Silica Wort Supplementation as an Alternative for Yeast Stress Relief on Corn Ethanol Production with Cell Recycling
Abstract
1. Introduction
2. Results
2.1. Cell Viability
2.2. Cellular Biomass
2.3. Total Reducing Sugar
2.4. Alcohol Content
2.5. Glycerol
2.6. Organic Acids
2.7. Trehalose
2.8. Fermentative Yield
2.9. Fermentation Productivity
3. Discussion
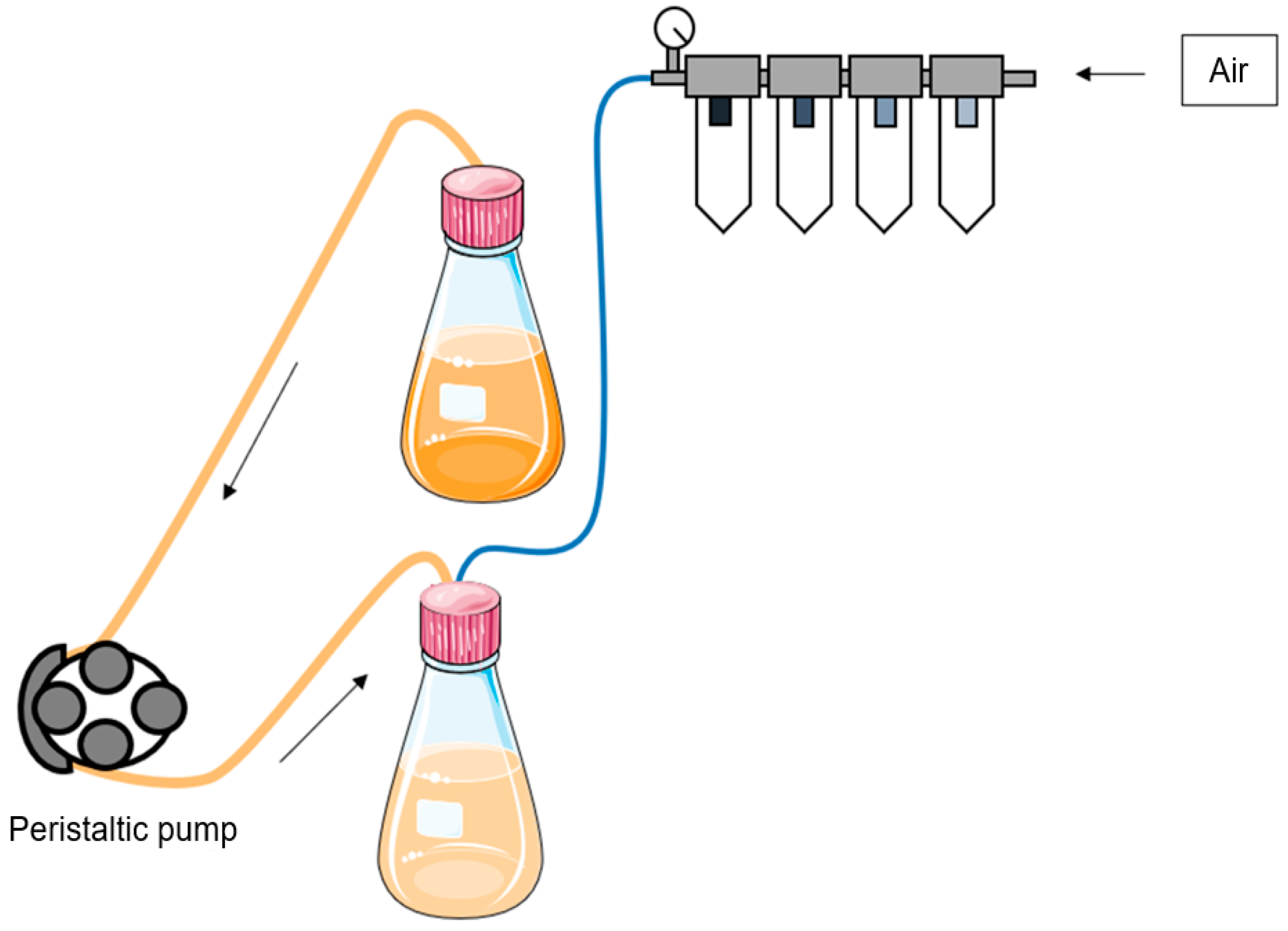
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Treatments
4.3. VHG Fermentation in Fed-Batch Mode
4.4. Cell Viability of Yeast
4.5. Yeast Biomass
4.6. Total Reducing Sugars and Glycerol Determination
4.7. Alcohol Content Determination
4.8. Organic Acids
4.9. Trehalose
4.10. Fermentative Yield
4.11. Fermentation Productivity
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol Fermentation Technologies from Sugar and Starch Feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Douradinho, R.; Sica, P.; Tonoli, F.; Mattos, E.; Oliveira, M.; Pinto, A.; Mota, L.; Faria, T.; Costa, V.F.; Leite, G.; et al. Osmotic Stress Alleviation in Saccharomyces cerevisiae for High Ethanol Fermentations with Different Wort Substrates. Stresses 2023, 3, 813–826. [Google Scholar] [CrossRef]
- Brazilian National Agency for Petroleum, Natural Gas and Biofuels—ANP. RenovaBio—Agência Nacional do Petróleo, Gás Natural e Biocombustíveis. Available online: www.gov.br (accessed on 26 April 2024).
- Douradinho, R.; Sica, P.; Oliveira, M.; Uchoa Pinto, A.; Mota, L.; Mattos, E.; Perecin, D.; Garcilasso, V.; de Almeida, J.M.A.R.; Piedade, S.; et al. Assessing Ionizing Radiation and Chlorine Dioxide (ClO2) as Potential Aseptization Treatments for Yeast Recycling on Mixed Wort of Corn and Sugarcane in Brazil. Stresses 2024, 4, 155–171. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Obulam, V.S.R.; Ko, S. Very High Gravity (VHG) Ethanolic Brewing and Fermentation: A Research Update. J. Ind. Microbiol. Biotechnol. 2011, 38, 1133–1144. [Google Scholar] [CrossRef]
- Tao, X.; Zheng, D.; Liu, T.; Wang, P.; Zhao, W.; Zhu, M.; Jiang, X.; Zhao, Y.; Wu, X. A Novel Strategy to Construct Yeast Saccharomyces cerevisiae Strains for Very High Gravity Fermentation. PLoS ONE 2012, 7, e31235. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Hao, X.-M.; Lin, Y.-H.; Bai, F.-W. Redox Potential Driven Aeration during Very-High-Gravity Ethanol Fermentation by Using Flocculating Yeast. Sci. Rep. 2016, 6, 25763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Li, Q.; Den Haan, R.; Li, F.; Liu, C.; Bai, F. Omics Analysis Reveals Mechanism Underlying Metabolic Oscillation during Continuous Very-high-gravity Ethanol Fermentation by Saccharomyces cerevisiae. Biotechnol. Bioeng. 2021, 118, 2990–3001. [Google Scholar] [CrossRef]
- Wang, P.-M.; Zheng, D.-Q.; Chi, X.-Q.; Li, O.; Qian, C.-D.; Liu, T.-Z.; Zhang, X.-Y.; Du, F.-G.; Sun, P.-Y.; Qu, A.-M.; et al. Relationship of Trehalose Accumulation with Ethanol Fermentation in Industrial Saccharomyces cerevisiae Yeast Strains. Bioresour. Technol. 2014, 152, 371–376. [Google Scholar] [CrossRef]
- Gomes, D.; Cruz, M.; de Resende, M.; Ribeiro, E.; Teixeira, J.; Domingues, L. Very High Gravity Bioethanol Revisited: Main Challenges and Advances. Fermentation 2021, 7, 38. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A Review of Recent Advances in High Gravity Ethanol Fermentation. Renew. Energy 2019, 133, 1366–1379. [Google Scholar] [CrossRef]
- Oliveira, M.R.B.; Silva, A.P.M.D.; Faria, T.M.; Basso, L.C.; Baptista, A.S. Protective Effect of Silica on Adaptation of Saccharomyces cerevisiae, Ethanol Red® for Very High Gravity Fermentation. Braz. Arch. Biol. Technol. 2023, 66, e23210416. [Google Scholar] [CrossRef]
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the Process and Costs of Fuel Ethanol Production by the Corn Dry-Grind Process. Ind. Crops Prod. 2006, 23, 288–296. [Google Scholar] [CrossRef]
- Betite, V.C.; Júnior, M.M.; Oliveira, J.E.; Ernandes, J.R. Very High Gravity Sucrose Fermentation by Brazilian Industrial Yeast Strains: Effect of Nitrogen Supplementation: Very High Gravity Sucrose Fermentation. J. Inst. Brew. 2012, 118, 174–178. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in Biotechnological Production of Fuel Ethanol from Different Feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zong, X.; Cui, C.; Mu, L.; Zhao, H. Peptide (Lys–Leu) and Amino Acids (Lys and Leu) Supplementations Improve Physiological Activity and Fermentation Performance of Brewer’s Yeast during Very High-gravity (VHG) Wort Fermentation. Biotechnol. Appl. Biochem. 2018, 65, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Charpentier, C. Biochemical Aspects of Stuck and Sluggish Fermentation in Grape Must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Ansanay-Galeote, V.; Blondin, B.; Dequin, S.; Sablayrolles, J.-M. Stress Effect of Ethanol on Fermentation Kinetics by Stationary-Phase Cells of Saccharomyces cerevisiae. Biotechnol. Lett. 2001, 23, 677–681. [Google Scholar] [CrossRef]
- Auesukaree, C.; Damnernsawad, A.; Kruatrachue, M.; Pokethitiyook, P.; Boonchird, C.; Kaneko, Y.; Harashima, S. Genome-Wide Identification of Genes Involved in Tolerance to Various Environmental Stresses in Saccharomyces cerevisiae. J. Appl. Genet. 2009, 50, 301–310. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.L. Mechanisms of Ethanol Tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 87, 829–845. [Google Scholar] [CrossRef]
- Veloso, I.I.K.; Rodrigues, K.C.S.; Esperança, M.N.; Batista, G.; Cruz, A.J.G.; Badino, A.C. A More Accurate Modeling for Fed-Batch Ethanol Fermentation with High Cell Density. Biochem. Eng. J. 2023, 193, 108855. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Deesuth, O.; Laopaiboon, P.; Laopaiboon, L. High Ethanol Production under Optimal Aeration Conditions and Yeast Composition in a Very High Gravity Fermentation from Sweet Sorghum Juice by Saccharomyces cerevisiae. Ind. Crops Prod. 2016, 92, 263–270. [Google Scholar] [CrossRef]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Effects of Particulate Materials and Osmoprotectants on Very-High-Gravity Ethanolic Fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1994, 60, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, Y.-L.; Fang, Y.; Zhao, H. Adaptive Evolution and Selection of Stress-Resistant Saccharomyces cerevisiae for Very High-Gravity Bioethanol Fermentation. Electron. J. Biotechnol. 2019, 41, 88–94. [Google Scholar] [CrossRef]
- Casey, E.; Sedlak, M.; Ho, N.W.Y.; Mosier, N.S. Effect of Acetic Acid and pH on the Cofermentation of Glucose and Xylose to Ethanol by a Genetically Engineered Strain of Saccharomyces cerevisiae: Effect of Acetic Acid/pH on Xylose Fermentation. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M. Traditional preservatives: Organic acids. In Encyclopedia of Food Microbiology; Robinson, R.K., Batt, C.A., Patel, P.D., Eds.; Academic Press: London, UK, 1999; Volume 3, pp. 1729–1737. [Google Scholar]
- Li, F.; Wei, X.; Sun, Q.; Guo, Y.; Liu, J. Production of L-Lactic Acid in Saccharomyces cerevisiae Through Metabolic Engineering and Rational Cofactor Engineering. Sugar Tech 2022, 24, 1272–1283. [Google Scholar] [CrossRef]
- Beckner, M.; Ivey, M.L.; Phister, T.G. Microbial Contamination of Fuel Ethanol Fermentations: Bioethanol Contamination. Lett. Appl. Microbiol. 2011, 53, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Xie, T.; Hui, M. Increase of Ethanol Productivity by Cell-Recycle Fermentation of Flocculating Yeast. Appl. Biochem. Microbiol. 2011, 47, 527–531. [Google Scholar] [CrossRef]
- Liu, S. Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2017; ISBN 9780444637833. [Google Scholar]
- Phukoetphim, N.; Khongsay, N.; Laopaiboon, P.; Laopaiboon, L. A Novel Aeration Strategy in Repeated-Batch Fermentation for Efficient Ethanol Production from Sweet Sorghum Juice. Chin. J. Chem. Eng. 2019, 27, 1651–1658. [Google Scholar] [CrossRef]
- Alfenore, S.; Cameleyre, X.; Benbadis, L.; Bideaux, C.; Uribelarrea, J.-L.; Goma, G.; Molina-Jouve, C.; Guillouet, S.E. Aeration Strategy: A Need for Very High Ethanol Performance in Saccharomyces cerevisiae Fed-Batch Process. Appl. Microbiol. Biotechnol. 2004, 63, 537–542. [Google Scholar] [CrossRef]
- Alfenore, S.; Cameleyre, X.; Benbadis, L.; Bideaux, C.; Uribelarrea, J.L.; Goma, G.; Molina-Jouve, C.; Guillouet, S.E. Improving Ethanol Production and Viability of Saccharomyces cerevisiae by a Vitamin Feeding Strategy during Fed-Batch Process. Appl. Microbiol. Biotechnol. 2002, 60, 67–72. [Google Scholar] [CrossRef]
- Chan-u-tit, P.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. High Level Ethanol Production by Nitrogen and Osmoprotectant Supplementation under Very High Gravity Fermentation Conditions. Energies 2013, 6, 884–899. [Google Scholar] [CrossRef]
- Oliveira, M.R.B.; Douradinho, R.S.; Sica, P.; Mota, L.A.; Pinto, A.U.; Faria, T.M.; Baptista, A.S. Evaluation of Aerobic Propagation of Yeasts as Additional Step in Production Process of Corn Ethanol. Stresses 2024, 4, 380–392. [Google Scholar] [CrossRef]
- Sica, P.; Prado, L.M.L.M.; Granja, P.; Carvalho, E.M.D.; Mattos, E.D.C.; Calegari, R.P.; Silverio, M.; Martins, B.C.; Baptista, A.S. Effects of Energy Cane (Saccharum spp.) Juice on Corn Ethanol (Zea mays) Fermentation Efficiency: Integration towards a More Sustainable Production. Fermentation 2021, 7, 30. [Google Scholar] [CrossRef]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 1992, 8, 501–517. [Google Scholar] [CrossRef]
- Pierce, J.S.; For the Analysis Committee. Institute of brewing: Analysis committee measurement of yeast viability. J. Inst. Brew. 1970, 76, 442–443. [Google Scholar] [CrossRef]
- Koshimizu, L.H.; Cruz, M.R.M.; Gomez, E.I.V.; Netto, C.L.B.; Gonçalves, A.C.R.; Borzani, W. Assessment of the Concentration of Yeast Suspended in an Aqueous Medium by Measuring the Volume Occupied by the Cells. Saccharum 1982, 4, 14–16. [Google Scholar]
- Eith, C.; Kolb, M.; Rumi, A.; Seubert, A.; Viehweger, K.H. Practices in Ion Chromatography: An Introduction; Metrohm: Herisau, Switzerland, 2006. [Google Scholar]
- Zago, E.A.; Silva, L.F.L.F.; Bernardino, C.D.; Amorim, H.V. Analytical Methods for Controlling Alcohol and Sugar Production; Fermentec: Piracicaba, Brazil, 1996. [Google Scholar]
- Silva, A.P.M.d.; Sica, P.; Pires, L.d.A.N.; Spironello, L.; Mota, L.A.; Peixoto, G.T.; Calegari, R.P.; Basso, T.O.; Tonso, A.; Gomes, M.P.; et al. Integration of Corn and Cane for Ethanol Production: Effects of Lactobacilli Contamination on Fermentative Parameters and Use of Ionizing Radiation Treatment for Disinfection. Fermentation 2023, 9, 89. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. 5. The trehalose content of baker’s yeast during anaerobic fermentation. Biochem. J. 1956, 62, 177–183. [Google Scholar] [CrossRef]
- Brin, M. Transketolase: Clinical aspects. Methods Enzymol. 1966, 9, 506–514. [Google Scholar]
- Ushima, A.K.; Ribeiro, A.M.M.; Souza, M.E.P.; Santos, N.F. Energy Conservation in the Sugar and Alcohol Industry; IPT: São Paulo, Brazil, 1990; p. 796. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006; Available online: https://www.R-project.org/ (accessed on 26 March 2024).
| Parameters | Treatments | Initial | 1st Cycle | 2nd Cycle | 3rd Cycle | 4th Cycle | 5th Cycle |
|---|---|---|---|---|---|---|---|
| Cell viability (%) | T1 | 88.1 Aa (±0.4) | 85.4 BCc (±0.4) | 85.9 Bb (±0.4) | 84.0 Eb (±0.4) | 84.7 Db (±0.4) | 85.1 CDb (±0.4) |
| T2 | 88.3 Ba (±0.4) | 88.2 Ba (±0.4) | 88.3 Ba (±0.4) | 87.5 Ca (±0.4) | 88.7 ABa (±0.4) | 89.2 Aa (±0.4) | |
| T3 | 88.2 Aa (±0.4) | 87.1 Cb (±0.4) | 88.0 ABa (±0.4) | 87.6 BCa (±0.4) | 88.1 ABa (±0.4) | 88.4 Aa (±0.4) | |
| Cell Biomass (g L−1) | T1 | 15.0 Ea (±0.2) | 15.4 Cc (±0.2) | 15.8 Bc (±0.2) | 14.6 Dc (±0.2) | 15.5 Cc (±0.2) | 16.3 Ac (±0.2) |
| T2 | 15.2 Ea (±0.2) | 17.4 Ca (±0.2) | 19.1 Aa (±0.2) | 16.3 Da (±0.2) | 18.4 Ba (±0.2) | 19.1 Aa (±0.2) | |
| T3 | 15.0 Ea (±0.2) | 15.9 CDb (±0.2) | 16.3 Bb (±0.2) | 15.6 Db (±0.2) | 16.1 BCb (±0.2) | 16.6 Ab (±0.2) | |
| Residual total reducing sugars (g L−1) | T1 | - | 9.8 Aa (±0.2) | 6.5 Ba (±0.2) | 5.2 Cb (±0,2) | 4.1 Da (±0.2) | 3.7 Da (±0.2) |
| T2 | - | 8.8 Ab (±0.2) | 6.6 Ba (±0.2) | 5.8 Ca (±0.2) | 4.5 CDa (±0.2) | 3.4 Da (±0.2) | |
| T3 | - | 8.5 Ab (±0.2) | 5.1 Bb (±0.2) | 4.7 BCc (±0.2) | 4.5 CDa (±0.2) | 3.6 Da (±0.2) | |
| Glycerol (g L−1) | T1 | 0.0 Da (±0.0) | 7.5 Ca (±0.2) | 7.6 Ca (±0.2) | 7.7 Ca (±0.2) | 8.2 Ba (±0.2) | 8.7 Aa (±0.2) |
| T2 | 0.0 Da (±0.0) | 6.8 Cb (±0.2) | 6.9 CBb (±0.2) | 7.3 Bb (±0.2) | 7.9 Aa (±0.20) | 8.2 Ab (±0.2) | |
| T3 | 0.0 Ea (±0.0) | 6.5 Db (±0.2) | 6.3 Dc (±0.49) | 6.9 Cb (±0.55) | 7.3 Bb (±0.2) | 7.7 Ac (±0.2) | |
| Alcohol content (v v−1) | T1 | 0.0 Fa (±0.0) | 13.9 Eb (±0.2) | 14.8 Db (±0.1) | 15.2 Cb (±0.2) | 15.7 Ba (±0.2) | 16.2 Aa (±0.2) |
| T2 | 0.0 Fa (±0.0) | 13.4 Ec (±0.1) | 13.7 Dc (±0.1) | 14.6 Cc (±0.2) | 15.4 Ba (±0.1) | 15.8 Ab (±0.2) | |
| T3 | 0.0 Da (±0.0) | 15.8 Ca (±0.2) | 15.8 Ba (±0.2) | 15.8 Ba (±0.2) | 15.6 Ba (±0.2) | 16.4 Aa (±0.2) |
| Parameters | Treatments | Initial | 1st Cycle | 2nd Cycle | 3rd Cycle | 4th Cycle | 5th Cycle |
|---|---|---|---|---|---|---|---|
| Trehalose (g 100 g−1) | T1 | 11.1 Fa (±0.2) | 13.1 Ea (±0.2) | 15.1 Da (±0.2) | 16.2 Ca (±0.2) | 17.3 Ba (±0.2) | 18.5 Aa (±0.2) |
| T2 | 11.1 Fa (±0.2) | 12.6 Ea (±0.1) | 14.0 Da (±0.1) | 14.9 Ca (±0.2) | 15.9 Ba (±0.1) | 16.6 Ab (±0.1) | |
| T3 | 11.1 Da (±0.2) | 12.3 Ea (±0.1) | 13.7 Da (±0.2) | 14.5 Ca (±0.1) | 15.4 Ba (±0.1) | 16.3 Ac (±0.1) | |
| Succinic acid (g L−1) | T1 | 0.0 Fa (±0.0) | 2.9 Aa (±0.2) | 2.4 Ba (±0.2) | 1.9 Ca (±0.1) | 1.3 Da (±0.1) | 1.1 Ea (±0.1) |
| T2 | 0.0 Ea (±0.0) | 2.5 Ab (±0.1) | 2.0 Bb (±0.1) | 1.4 Cb (±0.1) | 1.2 Ca (±0.1) | 1.0 Da (±0.1) | |
| T3 | 0.0 Fa (±0.0) | 2.4 Ab (±0.2) | 1.6 Bc (±0.1) | 1.4 Cb (±0.1) | 1.2 Da (±0.1) | 1.0 Ea (±0.1) | |
| Acetic acid (g L−1) | T1 | 0.0 Ea (±0.0) | 2.5 Aa (±0.1) | 2.3 Ba (±0.1) | 1.5 Ca (±0.1) | 1.3 Da (±0.1) | 1.2 Da (±0.1) |
| T2 | 0.0 Ca (±0.0) | 1.9 Ab (±0.1) | 1.8 Ab (±0.1) | 0.8 Bb (±0.1) | 0.8 Bb (±0.1) | 0.7 Bb (±0.2) | |
| T3 | 0.0 Da (±0.0) | 1.8 Ab (±0.1) | 1.7 Ab (±0.1) | 0.8 Bb (±0.1) | 0.7 Bb (±0.1) | 0.5 Cb (±0.1) | |
| Fermentative yield (%) | T1 | - | 71.6 Eb (±0.5) | 76.2 Db (±0.5) | 78.5 Cb (±0.5) | 80.7 Ba (±0.5) | 83.6 Ab (±0.3) |
| T2 | - | 68.8 Ec (±0.5) | 70.5 Dc (±0.5) | 75.0 Cc (±0.5) | 79.1 Bb (±0.5) | 81.3 Ac (±0.5) | |
| T3 | - | 76.0 Ca (±0.5) | 81.3 Ba (±0.5) | 81.5 Ba (±0.5) | 80.5 Ba (±0.5) | 84.4 Aa (±0.4) | |
| Fermentation productivity (mL L−1 h−1) | T1 | - | 2.1 Cab (±0.1) | 2.2 CBb (±0.1) | 2.3 Bb (±0.1) | 2.3 Ba (±0.1) | 2.4 Aa (±0.1) |
| T2 | - | 2.0 Cb (±0.1) | 2.0 Cc (±0.1) | 2.2 Bc (±0.1) | 2.3 Ba (±0.1) | 2.4 Aa (±0.1) | |
| T3 | - | 2.2 Ca (±0.1) | 2.4 Aa (±0.1) | 2.4 Aa (±0.1) | 2.3 Ba (±0.1) | 2.4 Aa (±0.1) |
| Treatments | |
|---|---|
| T1 | VHG fermentation in the absence of aeration injection (0 v v−1 min−1) and absence of vitamin supplementation in the medium. |
| T2 | VHG fermentation in the presence of aeration (0.2 v v−1 min−1) and vitamin supplementation: 5 mg L−1 thiamine (B1), 5 mg L−1 nicotinic acid (B3), 5 mg L−1 pantothenic acid (B5), 5 mg L−1 pyridoxine (B6), and 1 mg L−1 para-aminobenzoic acid in the medium. |
| T3 | VHG fermentation in the absence of aeration injection (0 v v−1 min−1) and supplementation with 500 mg L−1 of silica in the medium. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.R.B.; Douradinho, R.S.; Sica, P.; Mota, L.A.; Pinto, A.U.; Faria, T.M.; Baptista, A.S. Silica Wort Supplementation as an Alternative for Yeast Stress Relief on Corn Ethanol Production with Cell Recycling. Stresses 2024, 4, 421-435. https://doi.org/10.3390/stresses4030028
Oliveira MRB, Douradinho RS, Sica P, Mota LA, Pinto AU, Faria TM, Baptista AS. Silica Wort Supplementation as an Alternative for Yeast Stress Relief on Corn Ethanol Production with Cell Recycling. Stresses. 2024; 4(3):421-435. https://doi.org/10.3390/stresses4030028
Chicago/Turabian StyleOliveira, Matheus Ribeiro Barbosa, Rafael Soares Douradinho, Pietro Sica, Layna Amorim Mota, Alana Uchôa Pinto, Tamires Marques Faria, and Antonio Sampaio Baptista. 2024. "Silica Wort Supplementation as an Alternative for Yeast Stress Relief on Corn Ethanol Production with Cell Recycling" Stresses 4, no. 3: 421-435. https://doi.org/10.3390/stresses4030028
APA StyleOliveira, M. R. B., Douradinho, R. S., Sica, P., Mota, L. A., Pinto, A. U., Faria, T. M., & Baptista, A. S. (2024). Silica Wort Supplementation as an Alternative for Yeast Stress Relief on Corn Ethanol Production with Cell Recycling. Stresses, 4(3), 421-435. https://doi.org/10.3390/stresses4030028






