Response of Sunflower Genotypes to Salinity Stress Under Laboratory Conditions
Abstract
1. Introduction
2. Results
2.1. Germination Speed (GS)
2.2. Germination Percentage (GP)
2.3. Morphological Parameters
2.3.1. Root Length (RL)
2.3.2. Shoot Length (SL)
2.3.3. Total Seedling Length (TSL)
2.3.4. Root Dry Weight (RDW)
2.3.5. Shoot Dry Weight (SDW)
2.3.6. Total Dry Weight (TDW)
2.4. Seedling Water Content (WC)
2.5. Stress Tolerance Indices (STIs)
Correlations Between the Stress Tolerance Indices
3. Discussion
3.1. Effects of Salinity Stress on Germination
3.2. Effects of Salinity Stress on Seedling Morphology
3.3. Effects of Salinity Stress on Water Content
3.4. Effects of Salinity Stress on Biomass Accumulation
3.5. Stress Tolerance Indices
4. Materials and Methods
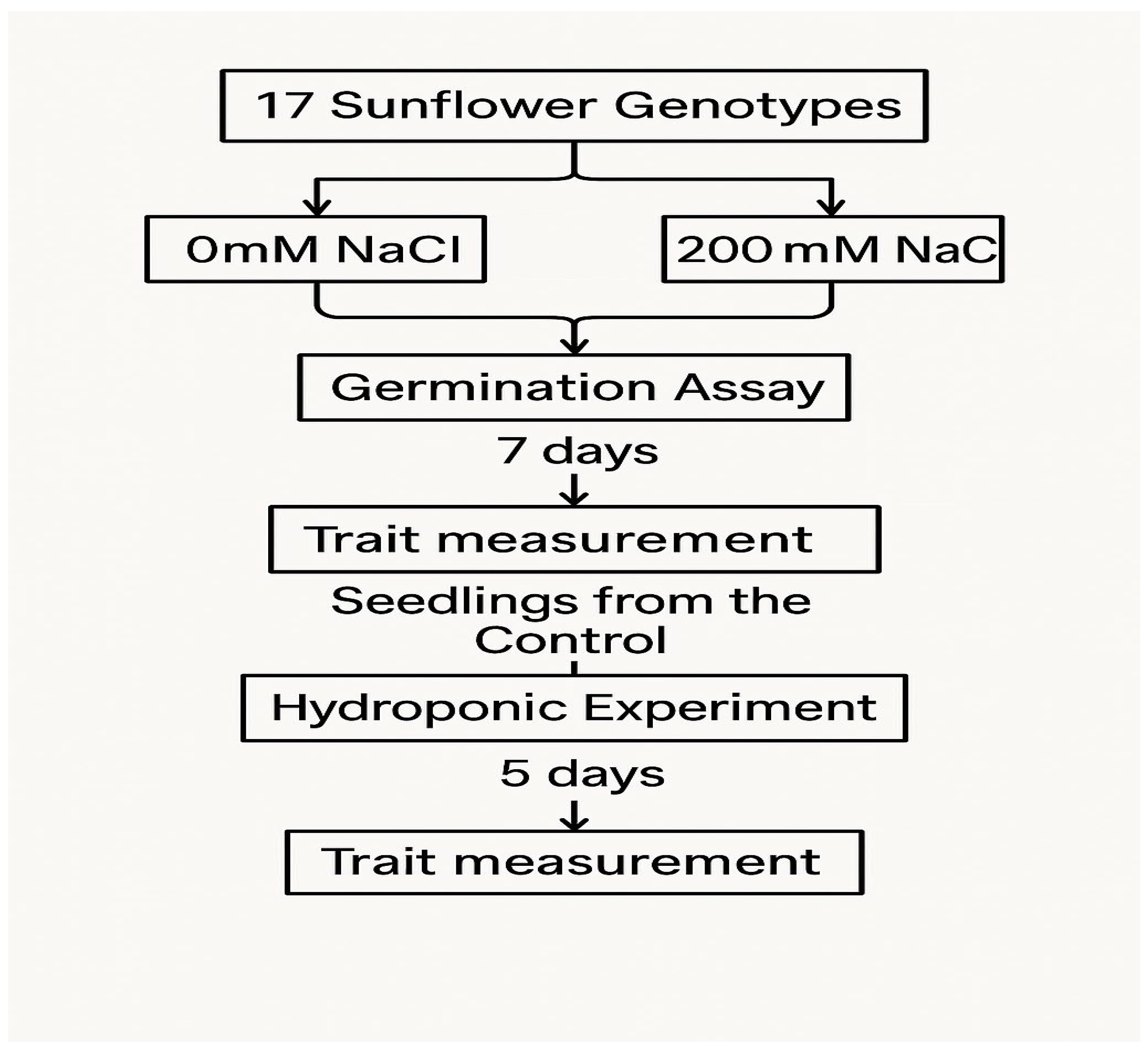
4.1. Experiment 1: Germination Assay
4.2. Experiment 2: Hydroponic Experiment
4.3. Data Collection
4.4. Statistical Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GP | Germination percentage |
| GS | Germination speed |
| SL | Shoot length |
| RL | Root length |
| RDW | Root dry weight |
| SDW | Shoot dry weight |
| TDW | Total dry weight |
| WC | Water content |
| STI | Stress tolerance index |
| GSTI | Germination stress tolerance index |
| RLSTI | Root length stress tolerance index |
| SLSTI | Shoot length stress tolerance index |
| DWSTI | Dry weight stress tolerance index |
| ANOVA | Analysis of variance |
| NaCl | Sodium chloride |
| ROS | Reactive oxygen species |
Appendix A
| SV | DF | GP | GS | RL | SL | TSL | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | η2p | SS | η2p | SS | η2p | SS | η2p | SS | η2p | ||
| Rep | 2 | 232.35 | 0.05 | 0.37 | 0.01 | 0.03 | 0.01 | 0.07 | 0.02 | 0.02 | 0 |
| Gen | 16 | 21718.63 | 0.82 | 60.8 | 0.56 | 170.26 | 0.98 | 219.95 | 0.98 | 538.55 | 0.99 |
| Env | 1 | 300,533.8 | 0.98 | 185.85 | 0.79 | 42.49 | 0.95 | 47.27 | 0.92 | 179.02 | 0.97 |
| G × E | 16 | 10,157.84 | 0.69 | 74.24 | 0.61 | 5.86 | 0.74 | 219.95 | 0.98 | 16.95 | 0.73 |
| Error | 168 | 4617.65 | 48.18 | 2.03 | 4.16 | 6.27 | |||||
| SV | DF | RDW | SDW | TDW | WC | ||||
|---|---|---|---|---|---|---|---|---|---|
| SS | η2p | SS | η2p | SS | η2p | SS | η2p | ||
| Rep | 2 | 0.02 | 0 | 2.78 | 0 | 4.98 | 0.01 | 0.19 | 0.01 |
| Gen(G) | 16 | 538.55 | 0.96 | 23,079.8 | 0.96 | 23,971.16 | 0.96 | 141.17 | 0.8 |
| Env(E) | 1 | 179.02 | 0.88 | 4340.6 | 0.83 | 5707.06 | 0.86 | 107.99 | 0.76 |
| G × E | 16 | 16.95 | 0.41 | 1379 | 0.61 | 1542.02 | 0.63 | 74.08 | 0.68 |
| Error | 168 | 24.11 | 866.73 | 919.19 | 34.5 | ||||
References
- Rauf, S.; Ortiz, R.; Shehzad, M.; Haider, W.; Ahmed, I. The exploitation of sunflower (Helianthus annuus L.) seed and other parts for human nutrition, medicine and the industry. Helia 2020, 43, 167–184. [Google Scholar] [CrossRef]
- Mokgolo, M.J.; Zerizghy, M.G.; Mzezewa, J. Sunflower Growth and Grain Yield under Different Tillage Systems and Sources of Organic Manure on Contrasting Soil Types in Limpopo Province of South Africa. Agronomy 2024, 14, 857. [Google Scholar] [CrossRef]
- Meyer, F.; Van der Burgh, G. The competitiveness of the South African sunflower value chain. Oilseeds Focus 2015, 1, 28–31. [Google Scholar]
- Nhundu, K.; Gandidzanwa, C.; Chaminuka, P.; Mamabolo, M.; Mahlangu, S.; Makhura, M.N. Agricultural supply response for sunflower in South Africa (1947–2016): The partial Nerlovian framework approach. AJSTID 2022, 14, 440–450. [Google Scholar] [CrossRef]
- Puttha, R.; Venkatachalam, K.; Hanpakdeesakul, S.; Wongsa, J.; Parametthanuwat, T.; Srean, P.; Pakeechai, K.; Charoenphun, N. Exploring the potential of sunflowers: Agronomy, applications, and opportunities within bio-circular-green economy. Horticulturae 2023, 9, 1079. [Google Scholar] [CrossRef]
- Agüera, E.; de la Haba, P. Climate change impacts on sunflower (Helianthus annus L.) plants. Plants 2021, 10, 2646. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Zia, M.A.; Najeeb-Alawadi, H.F.; Naqve, M.; Mahmood, A.; Shahzad, A.N. Exogenous application of selenium on sunflower (Helianthus annuus L.) to enhance drought stress tolerance by morpho-physiological and biochemical adaptations. Front. Plant Sci. 2024, 15, 1427420. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Beyaz, R.; Javani, M.; Gürsoy, M.; Yildiz, M. Morphological and biochemical changes in response to salinity in sunflower (Helianthus annus L.) cultivars. Ital. J. Agron. 2018, 13, 141–147. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Nomikou, A.; Malamos, N.; Liveri, E.; Roussos, P.A.; Papasotiropoulos, V. Salinity effect on plant growth parameters and fruit bioactive compounds of two strawberry cultivars, coupled with environmental conditions monitoring. Agronomy 2022, 12, 2279. [Google Scholar] [CrossRef]
- Shrivastata, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Jeppesen, E.; Beklioğlu, M.; Özkan, K.; Akyürek, Z. Salinization increase due to climate change will have substantial negative effects on inland waters: A call for multifaceted research at the local and global scale. Innovation 2020, 1, 100030. [Google Scholar] [CrossRef] [PubMed]
- Nell, J.P.; Huyssteen, W.V. Prediction of primary salinity, sodicity and alkalinity in South African soils. S. Afr. J. Plant Soil. 2018, 35, 173–178. [Google Scholar] [CrossRef]
- Ehtaiwesh, A.F. The effect of salinity on nutrient availability and uptake in crop plants. Sci. J. Appl. Sci. Sabratha Univ. 2022, 9, 55–73. [Google Scholar]
- Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; Sun, N.; Xu, M. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in Jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zeng, Y.; Xiang, L.; Lei, Z.; Huang, Q.; Li, T.; Shen, F.; Cheng, Q. A salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci. Rep. 2020, 10, 10626. [Google Scholar] [CrossRef]
- Machekposhti, M.F.; Shahnazari, A.; Ahmadi, M.Z.; Aghajani, G.; Ritzema, H. Effect of irrigation with sea water on soil salinity and yield of oleic sunflower. Agric. Water Manag. 2020, 188, 69–78. [Google Scholar] [CrossRef]
- Chowdhury, F.T.; Halim, M.A.; Hossain, F.; Akhtar, N. Effects of sodium chloride on germination and seedling growth of Sunflower (Helianthus annuus L.). Jahangirnagar Univ. J. Biol. Sci. 2018, 7, 35–44. [Google Scholar] [CrossRef][Green Version]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Cao, X.; Zhong, C.; Zhu, L.; Khaskheli, M.A.; Fiaz, S.; Zhang, J.; Jin, Q. Sodium chloride stress during early growth stages altered physiological and growth characteristics of rice. Chil. J. Agric. Res. 2018, 78, 183–197. [Google Scholar]
- Fellahi, Z.E.A.; Boubellouta, T.; Bentouati, I.; Safsaf, H.; Hannachi, A.; Utkina, A.O.; Rebouh, N.Y. Hydroponic Screening at Early Seedling Stage Identified Sources of Salinity Tolerance in Wheat (Triticum aestivum L.) Crop. Agronomy 2024, 14, 984. [Google Scholar] [CrossRef]
- Wu, G.Q.; Jiao, Q.; Shui, Q.Z. Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.). Plant Soil Environ. 2015, 11, 220–226. [Google Scholar] [CrossRef]
- Shtereva, L.; Vassilevska-Ivanova, R.; Kartzeva, T.; Kraptchev, B. Salt tolerance of two sunflower genotypes: Helianthus annuus and interspecific line Helianthus anuus × Helianthus mollis. Genet. Plant Physiol. 2015, 5, 61–73. [Google Scholar]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Hamaiel, A.F.; Hamada, M.S.; Ezzat, A.S.; ElHabashy, H.A. 2020. Mitigating of salinity stress and amelioration productivity of potato (Solanum tuberosum L.) using soil conditioners and foliar application of osmoprotectants. Middle East J. Agric. Res. 2020, 9, 737–748. [Google Scholar]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China-A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef]
- Crookes, C.; Hedden, S.; Donnenfeld, Z. A delicate balance: Water scarcity in South Africa. ISS S. Afr. Rep. 2018, 13, 1–24. [Google Scholar]
- Temme, A.A.; Kerr, K.L.; Masalia, R.R.; Burke, J.M.; Donovan, L.A. Key traits and genes associate with salinity tolerance independent from vigor in cultivated sunflower. Plant Physiol. 2020, 184, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Huqe, M.A.S.; Haque, M.S.; Sagar, A.; Uddin, M.N.; Hossain, M.A.; Hossain, A.Z.; Rahman, M.M.; Wang, X.; Al-Ashkar, I.; Ueda, A.; et al. Characterization of maize hybrids (Zea mays L.) for detecting salt tolerance based on morpho-physiological characteristics, ion accumulation and genetic variability at early vegetative stage. Plants 2021, 10, 2549. [Google Scholar] [CrossRef]
- Fellahi, Z.E.A.; Zaghdoudi, H.; Bensaadi, H.; Boutalbi, W.; Hannachi, A. Assessment of salt stress effect on wheat (Triticum aestivum L.) cultivars at seedling stage. Agric. Conspec. Sci. 2019, 84, 347–355. [Google Scholar]
- Chen, L.; Peng, L.; Ouyang, W.; Yao, H.; Ye, Y.; Shan, Z.; Cao, D.; Chen, S.; Yang, Z.; Huang, Y.; et al. Screening and identification of salt tolerance soybean varieties and germplasms. Oil Crop Sci. 2024, 9, 204–210. [Google Scholar] [CrossRef]
- Gitore, S.A.; Danga, B.; Henga, S.; Gurmu, F. Evaluating Drought tolerance indices for selection of drought tolerant Orange Fleshed Sweet Potato (OFSP) genotypes in Ethiopia. Int. J. Agric. Sci. Food Technol. 2021, 7, 249–254. [Google Scholar] [CrossRef]
- Shah, T.M.; Imran, M.; Atta, B.M.; Ashraf, M.Y.; Hameed, A.; Waqar, I.; Shafiq, M.; Hussain, K.; Naveed, M.; Aslam, M.; et al. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield trials. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and screening of agro-physiological indices for salinity stress tolerance in wheat at the seedling stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jagota, N.; Bindra, S.; Sharma, A. Optimization of stress tolerance index for screening of multiple abiotic stress tolerant genotype and determination of reliable trait in Cicer arieitnum L. Total Environ. Res. Themes. 2023, 8, 100073. [Google Scholar]
- Rajabi-Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas-Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- El-Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali-Raza, M.; Singh, K.; Anwar-Hossain, M. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants Under Salt Stress, 1st ed.; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar]
- Haouari, C.C.; Nasraoui, A.H.; Carrayol, E.; Gouia, H. Variations in a-, β-amylase and a-glycosidase activities in two genotypes of wheat under NaCl salinity stress. Afr. J. Agric. Res. 2013, 8, 2038–2043. [Google Scholar]
- El-Hendawy, S.; Elshafei, A.; Al-Suhaibani, N.; Alotabi, M.; Hassan, W.; Dewir, Y.H.; Abdella, K. Assessment of the salt tolerance of wheat genotypes during the germination stage based on germination ability parameters and associated SSR markers. J. Plant Interact. 2019, 14, 151–163. [Google Scholar] [CrossRef]
- Uçarlı, C. Effects of salinity on seed germination and early seedling stage. In Abiotic Stress in Plants, 1st ed.; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2020; pp. 211–232. [Google Scholar]
- Ahmed, R.; Howlader, M.H.K.; Shila, A.; Haque, M.A. Effect of salinity on germination and early seedling growth of maize. Progress. Agric. 2017, 28, 18–25. [Google Scholar] [CrossRef]
- Chakma, P.; Hossain, M.M.; Rabbani, M.G. Effects of salinity stress on seed germination and seedling growth of tomato. J Bangladesh Univ. 2019, 17, 490–499. [Google Scholar] [CrossRef]
- Irik, H.A.; Bikmaz, G. Effect of different salinity on seed germination, growth parameters and biochemical contents of pumpkin (Cucurbita pepo L.) seeds cultivars. Sci. Rep. 2024, 14, 6929. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants andits tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl–ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.L.; Coelho, E.F.; Coelho-Filho, M.A.; Santos, A.A.D. Salinity reduces nutrients absorption and efficiency of their utilization in cassava plants. Cienc. Rural. 2018, 48, e20180351. [Google Scholar] [CrossRef]
- Zhang, X.; Franzisky, B.L.; Eigner, L.; Geilfus, C.M.; Zörb, C. Antagonism of chloride and nitrate inhibits nitrate reductase activity in chloride-stressed maize. Plant Growth Regul. 2021, 93, 279–289. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Hualpa-Ramirez, E.; Carrasco-Lozano, E.C.; Madrid-Espinoza, J.; Tejos, R.; Ruiz-Lara, S.; Stange, C.; Norambuena, L. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol. Bioch. 2024, 208, 108507. [Google Scholar] [CrossRef] [PubMed]
- Gonzáles, H.H.S.; Peñuelas-Rubio, O.; Argentel-Martínez, L.; Ponce, A.L.; Andrade, M.H.H.; Hasanuzzaman, M.; Aguilera, J.G.; Teodoro, P.E. Salinity effects on water potential and the normalized difference vegetation index in four species of a saline semi-arid ecosystem. Peer J. 2021, 9, e12297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Zhang, M.; Wang, Y. The high pH value of alkaline salt destroys the root membrane permeability of Reaumuria trigyna and leads to its serious physiological decline. J. Plant Res. 2022, 135, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Cellular responses, osmotic adjustments, and role of osmolytes in providing salt stress resilience in higher plants: Polyamines and nitric oxide crosstalk. J. Plant Growth Regul. 2023, 42, 539–553. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupt, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Sultana, N.; Haque, M.A.; Hoque, M.F.; Hossain, M.B.; Satter, M.A.; Jahiruddin, M. Effect of silicon application on growth and biomass yield of rice under salinity stress. J.Bangladesh Agric. Univ. 2021, 19, 29–436. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mehrvar, M.R.; Sanjani, S.; Amini, A.; Nikkhah-Chamanabad, H.; Asadi, A. Effects of salinity stress on seedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiol. Plant. 2021, 43, 98. [Google Scholar] [CrossRef]
- Singh, D. Juggling with reactive oxygen species and antioxidant defense system–A coping mechanism under salt stress. Plant Stress 2020, 5, 100093. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Bioch. 2017, 118, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Basak, N.; Bhaduri, D.; Ray, S.; Vijayan, J.; Chattopadhyay, K.; Sarkar, R.K. Ionic basis of salt tolerance in plants: Nutrient homeostasis and oxidative stress tolerance. In Plant Nutrients and Abiotic Stress Tolerance, 1st ed.; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 325–362. [Google Scholar]
- Han, X.; Kang, Y.; Wan, S.; Li, X. Effect of salinity on oleic sunflower (Helianthus annuus L.) under drip irrigation in arid area of Northwest China. Agric. Water Manag. 2022, 259, 107267. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Chapter 7: Salty Soils. Available online: https://www.fao.org/4/r4082e/r4082e08.htm#TopOfPage (accessed on 10 April 2025).
- Kandil, A.A.; Sharief, A.E.; Ahmed, S.R.H. Germination and seedling growth of some chickpea cultivars (Cicer arietinum L.) under salinity stress. J. Basic Appl. Sci. 2012, 8, 561–571. [Google Scholar] [CrossRef]
- Abbasi, K.M.; Ghorbani, A.; Dadjou, F. Influence of nano-priming on Festuca ovina seed germination and early seedling traits under drought stress, in laboratory condition. Ecopersia 2019, 7, 133–139. [Google Scholar]
- Koch, M.J.; Bonos, S.A. Correlation of three salinity tolerance screening methods for cool-season turfgrasses. HortScience 2011, 46, 1198–1201. [Google Scholar] [CrossRef]
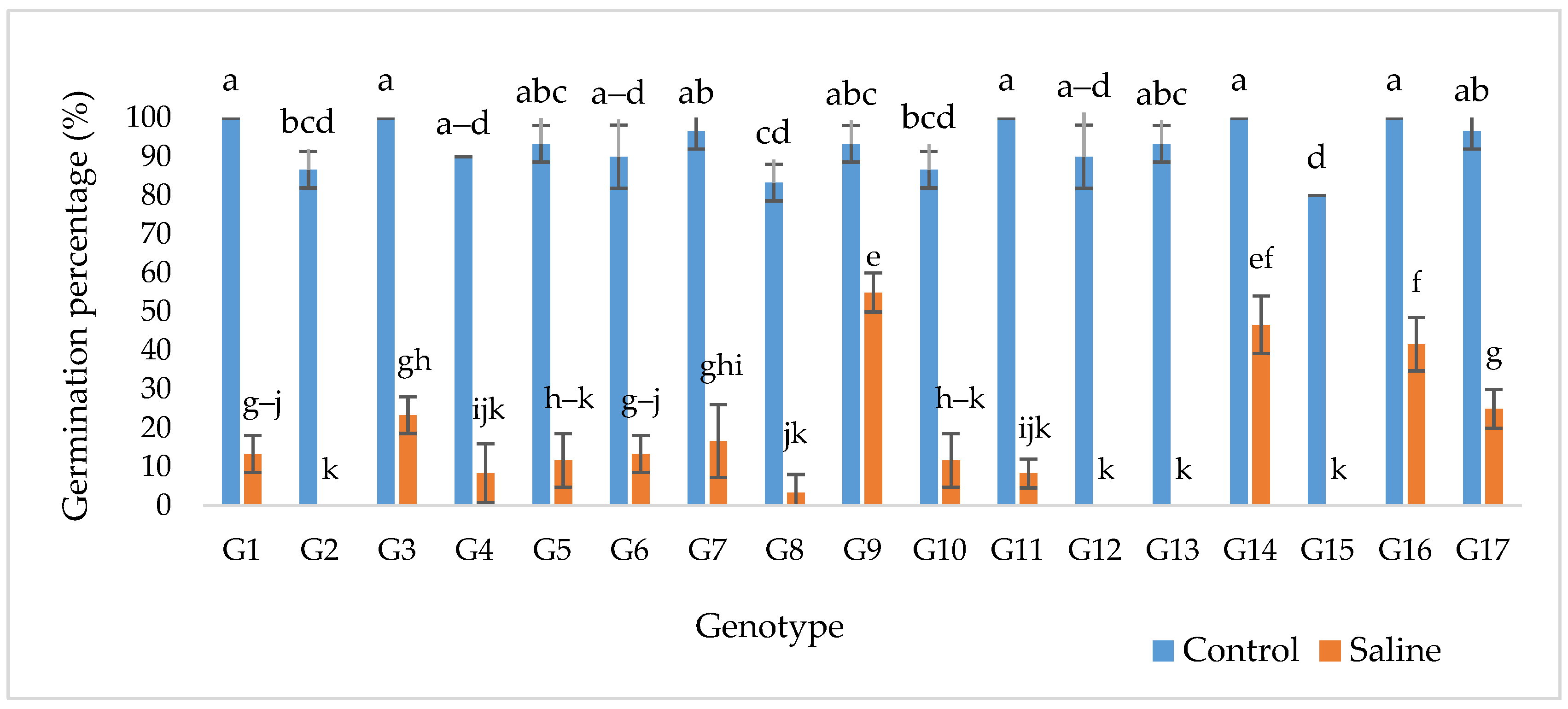
| SV | DF | GS | G% | RL | SL | TL | RDW | SDW | TDW | WC% |
|---|---|---|---|---|---|---|---|---|---|---|
| Rep | 2 | 0.19 ns | 116.20 ns | 0.02 ns | 0.04 ns | 0.01 ns | 0.28 ns | 1.39 ns | 2.50 ns | 0.10 ns |
| G | 16 | 3.80 *** | 1357.40 *** | 10.64 *** | 13.75 *** | 33.66 *** | 5.35 *** | 1442.49 *** | 1498.20 *** | 8.82 *** |
| E | 1 | 185.85 *** | 300,533.8 *** | 42.49 *** | 47.27 *** | 179.02 *** | 93.35 *** | 4340.60 *** | 5707.06 *** | 107.99 *** |
| G × E | 16 | 4.64 *** | 634,90 *** | 0.37 *** | 0.33 *** | 1.06 *** | 2.01 *** | 86.19 *** | 96.38 *** | 4.63 *** |
| Genotype | Control + | Saline + |
|---|---|---|
| G1 | 3.79 a ± 0.36 | 0.93 de ± 0.58 |
| G2 | 3.24 ab ± 0.48 | 0 e ± 0 |
| G3 | 2.55 bc± 0.19 | 1.48 cd ± 0.39 |
| G4 | 2.95 ab± 0.75 | 0.29 de ± 0.10 |
| G5 | 2.76 ab+ 0.71 | 0.81 de± 0.74 |
| G6 | 3.40 ab ± 0.19 | 1.12 de ±0.46 |
| G7 | 2.74 ab ± 0.19 | 1.12 de ± 1.02 |
| G8 | 3.00 ab± 0.88 | 0.29 de ± 0.44 |
| G9 | 2.69 ab ± 0.40 | 3.56 ab ± 0.93 |
| G10 | 2.83 ab ± 0.14 | 0.83 de ± 0.74 |
| G11 | 2.64 abc ± 0.27 | 0.56 de ± 0.43 |
| G12 | 3.07 ab ± 0.62 | 0 e ± 0 |
| G13 | 2.52 bc ± 0.39 | 0 e ± 0 |
| G14 | 2.98 ab ± 0.47 | 3.10 ab ± 0.97 |
| G15 | 3.62 ab ± 0.48 | 0 e ± 0 |
| G16 | 3.10 ab ± 0.35 | 2.95 ab ± 0.75 |
| G17 | 2.83 ab ± 0.25 | 1.21 d ± 0.45 |
| Root Length (cm) | Shoot Length (cm) | Total Seedling Length (cm) | ||||
|---|---|---|---|---|---|---|
| Genotype | Control + | Saline + | Control + | Saline + | Control + | Saline + |
| G1 | 8.80 f ± 0.09 | 7.58 kl ± 0.18 | 12.38 cd ± 0.08 | 10.92 kl ± 0.08 | 21.18 de ± 0.15 | 18.50 j ± 0.13 |
| G2 | 7.80 k ± 0.15 | 6.52 p ± 0.08 | 11.37 hij ± 0.05 | 10.68 l ± 0.34 | 19.17 i ± 0.12 | 17.20 lmn ± 0.40 |
| G3 | 7.15 mn ±0.10 | 6.20 q ± 0.11 | 11.92 ef ± 0.08 | 11.15 ijk ± 0.05 | 19.07 i ± 0.15 | 17.35 klm ± 0.15 |
| G4 | 8.53 gh ± 0.05 | 7.18 m ± 0.13 | 11.13 ijk ± 0.14 | 10.12 no ± 0.16 | 19.67 gh ± 0.12 | 17.30 klm ± 0.17 |
| G5 | 8.75 fg ± 0.12 | 7.37 lm ± 0.08 | 10.68 l ± 0.08 | 9.75 p ± 0.10 | 19.40 hi ± 0.09 | 17.12 mn ± 0.15 |
| G6 | 7.75 k ± 0.14 | 6.77 o ± 0.08 | 11.62 fgh ± 0.08 | 10.90 kl ± 0.09 | 19.37 hi ± 0.19 | 17.67 k ± 0.05 |
| G7 | 6.93 no ± 0.10 | 6.08 q ± 0.15 | 9.85 op ± 0.10 | 8.53 r ± 0.05 | 16.78 n ± 0.20 | 14.62 o ± 0.17 |
| G8 | 9.55 ab ± 0.05 | 8.27 ij ± 0.12 | 10.32 mn ± 0.08 | 9.15 q ± 0.05 | 19.87 fg ± 0.08 | 17.42 klm ± 0.12 |
| G9 | 9.10 de ± 0.11 | 8.70 fg ± 0.13 | 11.00 kl ± 0.09 | 10.67 lm ± 0.04 | 20.10 f ± 0.15 | 19.37 hi 0.08 |
| G10 | 8.05 j ± 0.19 | 7.23 m ± 0.08 | 10.97 kl ± 0.08 | 9.87 op ± 0.12 | 19.02 i ± 0.15 | 17.10 mn ± 0.13 |
| G11 | 9.08 de ± 0.04 | 8.40 hi ± 0.20 | 10.18 no ± 0.16 | 9.00 q ± 0.09 | 19.27 hi ± 0.16 | 17.40 klm ± 0.19 |
| G12 | 8.90 ef ± 0.11 | 7.80 k ± 0.13 | 11.12 jk ± 0.10 | 9.87 op ± 0.05 | 20.02 fg ± 0.19 | 17.67 k ± 0.10 |
| G13 | 9.35 bc ± 0.05 | 8.25 ij ± 0.10 | 11.48 ghi ± 0.08 | 9.95 op ± 0.66 | 20.83 e ± 0.10 | 18.20 j ± 0.69 |
| G14 | 9.70 a ± 0.06 | 9.37 bc ± 0.05 | 12.85 bc ± 0.14 | 12.15 de ± 0.05 | 22.55 ab ± 0.08 | 21.52 d ± 0.10 |
| G15 | 7.67 k ±0.10 | 6.80 o ± 0.06 | 11.75 fg ± 0.05 | 10.80 kl 0.08 | 19.42 hi ± 0.10 | 17.60 kl ± 0.11 |
| G16 | 9.63 a ± 0.05 | 9.27 cd ± 0.08 | 13.28 a ± 0.13 | 12.70 bc ± 0.07 | 22.92 a ± 0.10 | 21.97 c ± 0.12 |
| G17 | 9.32 bcd ± 0.10 | 8.77 fg ± 0.08 | 13.05 ab ± 0.08 | 12.38 cd ± 0.17 | 22.37 bc ± 0.18 | 21.17 de ± 0.24 |
| Genotype | Root Dry Weight (mg) | Shoot Dry Weight (mg) | Total Dry Weight (mg) | Water Content (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Control + | Saline + | Control + | Saline + | Control + | Saline + | Control + | Saline + | |
| G1 | 4.50 bcde ± 0.55 | 2.83 hijk ± 0.41 | 53.33 mn ± 2.66 | 40.67 q ± 4.23 | 57.83 lmn ± 3.06 | 43.50 r ± 4.32 | 89.70 efgh ± 0.61 | 87.20 k ± 1.33 |
| G2 | 4.67 bcd ± 0.52 | 2.17 jk ± 0.41 | 64.67 fghij ± 3.27 | 55.17 lm ± 2.93 | 69.33 fghij ± 3.08 | 57.33 mn ± 2.94 | 90.80 abcd ± 0.44 | 87.00 k ± 0.52 |
| G3 | 4.00 cdef ± 0 | 2.83 hijk ± 0.45 | 65.50 fghi ± 2.59 | 43.67 q ± 1.63 | 69.50 fghi ± 2.59 | 46.50 qr ± 1.64 | 90.80 abcd ± 0.27 | 88.40 ij ± 0.46 |
| G4 | 6.17 a ± 0.98 | 3.67 efgh ± 0.52 | 52.33 mn ± 1.75 | 40.00 q ± 0.63 | 58.50 lm ± 1.76 | 43.67 r ± 0.52 | 91.00 abcd ± 2.34 | 89.70 efgh ± 0.23 |
| G5 | 4.67 bcd ± 0.52 | 2.00 k ± 0 | 52.33 mn ± 3.39 | 46.00 op ± 4.20 | 57.00 mn ± 3.03 | 48.00 pqr ± 4.20 | 90.90 abcde ± 2.66 | 90.40 bcdef ± 0.89 |
| G6 | 5.00 b ± 0 | 3.83 defg ± 0.41 | 62.33 hijk ± 1.37 | 53.50 mn ± 2.07 | 67.33 ghijk ± 1.4 | 57.33 mn ± 2.34 | 90.80 abcd ± 0.22 | 89.10 ghi ± 0.44 |
| G7 | 4.33 bc ± 0.46 | 2.83 hijk ± 0.41 | 46.33 op ± 0.82 | 31.83 r ± 2.48 | 51.17 opq ± 0.98 | 34.67 s ± 2.66 | 90.80 abcd ± 0.18 | 87.60 jk ± 1.03 |
| G8 | 4.00 cdef ± 0 | 3.83 defg ± 0 | 58.67 kl ± 1.51 | 49.00 no ± 2.19 | 62.67 kl ± 1.51 | 52.83 nop ± 2.40 | 90.90 abcd ± 0.24 | 88.90 hi ± 0.48 |
| G9 | 5.00 b ± 0.13 | 3.17 fghi ± 0.13 | 59.83 jkl ± 0.75 | 53.50 mn ± 0.84 | 64.83 ijk ± 0.75 | 56.67 mn ± 1.1 | 91.00 abcd ± 0.11 | 90.70 abcde ± 0.16 |
| G10 | 5.00 b ± 0.05 | 3.83 defg ± 0.37 | 67.50 efg ± 1.05 | 63.00 ghijk ± 2.00 | 72.50 efg ± 1.05 | 66.83 hijk ± 2.40 | 90.50 bcdef ± 0.18 | 90.10 cdefg ± 0.33 |
| G11 | 6.00 a ± 0 | 3.83 defg ± 0.41 | 69.67 def ± 1.03 | 52.83 mn ± 1.33 | 75.67 cde ± 1.03 | 56.67 mn ± 1.03 | 90.90 abcd ± 0.10 | 87.60 jk ± 0.24 |
| G12 | 3.17 fghi ± 0.41 | 3.00 ghij ± 0 | 61.00 ijk ± 0.63 | 53.00 mn ± 1.55 | 64.17 jk ± 0.98 | 56.00 mno ± 1.6 | 90.10 abcd ± 0.19 | 89.10 jk ± 0.29 |
| G13 | 3.00 ghij ± 0 | 2.33 ijk ± 0.50 | 66.17 fgh ± 2.04 | 55.00 lm ± 1.10 | 69.17 fghij ± 2.04 | 57.33 mn ± 1.21 | 91.10 abcde ± 0.26 | 90.10 defg ± 0.25 |
| G14 | 5.00 b ± 0 | 4.67 bcd ± 0.52 | 74.83 bc ± 1.47 | 73.33 bcd ± 1.03 | 79.83 bc ± 1.47 | 78.00 bcd ± 1.10 | 91.20 ab ± 0.18 | 91.10 abc ± 0.15 |
| G15 | 4.17 bcde ± 0.41 | 3.17 fghi ± 0.41 | 71.67 cde ± 4.13 | 68.83 def ± 3.71 | 75.83 cde ± 4.54 | 72.00 efgh ± 4.1 | 91.20 ab ± 0.53 | 91.00 abcd ± 0.47 |
| G16 | 5.00 b ± 0 | 4.00 cdef ± 0 | 85.67 a ± 1.97 | 78.17 b ± 2.23 | 90.67 a ± 1.97 | 82.17 b ± 2.23 | 90.90 abcd ± 0.20 | 90.80 abcd ± 0.25 |
| G17 | 4.83 bc ± 0.41 | 4.00 cdef ± 0 | 72.00 cde ± 1.55 | 69.50 def ± 2.26 | 76.83 cde ± 1.17 | 73.50 def ± 2.26 | 91.60 a ± 0.19 | 91.30 ab ± 0.26 |
| G | G | Rank | G | RL | Rank | G | SL | Rank | G | TFW | G | TDW | Rank | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | STI | Code | STI | Code | STI | Code | STI | Rank | Code | STI | STI | |||
| G9 | 0.59 | 1 | G14 | 0.97 | 1 | G9 | 0.97 | 1 | G14 | 0.96 | 1 | G14 | 0.98 | 1 |
| G14 | 0.47 | 2 | G9 | 0.96 | 2 | G16 | 0.96 | 2 | G15 | 0.93 | 2 | G17 | 0.96 | 2 |
| G16 | 0.42 | 3 | G16 | 0.96 | 3 | G17 | 0.95 | 3 | G17 | 0.92 | 3 | G15 | 0.95 | 3 |
| G17 | 0.26 | 4 | G17 | 0.94 | 4 | G14 | 0.95 | 4 | G16 | 0.89 | 4 | G10 | 0.92 | 4 |
| G3 | 0.23 | 5 | G10 | 0.94 | 5 | G6 | 0.94 | 5 | G10 | 0.89 | 5 | G16 | 0.91 | 5 |
| G7 | 0.17 | 6 | G11 | 0.92 | 6 | G3 | 0.94 | 6 | G9 | 0.84 | 6 | G9 | 0.87 | 6 |
| G6 | 0.15 | 7 | G6 | 0.87 | 7 | G2 | 0.94 | 7 | G5 | 0.80 | 7 | G12 | 0.87 | 7 |
| G10 | 0.13 | 8 | G15 | 0.89 | 8 | G15 | 0.92 | 8 | G12 | 0.76 | 8 | G6 | 0.85 | 8 |
| G5 | 0.13 | 9 | G12 | 0.88 | 9 | G5 | 0.91 | 9 | G13 | 0.75 | 9 | G5 | 0.84 | 9 |
| G1 | 0.13 | 10 | G13 | 0.88 | 10 | G4 | 0.91 | 10 | G6 | 0.72 | 10 | G8 | 0.84 | 10 |
| G4 | 0.09 | 11 | G7 | 0.88 | 11 | G10 | 0.90 | 11 | G8 | 0.69 | 11 | G13 | 0.83 | 11 |
| G11 | 0.08 | 12 | G3 | 0.87 | 12 | G12 | 0.89 | 12 | G4 | 0.65 | 12 | G2 | 0.82 | 12 |
| G8 | 0.04 | 13 | G8 | 0.87 | 13 | G11 | 0.88 | 13 | G1 | 0.61 | 13 | G1 | 0.75 | 13 |
| G15 | 0 | 14 | G5 | 0.84 | 14 | G1 | 0.88 | 14 | G2 | 0.58 | 14 | G4 | 0.75 | 14 |
| G13 | 0 | 15 | G2 | 0.84 | 15 | G13 | 0.87 | 15 | G11 | 0.55 | 15 | G11 | 0.75 | 15 |
| G12 | 0 | 16 | G4 | 0.84 | 16 | G8 | 0.87 | 16 | G3 | 0.53 | 16 | G7 | 0.68 | 16 |
| G2 | 0 | 17 | G1 | 0.82 | 17 | G7 | 0.87 | 17 | G7 | 0.50 | 17 | G3 | 0.67 | 17 |
| GSTI | RLSTI | SLSTI | TFWSTI | TDWSTI | |
|---|---|---|---|---|---|
| GSTI | 1 | ||||
| RLSTI | 0.69 ** | 1 | |||
| SLSTI | 0.69 ** | 0.60 ** | 1 | ||
| TFWSTI | 0.39 ns | 0.63 ** | 0.58 * | 1 | |
| TDWSTI | 0.25 ns | 0.58 * | 0.54 * | 0.94 *** | 1 |
| Nutritional Element | K | Ca | N | Mg | Na | Cu | Cl | Mn | Mo | Zn | B | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 23.5 | 48.1 | 42.6 | 14.6 | 0.06 | 0.06 | 0.03 | 0.03 | 0.03 | 0.16 | 0.32 | 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiradza, T.O.; Mutengwa, C.S.; Chiuta, N.E. Response of Sunflower Genotypes to Salinity Stress Under Laboratory Conditions. Stresses 2025, 5, 50. https://doi.org/10.3390/stresses5030050
Chiradza TO, Mutengwa CS, Chiuta NE. Response of Sunflower Genotypes to Salinity Stress Under Laboratory Conditions. Stresses. 2025; 5(3):50. https://doi.org/10.3390/stresses5030050
Chicago/Turabian StyleChiradza, Tatenda Ocean, Charles Shelton Mutengwa, and Nyasha Esnath Chiuta. 2025. "Response of Sunflower Genotypes to Salinity Stress Under Laboratory Conditions" Stresses 5, no. 3: 50. https://doi.org/10.3390/stresses5030050
APA StyleChiradza, T. O., Mutengwa, C. S., & Chiuta, N. E. (2025). Response of Sunflower Genotypes to Salinity Stress Under Laboratory Conditions. Stresses, 5(3), 50. https://doi.org/10.3390/stresses5030050






