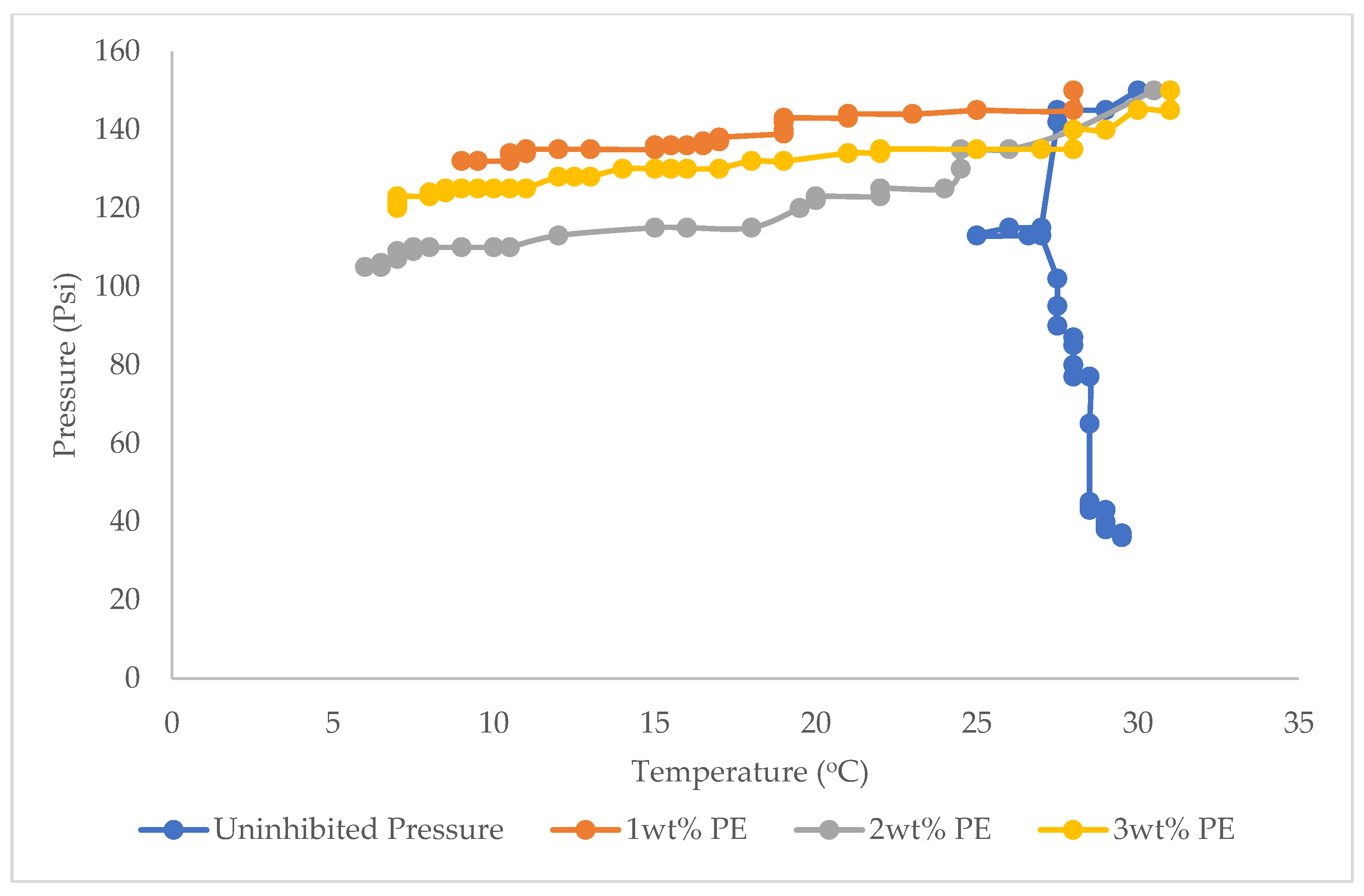
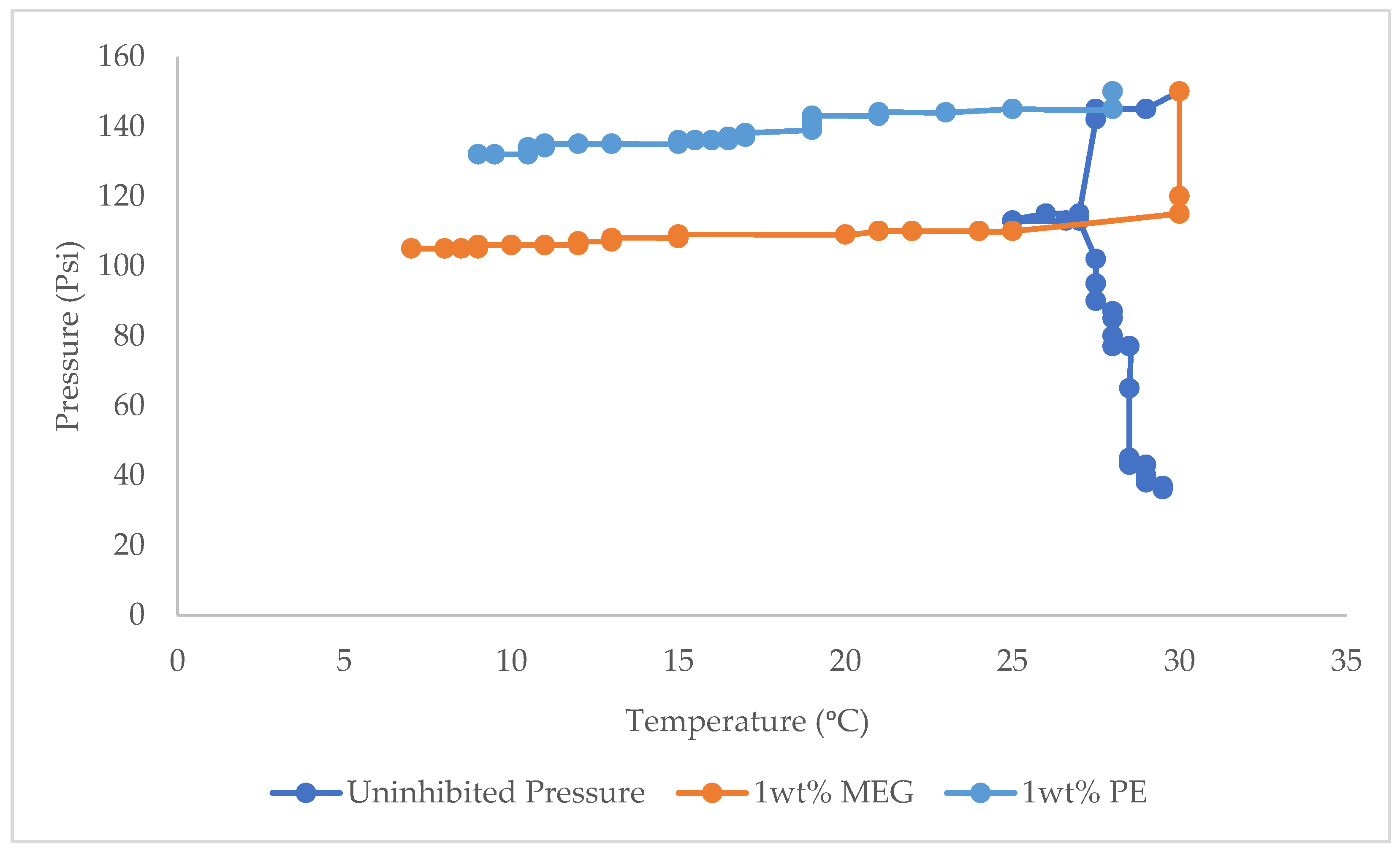
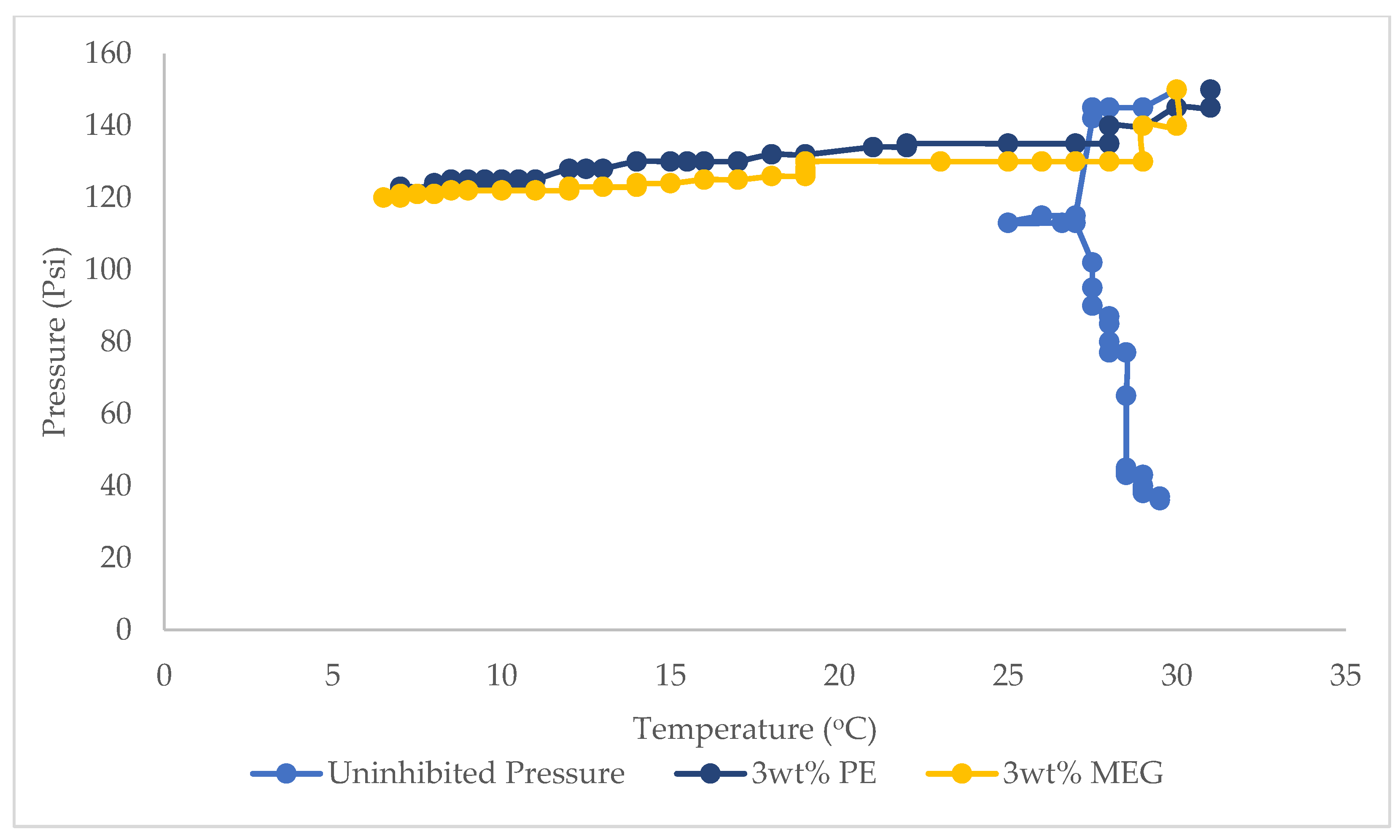
3.1. Hydrate Equilibrium Pressure-Temperature Plots for 1, 2, and 3 wt% PE and Uninhibited Experiment (Water and Gas)
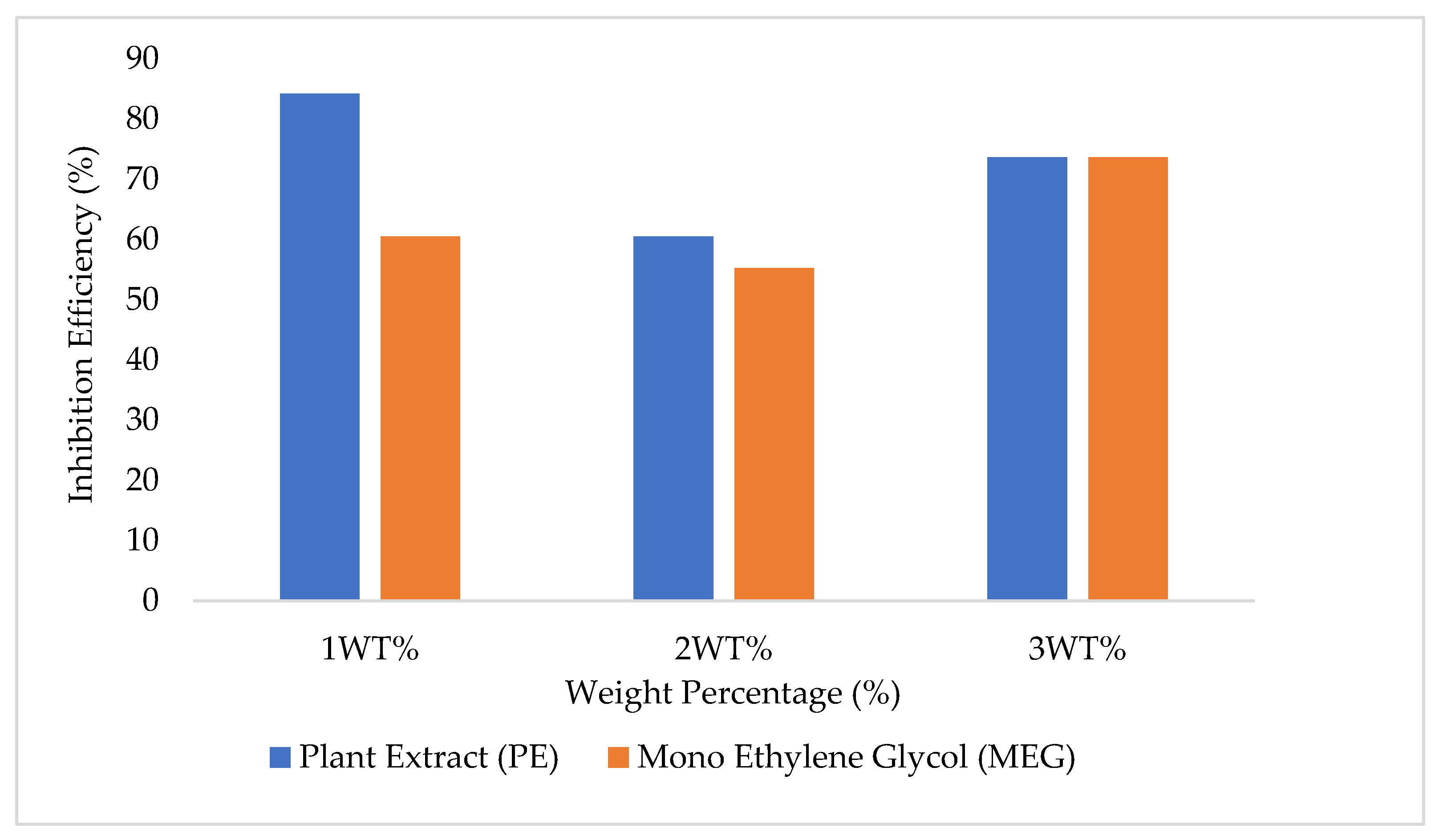
3.2. Comparison of the Inhibitory Capacities of Plant Extract (PE) and Mono Ethylene Glycol (MEG)
3.3. Hydrate Equilibrium Pressure-Temperature Plots for 1, 2, and 3 wt% PE, MEG and Uninhibited Experiment (Water and Gas)
3.4. Initial and Final Pressure versus Time for 1, 2, and 3 wt% of PE, MEG, and Uninhibited Experiment
3.5. Inhibition Efficiency for 1, 2, and 3 wt% of PE, MEG Inhibited Experiment
- Pi is the initial pressure for both gas and water alone and inhibited systems using PE and MEG.
- Pinhibited is the final pressure of PE and MEG-inhibited systems
- Puninhibited is the final pressure for the gas and water system only.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammerschmidt, E.G. Gas Hydrates Formations: A Further Study on Their Prevention and Elimination from Natural Gas Pipelines. GPSA 1939, 15, 30–35. [Google Scholar]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J. Natural Gas Hydrates. Guide for Engineers, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 17–22. [Google Scholar]
- Sloan, E.D. Clathrates of Hydrates of Natural Gas, 2nd ed.; Marcell Dekker, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Seo, Y.-T.; Kang, S.-P.; Lee, H. Experimental determination and thermodynamic modeling of methane and nitrogen hydrates in the presence of THF, propylene oxide, 1,4-dioxane and acetone. Fluid Phase Equilibria 2001, 189, 99–110. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A.; Sum, A.K.; Ballard, A.L.; Shoup, G.J.; McMullen, N.; Creek, J.L.; Palermo, T. Hydrates: State of the Art Inside and Outside Flowlines. J. Pet. Technol. 2009, 61, 89–94. [Google Scholar] [CrossRef]
- Dorstewitz, F.; Mewes, D. Hydrate Formation in Pipelines. In Proceedings of the 5th International Offshore and Polar Engineering Conference, The Hague, The Netherlands, 11–16 June 1995. ISOPE-1-95-038. [Google Scholar]
- Baker, J.W.; Gomez, R.K. Formation of Hydrates during Deep Water Drilling Operations. In Proceedings of the SPE/IADC Drilling Conference, New Orleans, LA, USA, 28 February–3 March 1989. [Google Scholar]
- Gbaruko, B.C. Asphaltenes Oil Recovery and Down-hole Upgrading in Nigerian Petroleum Industry. In Proceedings of the 228 ACS National Meeting, Philadelphia, PA, USA, 22–26 August 2004. [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2008; 758p. [Google Scholar]
- Botrel, T. Hydrate Prevention and Removal in Ultra-Deepwater Drilling Systems. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April-3 May 2001. OTC-12962. [Google Scholar]
- Szymczak, S.; Sanders, K.B.; Pakulsi, M.K.; Higgins, T.O. Chemical Compromise, a Thermodynamic and Low-Dosage Hydrate Inhibitor Solution for Hydrate Control in the Gulf of Mexico. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 9–12 October 2005. SPE P6418. [Google Scholar]
- Owodunni, O.L.; Ajienka, J.A. Use of Thermal Insulation to Prevent Hydrate and Paraffin Wax Deposition. In Proceedings of the 31st Nigerian International Conference and Exhibition, Abuja, Nigeria, 6–8 August 2007; p. 111903. [Google Scholar]
- Kelland, M.A.; Svartaas, T.M.; Dybvik, L. A New Generation of Gas Hydrate Inhibitors. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 22–25 October 1995; p. 30695. [Google Scholar]
- Qureshi, M.F.; Atilhan, M.; Altamash, T.; Tariq, M.; Khraisheh, M.; Aparicio, S.; Tohidi, B. Gas Hydrate Prevention and Flow Assurance by Using Mixtures of Ionic Liquids and Synergent Compounds: Combined Kinetics and Thermodynamic Approach. Energy Fuels 2016, 30, 3541–3548. [Google Scholar] [CrossRef]
- Tariq, M.; Rooney, D.; Othman, E.; Aparicio, S.; Atilhan, M.; Khraisheh, M. Gas Hydrate Inhibition: A review of the role of Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 17855–17868. [Google Scholar] [CrossRef]
- Swanson, T.A.; Petrie, M.; Sifferman, C.R. The Successful Use of Both Kinetic Hydrate and Paraffin Inhibitors together in a Deep-Water Pipeline with a High Water Cut in the Gulf of Mexico. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 2–4 February 2005; p. 93158. [Google Scholar]
- Makogun, T.; Sloan, D. Mechanism of Kinetic Inhibitors. In Proceedings of the 4th International Conference on Gas Hydrates, Yokhama, Japan, 19–23 May 2002. [Google Scholar]
- Zheng, J.; Musa, O.; Lei, C.; Zhang, Y.; Alexandre, M.; Edris, S.A. Innovative KHI Polymers for Gas Hydrate Control. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2011. OTC-21275. [Google Scholar]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Perfeldt, C.M.; Chua, P.C.; Daraboina, N.; Frus, D.; Kristiansen, E.; Ranlov, H. Inhibition of Gas Hydrate Nucleation and Growth: Efficacy of an Anti-Freeze Protein from the Long horn Beetle, Rhagium Mordax. Energy Fuels 2014, 28, 3666–3672. [Google Scholar] [CrossRef]
- Daraboina, N.; Ripmeester, J.A.; Walker, V.; Englezos, P. Natural Gas Hydrate Formation and Decomposition in the Presence of Kinetic Inhibitor 1. High Calorimetry. Energy Fuels 2011, 25, 4392–4397. [Google Scholar] [CrossRef]
- Ohno, H.; Susilo RGordienko, R.; Ripmeester, J.; Walker, V.K. Interactions of Anti-freeze Proteins with Hydrocarbon Hydrates. Chem. Eur. J. 2010, 16, 10407–10417. [Google Scholar] [CrossRef]
- Jensen, L.; Thomsen, L.; Nicolas, V.S. Inhibition of Structure I and II Gas Hydrates using Synthetic and Biological Kinetic Inhibitors. Energy Fuels 2011, 25, 17–23. [Google Scholar] [CrossRef]
- Pal, S.; Mal, D.; Singh, R. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Fang, S.; Lang, X.; Wang, Y.; Chen, J. Pectin as an Extraordinary Natural Kinetic Hydrate Inhibitor. Sci. Rep. 2016, 6, 23220. [Google Scholar] [CrossRef] [PubMed]
- Rasoolzadeh, A.; Javanmardi, J.; Esiamimanesh, A.; Amir, H.A. Experimental Studyand Modelling of Methane Hydrate Formation Induction Times in the presence of Ionic Liquids. J. Mol. Liq. 2016, 221, 149–155. [Google Scholar] [CrossRef]
- Argo, C.B.; Blaine, R.A.; Osborne, C.C. Commercial Deployment of Low Dosage Hydrate Inhibitor in a Southern North Sea 69Km Wet-Gas Subsea Pipeline. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997; p. 37255. [Google Scholar]
- Talley, L.D.; Mitchell, G.F.; Oelfke, R.H. Comparison of Laboratory Results on Hydrate Induction Rates in a THF Rig, High-Pressure Rocking Cell, Miniloop, and Large Flowloop. Ann. N. Y. Acad. Sci. 2000, 912, 314–321. [Google Scholar] [CrossRef]
- Frostman, L.M. Anti-Agglomerant Hydrate Inhibitor for Prevention of Hydrate Plugs in Deep Water Systems. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1–4 October 2000; p. 63122. [Google Scholar] [CrossRef]
- Lee, J.D.; Englezos, P. Enhancement of the performance of gas hydrate kinetic inhibitors with polyethylene oxide. Chem. Eng. Sci. 2005, 60, 5323–5330. [Google Scholar] [CrossRef]
- Odutola, T.O.; Ikiensikimama, S.S.; Ajienka, J.A. Effective Hydrate Management during Gas Expansion. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 4–6 August 2015. SPE 178342. [Google Scholar]
- Elechi, V.U.; Ikiensikimama, S.S.; Akaranta, O.; Ajienka, J.A.; Onyekonwu, M.O.; Okon, O.E. Gas Hydrate Mitigation Using an Organic Extract in a Simulated Offshore Environment. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. OTC-30774-MS. [Google Scholar] [CrossRef]
- Elechi, V.U.; Ikiensikimama, S.S.; Akaranta, O.E.; Ajienka, J.A.; Onyekonwu, M.O.; Okon, O.E. The use of a bench-scale high pressure apparatus to evaluate the effectiveness of a locally sourced material as gas hydrate inhibitor. Sci. Afr. 2020, 8, e00300. [Google Scholar] [CrossRef]
- Elechi, V.U.; Ikiensikimama, S.S.; Ajienka, J.A.; Akaranta, O.; Okon, O.E. Mitigation capacity of an eco-friendly locally sourced surfactant for gas hydrate inhibition in an offshore environment. J. Pet. Explor. Prod. Technol. 2021, 11, 1797–1808. [Google Scholar] [CrossRef]
- Elechi, V.U.; Ikiensikimama, S.S.; Akaranta, O.E.; Ajienka, J.A.; Onyekonwu, M.O.; Okon, O.E. Evaluation of the Inhibition Efficiency of Plant Extract PE as Gas Hydrate Inhibitor in a Simulated Offshore Environment. In Proceedings of the Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2019; p. 198781. [Google Scholar]
- Okon, O.E.; Ajienka, J.A.; Ikiensikimama, S.S.; Elechi, V.U.; Odutola, T.O. Use of locally formulated inhibitor from agro waste for gas hydrate inhibition in a mini flow loop. Int. J. Sci. Eng. Inv. (IJSEI) 2018, 7, 104–112. [Google Scholar]
- Elechi, V.U.; Ikiensikimama, S.S.; Ajienka, J.A.; Akaranta, O.; Onyekonwu, M.O.; Odutola, T.O.; Okon, O.E. Gas Hydrate Inhibition in a Simulated Offshore Environment using Local Inhibitor. In Proceedings of the Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 6–8 August 2018; p. 193439. [Google Scholar]
- Odutola, T.O.; Ajienka, J.A.; Onyekonwu, M.O.; Ikiensikimama, S.S. Design, Fabrication and Validation of a Laboratory Flow Loop for Hydrate Studies. Am. J. Chem. Eng. 2017, 5, 28–41. [Google Scholar] [CrossRef]
- Ababio, O.Y. New School Chemistry, 3rd ed.; African First Publisher: Onitsha, Nigeria, 2001; 550p. [Google Scholar]
- El-Etre, A.Y. Inhibition of Aluminium Corrosion using Opuntia Extract. Corros. Sci. 2003, 45, 2485–2495. [Google Scholar] [CrossRef]
- Ezeagu, I.E.; Fafunso, M.A. Biochemical Constituents of Arecaceae Extract. Ecol. Food Nutr. 2003, 42, 213–222. [Google Scholar] [CrossRef]
- Quideau, S. Why Bother with Poly Phenols? Groupe Poly Phenols. 2011. Available online: https://www.groupepolyphenols.com/the-society/why-bother-with-polyphenols/ (accessed on 26 March 2022).
- Valberg, T. Efficiency of Thermodynamic Hydrate Inhibitors for Melting Gas Hydrates; University of Science & Technology (NTNU): Trondheim, Norway, 2006. [Google Scholar]
- Bishnoi, P.R.; Natarjan, V. Formation and Decomposition of Gas Hydrates. Fluid Phase Equilibria 1996, 117, 168–177. [Google Scholar] [CrossRef]
- Jensen, L.; Thomsen, K.; von Solms, N. Propane hydrate nucleation: Experimental investigation and correlation. Chem. Eng. Sci. 2008, 63, 3069–3080. [Google Scholar] [CrossRef]
- Sloan, E.D. Clathrate Hydrates of Natural Gases, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Ellison, B.T.; Gallagher, C.T.; Lorimer, S.E. The Physical Chemistry of Wax, Hydrates and Asphaltenes. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2000; p. 11963. [Google Scholar]
- Bai, Y.; Bai, Q. Hydrates. In Subsea Engineering Handbook; O’Reilly: Springfield, MO, USA, 2019; pp. 409–434. [Google Scholar] [CrossRef]
- Magnusson, C.; Abrahamsen, E.; Kelland, M.A.; Cely, A.; Kinnari, K.; Li, X.; Askvik, K.M. As Green As It Gets: An Abundant Kinetic Hydrate Inhibitor from Nature. Energy Fuels 2018, 32, 5772–5778. [Google Scholar] [CrossRef]
- Edmonds, B.; Moorwood RA, S.; Szcpanski, R.; Zhang, X. Latest Development on Integrated Prediction Modeling Hydrates, Waxes and Asphaltenes, Focus on Controlling Hydrates, Waxes and Asphaltenes; IBC: Aberdeen, Scotland, 1999. [Google Scholar]
- Zubin, D. Patel and Jim Russel Flow Assurance: Chemical Inhibition of Gas Hydrates in Deep Water Production Systems. Article Extracted from―OFFSHORE Magazine‖. Offshore J. 24 June 2010. Available online: https://www.offshore-mag.com/pipelines/article/16763699/flow-assurance-chemical-inhibition-of-gas-hydrates-in-deepwater-production-systems (accessed on 20 July 2022).
- Brustard, S.; Loken, K.P.; Waalman, J.G. Hydrate Prevention using MEG instead of MeOH: Impact of Experience from Major Norwegian Development on Technology Selection for Injection and Recovery of MEG. In Proceedings of the 2005 Offshore Technology Conference, Houston, TX, USA, 2–5 May 2005; p. 17355. [Google Scholar]





| Components | Molecular Weight (Mw) | Mole Fraction (%) |
|---|---|---|
| Methane, CH4 | 16 | 98.44 |
| Carbondioxide, CO2 | 44 | 1.56 |
| Hydrogen sulfide (H2S) | 34.08 | - |
| Total Sulfur | 32.065 | - |
| Oxygen (O2) | 16 | - |
| 1 wt% | Δp (psi) | 2 wt%(psi) | Δp (psi) | 3 wt%(psi) | Δp (psi) | |
|---|---|---|---|---|---|---|
| Gas and water | 150 − 36 | 114 | 150 − 36 | 114 | 150 − 36 | 114 |
| Plant Extract (PE) | 150 − 132 | 18 | 150 − 105 | 45 | 150 − 120 | 30 |
| Mono Ethylene Glycol (MEG) | 150 − 105 | 45 | 150 − 99 | 51 | 150 − 120 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachikwu-Elechi, V.U.; Ikiensikimama, S.S.; Ajienka, J.A. The Influence of Plant Extract on the Phase Equilibrium of Structure I Gas Hydrate in a Simulated Offshore Environment. Thermo 2023, 3, 21-37. https://doi.org/10.3390/thermo3010002
Wachikwu-Elechi VU, Ikiensikimama SS, Ajienka JA. The Influence of Plant Extract on the Phase Equilibrium of Structure I Gas Hydrate in a Simulated Offshore Environment. Thermo. 2023; 3(1):21-37. https://doi.org/10.3390/thermo3010002
Chicago/Turabian StyleWachikwu-Elechi, Virtue Urunwo, Sunday Sunday Ikiensikimama, and Joseph Atubokiki Ajienka. 2023. "The Influence of Plant Extract on the Phase Equilibrium of Structure I Gas Hydrate in a Simulated Offshore Environment" Thermo 3, no. 1: 21-37. https://doi.org/10.3390/thermo3010002
APA StyleWachikwu-Elechi, V. U., Ikiensikimama, S. S., & Ajienka, J. A. (2023). The Influence of Plant Extract on the Phase Equilibrium of Structure I Gas Hydrate in a Simulated Offshore Environment. Thermo, 3(1), 21-37. https://doi.org/10.3390/thermo3010002