Dose-Dependent Effect of Foliar ZnO Nanoparticles on the Physiology, Mineral Nutrition, and Redox Status of Coffea arabica Seedlings Under Soil Acidity
Abstract
1. Introduction
2. Results
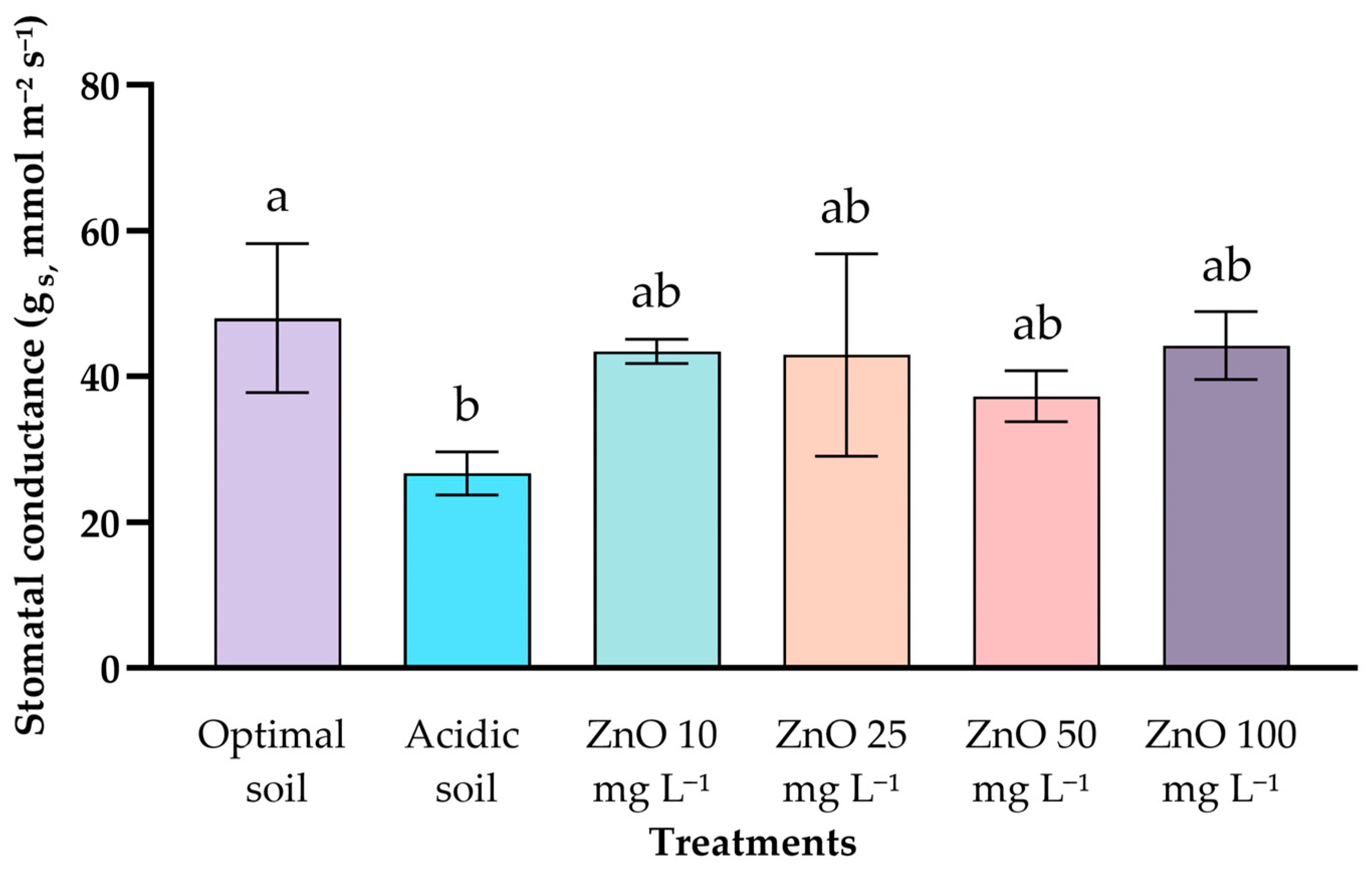
2.1. Relative Chlorophyll Content and Stomatal Conductance (Gs)
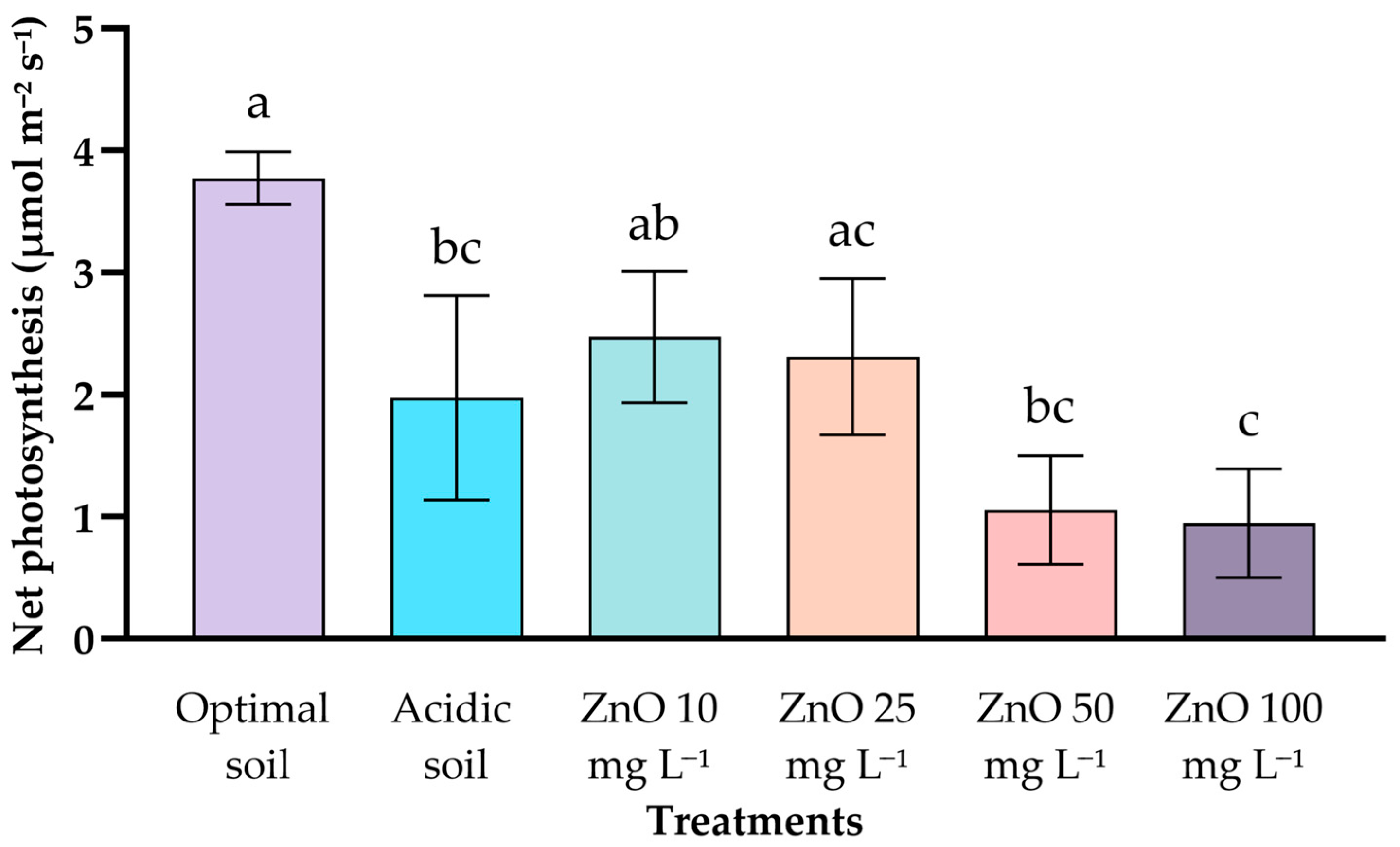
2.2. Net Photosynthesis Rate (A, μmol CO2 m−2 s−1)
2.3. Elemental Content in Leaves and Roots
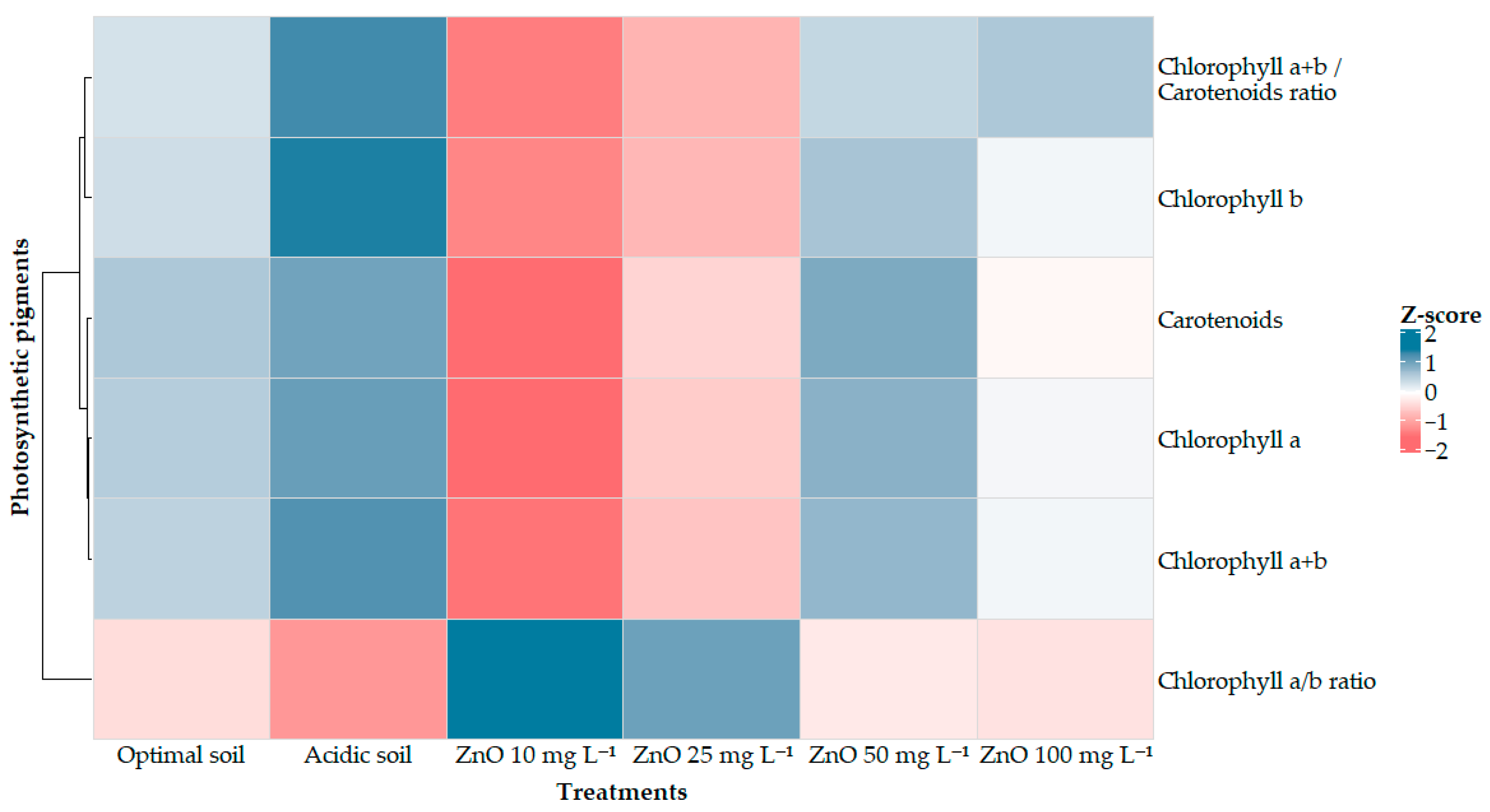
2.4. Photosynthetic Pigment Content
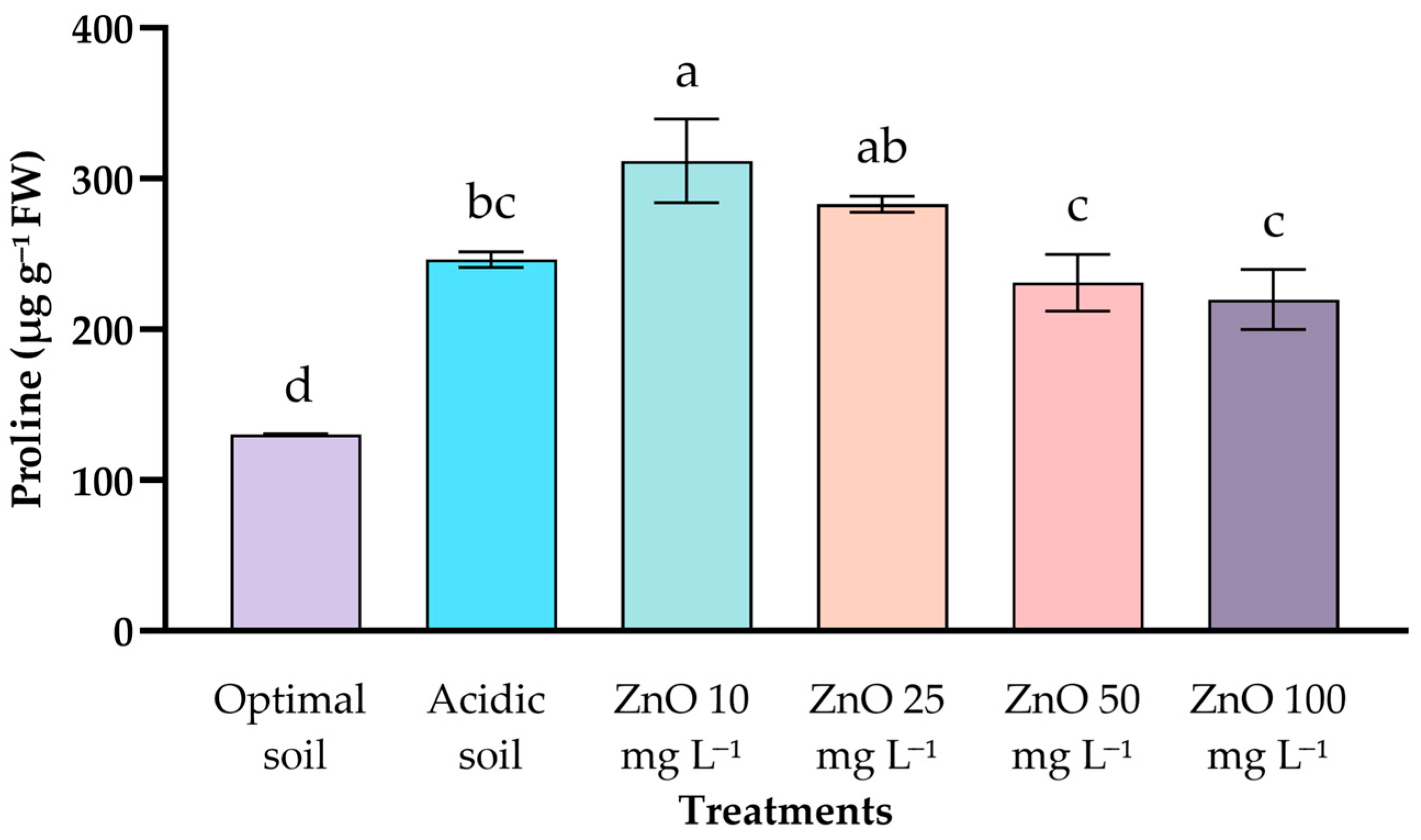
2.5. Proline Content
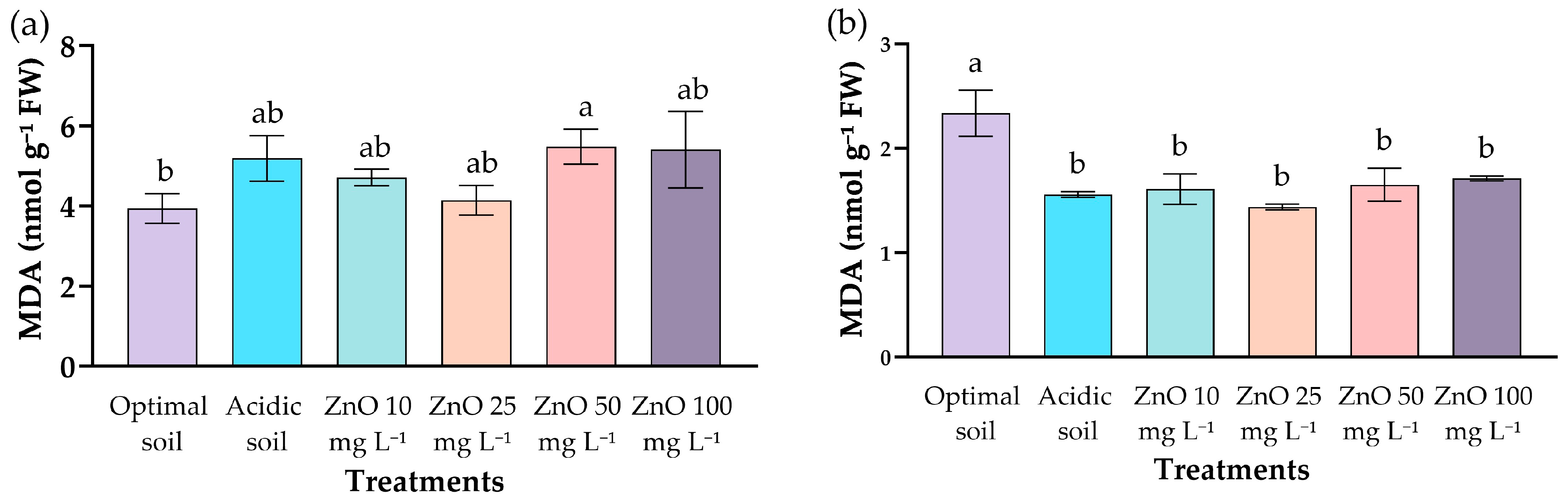
2.6. Lipid Peroxidation in Leaves and Roots
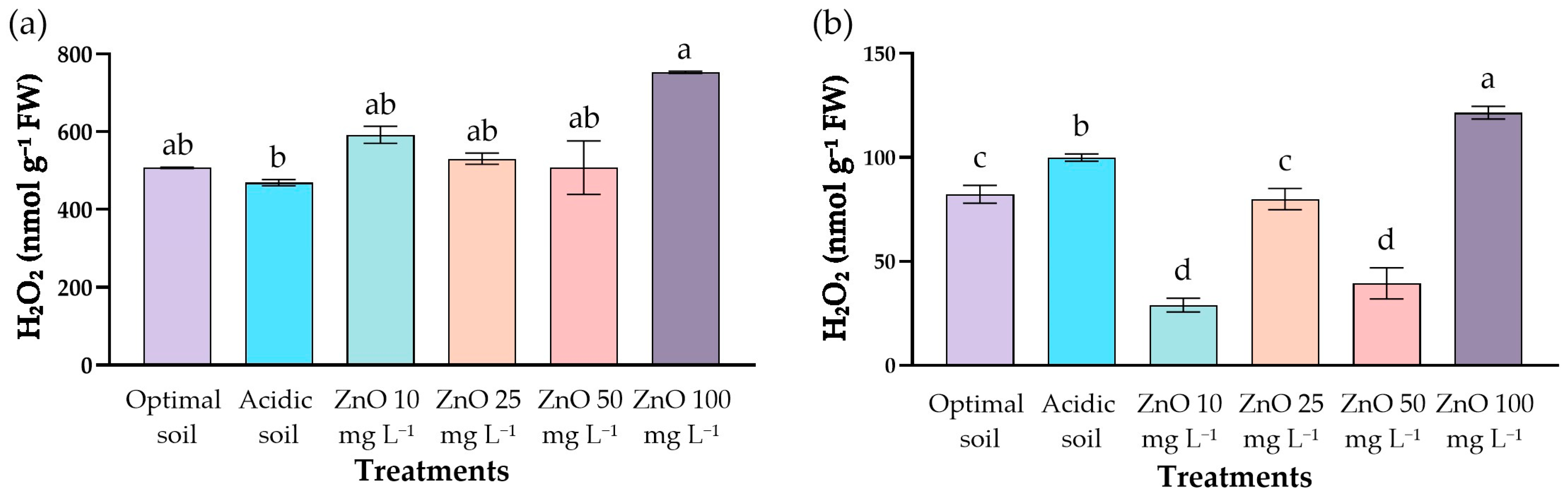
2.7. Hydrogen Peroxide Content
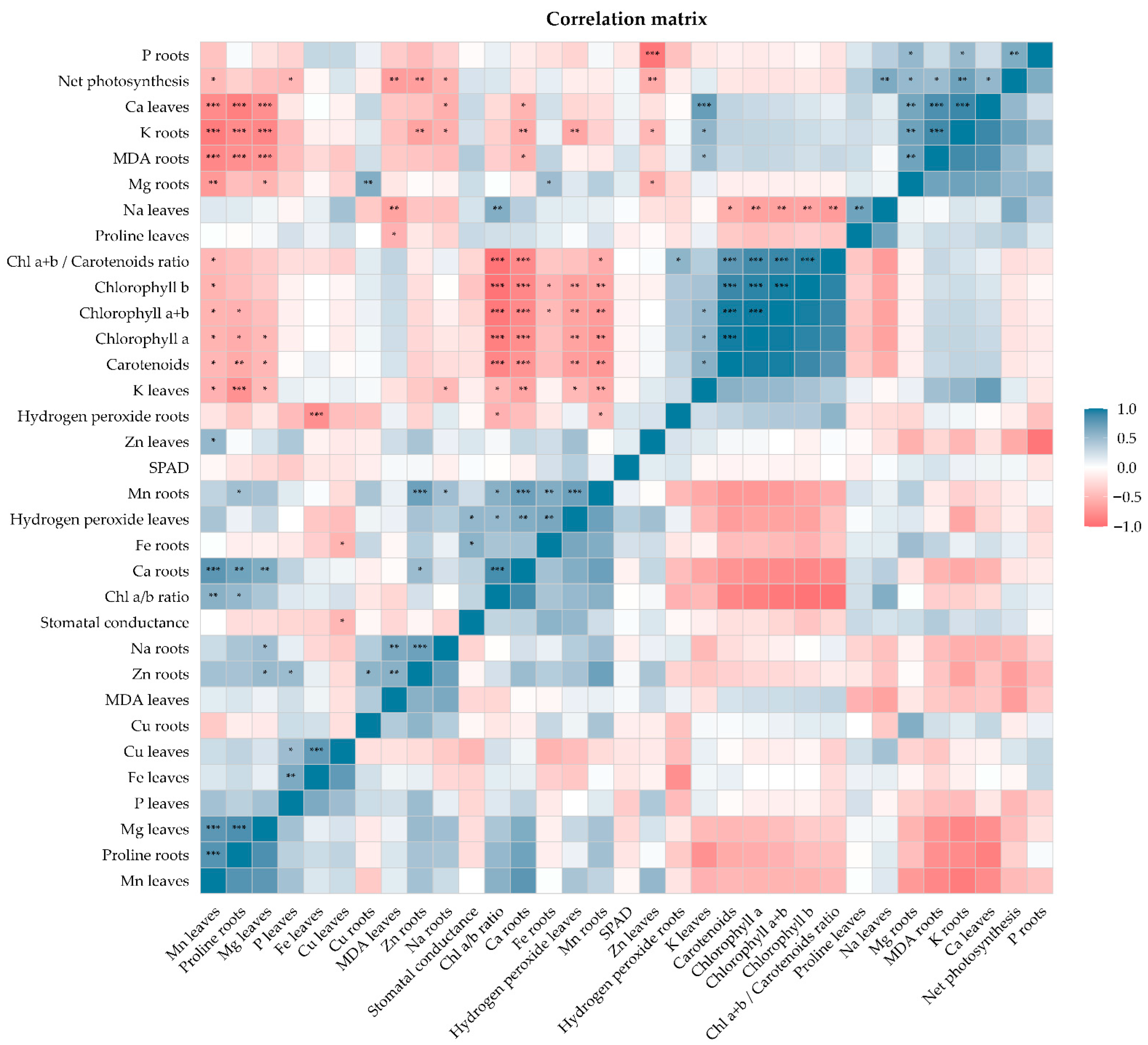
2.8. Physiological, Biochemical, and Nutritional Correlation Matrix
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Determination of Relative Chlorophyll Content and Stomatal Conductance
4.3. Measurement of the Net Photosynthesis Rate (A, μmol CO2 m−2 s−1)
4.4. Photochemical Analysis of Photosynthetic Pigments
4.5. Elemental Analysis of Leaves and Roots
4.6. Colorimetric Quantification of Proline Content
4.7. Quantification of MDA (Lipid Peroxidation)
4.8. Spectrophotometric Quantification of H2O2 Content
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZnO NPs | Zinc oxide nanoparticles |
| Al3+ | Aluminum ion |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| gs | Stomatal conductance |
| To | Net photosynthetic rate |
| SPAD | Chlorophyll content index (SPAD units) |
| FW | Fresh weight |
| DW | Dry weight |
| PSII | Photosystem II |
| OEC | Oxygen-evolving complex |
| Pi | Inorganic phosphate |
| NR | Nitrate reductase |
| Nir | Nitrite reductase |
| GS | Glutamine synthetase |
| GOGAT | Glutamate synthase |
| H+-ATPase | Proton-translocating ATPase |
| CBL–CIPK | Calcineurin B-like/CBL-interacting protein kinase |
| TBARS | Thiobarbituric acid reactive substances |
| ZIP | Zinc-regulated transporter/Iron-regulated transporter-like protein |
| HMA | Heavy-metal ATPase |
| IRT | Iron-regulated transporter |
| NRAMP | Natural resistance-associated macrophage protein |
References
- Ovalle-Rivera, O.; Läderach, P.; Bunn, C.; Obersteiner, M.; Schroth, G. Projected Shifts in Coffea arabica Suitability among Major Global Producing Regions Due to Climate Change. PLoS ONE 2015, 10, e0124155. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Coffee. Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2025; Available online: https://www.fao.org/markets-and-trade/commodities-overview/beverages/coffee/en (accessed on 8 September 2025).
- Daily Coffee News Staff. Major ICO Green Coffee Report Notes “Growing Americas” and “Shrinking Rest of the World.” Daily Coffee News by Roast Magazine. 2023. Available online: https://dailycoffeenews.com/2023/12/05/major-ico-green-coffee-report-notes-growing-americas-and-shrinking-rest-of-the-world/ (accessed on 9 September 2025).
- Nogueira, V. Recent Developments and Prospects in the Coffee Value Chain; UNCTAD: Geneva, Switzerland, 2024; Available online: https://unctad.org/system/files/non-official-document/vanusia-nogueira_myem2024.pdf (accessed on 13 September 2025).
- Yang, Z.-B.; Rao, I.M.; Horst, W.J. Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 2013, 372, 3–25. [Google Scholar] [CrossRef]
- Quispe, K.; Hermoza, N.; Mejia, S.; Romero-Chavez, L.E.; Ottos, E.; Arce, A.; Solórzano Acosta, R. Spatial Analysis of Soil Acidity and Available Phosphorus in Coffee-Growing Areas of Pichanaqui: Implications for Liming and Site-Specific Fertilization. Agriculture 2025, 15, 1632. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, J.E.D.A.; Sánchez-Cach, L.A.; Ku-González, Á.; de los Santos-Briones, C.; de Fátima Medina-Lara, M.; Echevarría-Machado, I.; Muñoz-Sánchez, J.A.; Teresa Hernández Sotomayor, S.M.; Estévez, M.M. Differential effects of aluminum on in vitro primary root growth, nutrient content and phospholipase C activity in coffee seedlings (Coffea arabica). J. Inorg. Biochem. 2014, 134, 39–48. [Google Scholar] [CrossRef]
- Poot-Poot, W.; Rodas-Junco, B.A.; Muñoz-Sánchez, J.A.; Hernández-Sotomayor, S.M.T. Protoplasts: A friendly tool to study aluminum toxicity and coffee cell viability. SpringerPlus 2016, 5, 1452. [Google Scholar] [CrossRef]
- Braccini, M.C.L.; Martinez, H.E.P.; Pereira, P.R.G.; Sampaio, N.F.; Silva, E.a.M. Aluminum tolerance of coffee genotypes in nutrient solution. I. Root and shoot growth and development. Rev. Bras. Ciênc. Solo 1998, 22, 435–442. [Google Scholar] [CrossRef]
- Silva Rincón, C.I.; Alarcón Gutiérrez, E.; García Pérez, J.A.; Sánchez-Velásquez, L.R.; Iglesias Andreu, L.G. La variedad de café Geisha y su estatus en el mundo y en México. Rev. Mex. Cienc. Agríc. 2024, 15. [Google Scholar] [CrossRef]
- Bauduin, S.; Latini, M.; Belleggia, I.; Migliore, M.; Biancucci, M.; Mattioli, R.; Francioso, A.; Mosca, L.; Funck, D.; Trovato, M. Interplay between Proline Metabolism and ROS in the Fine Tuning of Root-Meristem Size in Arabidopsis. Plants 2022, 11, 1512. [Google Scholar] [CrossRef]
- Schmitt, M.; Boras, S.; Tjoa, A.; Watanabe, T.; Jansen, S. Aluminium Accumulation and Intra-Tree Distribution Patterns in Three Arbor aluminosa (Symplocos) Species from Central Sulawesi. PLoS ONE 2016, 11, e0149078. [Google Scholar]
- Zhao, Y.; Tang, Y.; Hu, L.; Xu, J.; Zhang, X.; Dou, X.; Zhang, S.; Huang, L.; Wang, X. Growth and physiology effects of seed priming and foliar application of ZnO nanoparticles on Hibiscus syriacus L. Sci. Rep. 2025, 15, 22606. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, L.V.; Puranik, N.; Lekkala, V.V.V.; Lomada, D.; Reddy, M.C.; Maurya, A.K. ZnO Nanoparticles: Advancing Agricultural Sustainability. Plants 2025, 14, 2430. [Google Scholar] [CrossRef] [PubMed]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Exogenous Kinetin Promotes the Nonenzymatic Antioxidant System and Photosynthetic Activity of Coffee (Coffea arabica L.) Plants Under Cold Stress Conditions. Plants 2020, 9, 281. [Google Scholar] [CrossRef]
- Maxiselly, Y.; Anusornwanit, P.; Rugkong, A.; Chiarawipa, R.; Chanjula, P. Morpho-Physiological Traits, Phytochemical Composition, and Antioxidant Activity of Canephora Coffee Leaves at Various Stages. Int. J. Plant Biol. 2022, 13, 106–114. [Google Scholar] [CrossRef]
- Baloch, S.B.; Ali, S.; Bernas, J.; Moudrý, J.; Konvalina, P.; Mushtaq, Z.; Murindangabo, Y.T.; Onyebuchi, E.F.; Baloch, F.B.; Ahmad, M.; et al. Wood ash application for crop production, amelioration of soil acidity and contaminated environments. Chemosphere 2024, 357, 141865. [Google Scholar] [CrossRef]
- Parecido, R.J.; Soratto, R.P.; Perdoná, M.J.; Gitari, H.I.; Dognani, V.; Santos, A.R.; Silveira, L. Liming Method and Rate Effects on Soil Acidity and Arabica Coffee Nutrition, Growth, and Yield. J. Soil Sci. Plant Nutr. 2021, 21, 2613–2625. [Google Scholar] [CrossRef]
- Agegnehu, G.; Amede, T.; Erkossa, T.; Yirga, C.; Henry, C.; Tyler, R.; Nosworthy, M.G.; Beyene, S.; Sileshi, G.W. Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2021, 71, 852–869. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Rajpal, V.R.; Nongthongbam, B.; Bhatia, M.; Singh, A.; Raina, S.N.; Minkina, T.; Rajput, V.D.; Zahra, N.; Husen, A. The nano-paradox: Addressing nanotoxicity for sustainable agriculture, circular economy and SDGs. J. Nanobiotechnol. 2025, 23, 314. [Google Scholar] [CrossRef]
- Meléndez-Mori, J.B.; Lapiz-Culqui, Y.K.; Huaman-Huaman, E.; Zuta-Puscan, M.; Oliva-Cruz, M. Can Zinc Oxide Nanoparticles Alleviate the Adverse Effects of Salinity Stress in Coffea arabica? Agronomy 2025, 15, 1239. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Chapter Six—Management of Soil Acidity of South American Soils for Sustainable Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 221–275. Available online: https://www.sciencedirect.com/science/article/pii/B9780128021392000068 (accessed on 20 October 2025).
- Melash, A.A.; Bytyqi, B.; Nyandi, M.S.; Vad, A.M.; Ábrahám, É.B. Chlorophyll Meter: A Precision Agricultural Decision-Making Tool for Nutrient Supply in Durum Wheat (Triticum turgidum L.) Cultivation under Drought Conditions. Life 2023, 13, 824. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alhammad, B.A.; Tola, E. Exogenous Application of Zinc Oxide Nanoparticles Improved Antioxidants, Photosynthetic, and Yield Traits in Salt-Stressed Maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- Velasco, E.A.P.; Valdez-Aguilar, L.A.; Galindo, R.B.; Palomino, A.B.; Urbina, B.A.P. Morphology and Coating of ZnO Nanoparticles Affect Growth and Gas Exchange Parameters of Bell Pepper Seedlings. Agronomy 2025, 15, 1579. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Święciło, A.; Gawroński, J. The Effect of Zinc Oxide Nanoparticles on the Quantitative and Qualitative Traits of Scutellaria baicalensis Georgi in In Vitro Culture. Int. J. Mol. Sci. 2025, 26, 5836. [Google Scholar] [CrossRef] [PubMed]
- Azarin, K.; Usatov, A.; Minkina, T.; Duplii, N.; Kasyanova, A.; Fedorenko, A.; Khachumov, V.; Mandzhieva, S.; Rajput, V.D. Effects of bulk and nano-ZnO particles on functioning of photosynthetic apparatus in barley (Hordeum vulgare L.). Environ. Res. 2023, 216, 114748. [Google Scholar] [CrossRef]
- Ware, M.A.; Dall’Osto, L.; Ruban, A.V. An In Vivo Quantitative Comparison of Photoprotection in Arabidopsis Xanthophyll Mutants. Front. Plant Sci. 2016, 7, 841. [Google Scholar] [CrossRef]
- Dau, H.; Grundmeier, A.; Loja, P.; Haumann, M. On the structure of the manganese complex of photosystem II: Extended-range EXAFS data and specific atomic-resolution models for four S-states. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1237–1244. [Google Scholar] [CrossRef]
- Fallah, S.; Yusefi-Tanha, E.; Peralta-Videa, J.R. Interaction of nanoparticles and reactive oxygen species and their impact on macromolecules and plant production. Plant Nano Biol. 2024, 10, 100105. [Google Scholar] [CrossRef]
- Baksi, S.; Singh, K.M.; Rani, S.; Sharma, P. Melatonin-functionalized zinc oxide nanoparticles enhance salt stress tolerance in Vigna mungo L. by regulating antioxidants and ion homeostasis. Environ. Sci. Nano 2025, 12, 5056–5073. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Chernikova, N.; Hassan, T.; Mandzhieva, S.; Sushkova, S.; Lysenko, V.; Soldatov, M.A.; Burachevskaya, M. Effects of Zinc Oxide Nanoparticles on Physiological and Anatomical Indices in Spring Barley Tissues. Nanomaterials 2021, 11, 1722. [Google Scholar] [CrossRef]
- Wang, R.; Mi, K.; Yuan, X.; Chen, J.; Pu, J.; Shi, X.; Yang, Y.; Zhang, H.; Zhang, H. Zinc Oxide Nanoparticles Foliar Application Effectively Enhanced Zinc and Aroma Content in Rice (Oryza sativa L.) Grains. Rice 2023, 16, 36. [Google Scholar] [CrossRef]
- Kang, M.; Liu, Y.; Weng, Y.; Wang, H.; Bai, X. A critical review on the toxicity regulation and ecological risks of zinc oxide nanoparticles to plants. Environ. Sci. Nano 2024, 11, 14–35. [Google Scholar] [CrossRef]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-Based Nanotoxicity and Detoxification Pathways in Higher Plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of Proline in Plants under Contaminated Soils—Are We on the Same Page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Rodríguez-Jurado, S.; Guevara-González, R.G.; Aguirre-Becerra, H.; Esquivel-Escalante, K.; Feregrino-Pérez, A.A. Nanoparticles as Potential Eustressors in Plants. Agronomy 2025, 15, 2186. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Wang, Y.; Wang, H.; Ding, Z.; Fan, K. Zno nanoparticles: Improving photosynthesis, shoot development, and phyllosphere microbiome composition in tea plants. J. Nanobiotechnol. 2024, 22, 389. [Google Scholar] [CrossRef]
- Dang, K.; Wang, Y.; Tian, H.; Bai, J.; Cheng, X.; Guo, L.; Zhang, Q.; Geng, Y.; Shao, X. Impact of ZnO NPs on photosynthesis in rice leaves plants grown in saline-sodic soil. Sci. Rep. 2024, 14, 16233. [Google Scholar] [PubMed]
- Gupta, A.; Bharati, R.; Kubes, J.; Popelkova, D.; Praus, L.; Yang, X.; Severova, L.; Skalicky, M.; Brestic, M. Zinc oxide nanoparticles application alleviates salinity stress by modulating plant growth, biochemical attributes and nutrient homeostasis in Phaseolus vulgaris L. Front. Plant Sci. 2024, 15, 1432258. [Google Scholar]
- Rahman, H.S.; Othman, H.H.; Abdullah, R.; Edin, H.Y.A.S.; AL-Haj, N.A. Beneficial and toxicological aspects of zinc oxide nanoparticles in animals. Vet. Med. Sci. 2022, 8, 1769–1779. [Google Scholar] [PubMed]
- Bao, L.; Liu, J.; Mao, T.; Zhao, L.; Wang, D.; Zhai, Y. Nanobiotechnology-mediated regulation of reactive oxygen species homeostasis under heat and drought stress in plants. Front. Plant Sci. 2024, 15, 1418515. [Google Scholar] [CrossRef]
- Lv, W.; Geng, H.; Zhou, B.; Chen, H.; Yuan, R.; Ma, C.; Liu, R.; Xing, B.; Wang, F. The behavior, transport, and positive regulation mechanism of ZnO nanoparticles in a plant-soil-microbe environment. Environ. Pollut. 2022, 315, 120368. [Google Scholar]
- Salt, D.; Baxter, I.; Lahner, B. Ionomics and the Study of the Plant Ionome. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Cho, H.-K.; Sandhu, J.; Bouain, N.; Prom-u-thai, C.; Rouached, H. Towards a Discovery of a Zinc-Dependent Phosphate Transport Road in Plants. Plants 2022, 11, 3066. [Google Scholar] [CrossRef]
- Ajmera, I.; Hodgman, T.C.; Lu, C. An Integrative Systems Perspective on Plant Phosphate Research. Genes 2019, 10, 139. [Google Scholar] [CrossRef]
- Chérel, I.; Gaillard, I. The Complex Fine-Tuning of K+ Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 715. [Google Scholar] [CrossRef]
- Kabała, K.; Janicka, M. Structural and Functional Diversity of Two ATP-Driven Plant Proton Pumps. Int. J. Mol. Sci. 2023, 24, 4512. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. Structural and Functional Insights into the Role of Guard Cell Ion Channels in Abiotic Stress-Induced Stomatal Closure. Plants 2021, 10, 2774. [Google Scholar] [CrossRef]
- Shabala, S. Regulation of Potassium Transport in Leaves: From Molecular to Tissue Level. Ann. Bot. 2003, 92, 627–634. [Google Scholar] [CrossRef]
- Gupta, R. The oxygen-evolving complex: A super catalyst for life on earth, in response to abiotic stresses. Plant Signal. Behav. 2020, 15, 1824721. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current Understandings on Magnesium Deficiency and Future Outlooks for Sustainable Agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Takahashi, D.; Uemura, M.; Abadía, J.; López-Millán, A.F.; Rodríguez-Celma, J. Effects of Fe and Mn Deficiencies on the Root Protein Profiles of Tomato (Solanum lycopersicum) Using Two-Dimensional Electrophoresis and Label-Free Shotgun Analyses. Int. J. Mol. Sci. 2022, 23, 3719. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Levengood, H.; Fan, J.; Zhang, C. Plants Under Stress: Exploring Physiological and Molecular Responses to Nitrogen and Phosphorus Deficiency. Plants 2024, 13, 3144. [Google Scholar] [CrossRef]
- Briat, J.-F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Ma, T.; Liang, Y.; Huo, Z.; Yang, F. Synthesis of Zinc Oxide Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review. Agronomy 2023, 13, 3060. [Google Scholar] [CrossRef]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Contents of photosynthetic pigments and ratios of chlorophyll a/b and chlorophylls to carotenoids (a + b)/(x + c) in C4 plants as compared to C3 plants. Photosynthetica 2022, 60, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Moustaka, J.; Adamakis, I.-D.S.; Apostolova, E. Photosystem II Tolerance to Excess Zinc Exposure and High Light Stress in Salvia sclarea L. Agronomy 2024, 14, 589. [Google Scholar] [CrossRef]
- ur Rehman, F.; Paker, N.P.; Khan, M.; Zainab, N.; Ali, N.; Munis, M.F.H.; Iftikhar, M.; Chaudhary, H.J. Assessment of application of ZnO nanoparticles on physiological profile, root architecture and antioxidant potential of Solanum lycopersicum. Biocatal. Agric. Biotechnol. 2023, 53, 102874. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S. Effect of foliar spray of ZnO-NPs on the physiological parameters and antioxidant systems of Lycopersicon esculentum. Pol. J. Nat. Sci. 2019, 34, 87–105. [Google Scholar]
- Instituto Nacional de Calidad (INACAL). Laboratorio de Investigación en Suelo y Aguas-LABISAG-Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas. Available online: https://www.gob.pe/es/i/2825857 (accessed on 10 November 2025).
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 39. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, B.; Sun, Z.; Zhu, C. Relationship Between Proline and Hg2+-Induced Oxidative Stress in a Tolerant Rice Mutant. Arch. Environ. Contam. Toxicol. 2009, 56, 723–731. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. Available online: https://www.r-project.org/ (accessed on 5 August 2025).
- RStudio Team. RStudio: Integrated Development Environment for R; Posit PBC: Boston, MA, USA, 2025. Available online: https://www.rstudio.com/ (accessed on 5 August 2025).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T.; et al. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2025. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 4 November 2025).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2023. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 4 November 2025).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2025. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 4 November 2025).
- Ogle, D. FSAdata: Data to Support Fish Stock Assessment (‘FSA’) Package. 2023. Available online: https://cran.rstudio.com/web/packages/FSAdata/index.html (accessed on 4 November 2025).
- Wickham, H.; Henry, L.; Posit Software, PBC. Purrr: Functional Programming Tools. 2025. Available online: https://cran.r-project.org/web/packages/purrr/index.html (accessed on 4 November 2025).

| Treatment | P | K | Ca | Mg | Fe | Zn | Cu | Mn | Na |
|---|---|---|---|---|---|---|---|---|---|
| Optimal soil | 1773.3 ± 168.82 b | 15,354.94 ± 963.14 a | 14,822.83 ± 829.93 a | 2599.46 ± 113.82 b | 89.07 ± 6.07 bc | 13.78 ± 0.51 c | 9.51 ± 1.21 ab | 71.04 ± 1.72 e | 1272.93 ± 141.6 a |
| Acidic soil | 1847.94 ± 33.46 ab | 13,863.24 ± 819.12 ab | 9136.7 ± 91.03 bc | 3356.89 ± 251.87 a | 92.86 ± 0.45 b | 8.65 ± 1.82 c | 10.22 ± 0.27 a | 145.83 ± 1.98 d | 1041.86 ± 7.79 b |
| ZnO 10 mg L−1 | 1928.83 ± 45.11 ab | 12,047.39 ± 467.54 b | 9251.7 ± 483.48 bc | 3667.25 ± 58.12 a | 97.48 ± 9.52 ab | 10.66 ± 0.15 c | 9.91 ± 0.51 ab | 207.88 ± 5.87 b | 1280.23 ± 57.36 a |
| ZnO 25 mg L−1 | 1934.9 ± 76.83 ab | 13,538.12 ± 1128.52 ab | 8652.16 ± 746.35 c | 3548.52 ± 216.42 a | 94.55 ± 1.47 ab | 44.05 ± 3.46 b | 10.9 ± 0.82 a | 263.26 ± 0.63 a | 1361.52 ± 111.12 a |
| ZnO 50 mg L−1 | 2101.29 ± 145.36 a | 14,605.12 ± 441.41 a | 10,902.36 ± 570.84 b | 3224.31 ± 321.74 a | 107.98 ± 4.19 a | 46.91 ± 1.84 ab | 10.4 ± 0.32 a | 188.23 ± 8.17 c | 1022.78 ± 51.44 b |
| ZnO 100 mg L−1 | 1873.41 ± 69.42 ab | 13,838.6 ± 612.09 ab | 9636.13 ± 933.82 bc | 3421.45 ± 199.44 a | 77.26 ± 4.8 c | 52.71 ± 3.67 a | 8.16 ± 0.23 b | 193.1 ± 4.89 c | 1044.54 ± 52.57 b |
| CV % | 7.09 | 8.89 | 21.43 | 12.01 | 11.26 | 65.53 | 10.67 | 34.20 | 13.46 |
| p-value | 0.0355 | 0.0050 | <0.0001 | 0.0007 | 0.0004 | <0.0001 | 0.0046 | <0.0001 | 0.0006 |
| Treatment | P | K | Ca | Mg | Fe | Zn | Cu | Mn | Na |
|---|---|---|---|---|---|---|---|---|---|
| Optimal soil | 1891.14 ± 75.89 a | 13,272.36 ± 663.17 a | 10,092.83 ± 499.74 c | 3334.63 ± 104.2 a | 541.74 ± 69.91 a | 9.27 ± 1.01 c | 7.35 ± 0.65 ab | 66.45 ± 10.71 d | 380.88 ± 25.83 d |
| Acidic soil | 1806.76 ± 96.77 a | 6170.85 ± 273.35 b | 9639.03 ± 345.75 c | 2112.5 ± 111.6 b | 294.46 ± 10.66 b | 15.66 ± 0.93 b | 6.75 ± 0.37 b | 52.13 ± 1.69 d | 584.18 ± 62.16 ab |
| ZnO 10 mg L−1 | 1974.24 ± 109.65 a | 5054.15 ± 416.93 bc | 23,225.79 ± 653.29 a | 3006.13 ± 174.92 a | 578.56 ± 40.52 a | 27.42 ± 0.33 a | 8.26 ± 0.97 ab | 168.69 ± 6.89 a | 600.23 ± 84.47 ab |
| ZnO 25 mg L−1 | 1402.9 ± 163.68 b | 3791.54 ± 612.25 d | 20,555.18 ± 2506.34 ab | 1415.77 ± 106.42 c | 437.23 ± 124.49 ab | 14.61 ± 2.31 b | 4.49 ± 0.13 c | 68.37 ± 4.29 cd | 435.57 ± 26.55 cd |
| ZnO 50 mg L−1 | 1319.34 ± 32.86 b | 5187.19 ± 209.03 bc | 15,641.65 ± 1271.71 b | 2293.7 ± 387.06 b | 479.73 ± 95.33 ab | 28.13 ± 0.78 a | 8.83 ± 0.22 a | 89.43 ± 8.52 c | 515.07 ± 7.65 bc |
| ZnO 100 mg L−1 | 950.51 ± 79.87 c | 4324.06 ± 159.33 cd | 16,660.11 ± 3378.5 b | 2245.29 ± 113.84 b | 590.75 ± 70.19 a | 28.67 ± 0.52 a | 7.36 ± 0.64 ab | 113.13 ± 10.25 b | 656.94 ± 34.69 a |
| CV % | 24.68 | 52.66 | 33.51 | 27.55 | 25.27 | 38.77 | 20.87 | 43.71 | 20.17 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0048 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle-Lopez, A.; Meléndez-Mori, J.B.; Huaman, E.; Oliva-Cruz, M. Dose-Dependent Effect of Foliar ZnO Nanoparticles on the Physiology, Mineral Nutrition, and Redox Status of Coffea arabica Seedlings Under Soil Acidity. Stresses 2025, 5, 70. https://doi.org/10.3390/stresses5040070
Valle-Lopez A, Meléndez-Mori JB, Huaman E, Oliva-Cruz M. Dose-Dependent Effect of Foliar ZnO Nanoparticles on the Physiology, Mineral Nutrition, and Redox Status of Coffea arabica Seedlings Under Soil Acidity. Stresses. 2025; 5(4):70. https://doi.org/10.3390/stresses5040070
Chicago/Turabian StyleValle-Lopez, Amilcar, Jegnes Benjamín Meléndez-Mori, Eyner Huaman, and Manuel Oliva-Cruz. 2025. "Dose-Dependent Effect of Foliar ZnO Nanoparticles on the Physiology, Mineral Nutrition, and Redox Status of Coffea arabica Seedlings Under Soil Acidity" Stresses 5, no. 4: 70. https://doi.org/10.3390/stresses5040070
APA StyleValle-Lopez, A., Meléndez-Mori, J. B., Huaman, E., & Oliva-Cruz, M. (2025). Dose-Dependent Effect of Foliar ZnO Nanoparticles on the Physiology, Mineral Nutrition, and Redox Status of Coffea arabica Seedlings Under Soil Acidity. Stresses, 5(4), 70. https://doi.org/10.3390/stresses5040070








