Unleashing the Power of Fungi: Utilizing the Arbuscular Mycorrhizal Fungi Rhizophagus clarus to Mitigate Salinity Stress and Boost Cowpea Bean Productivity for Food Security
Abstract
1. Introduction
2. Results
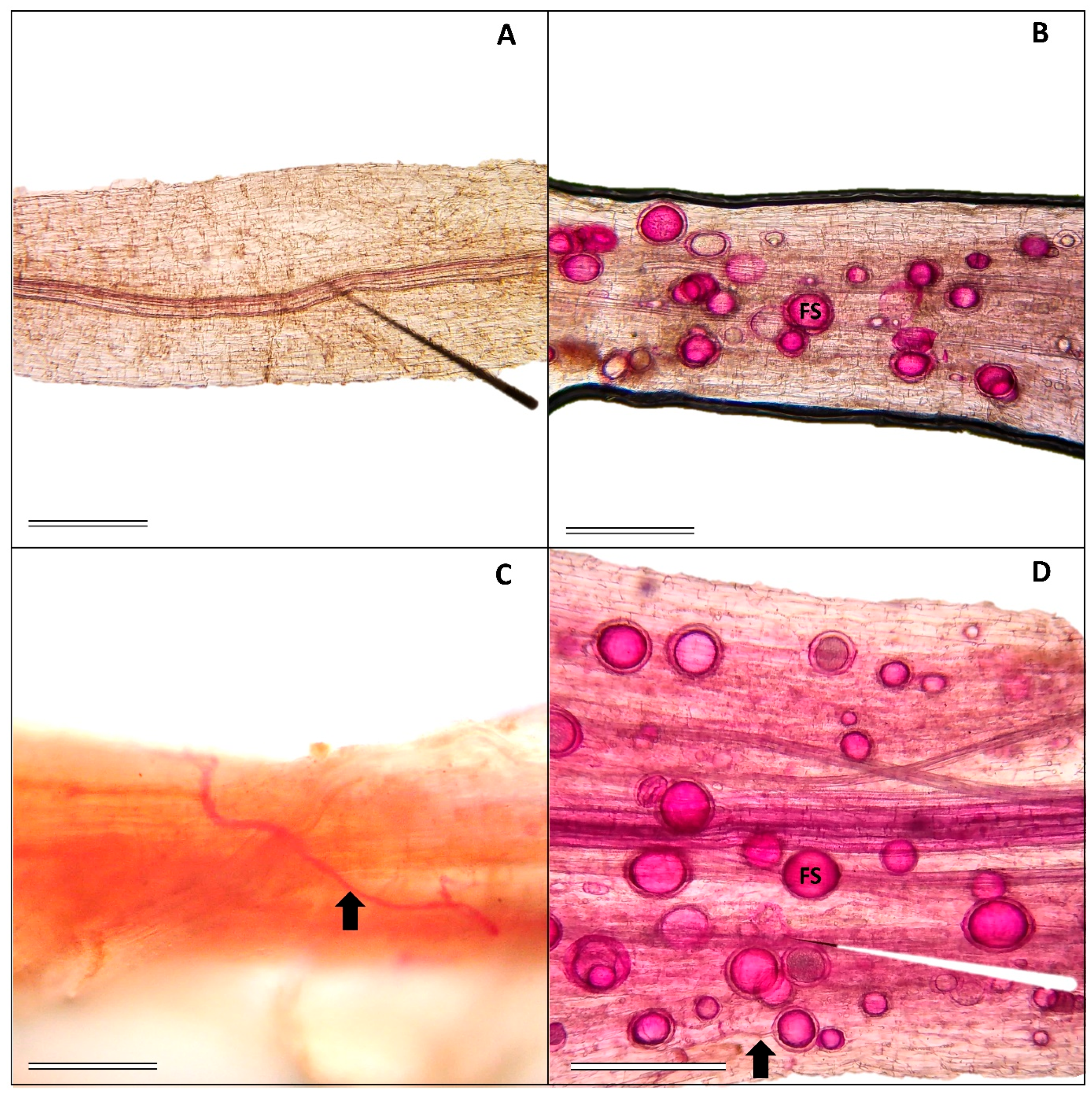
2.1. Mycorrhizal Colonization
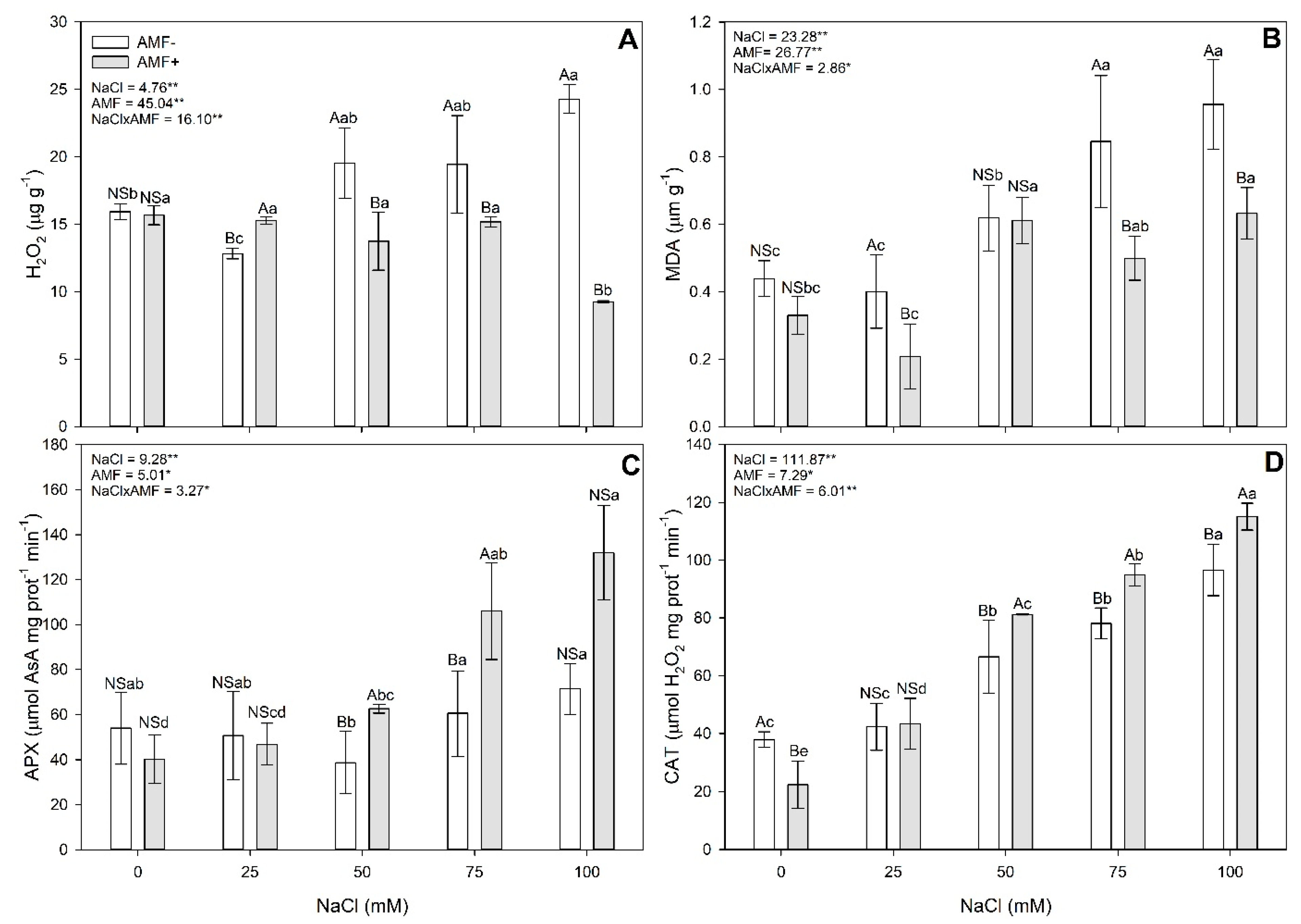
2.2. Biochemical Evaluations
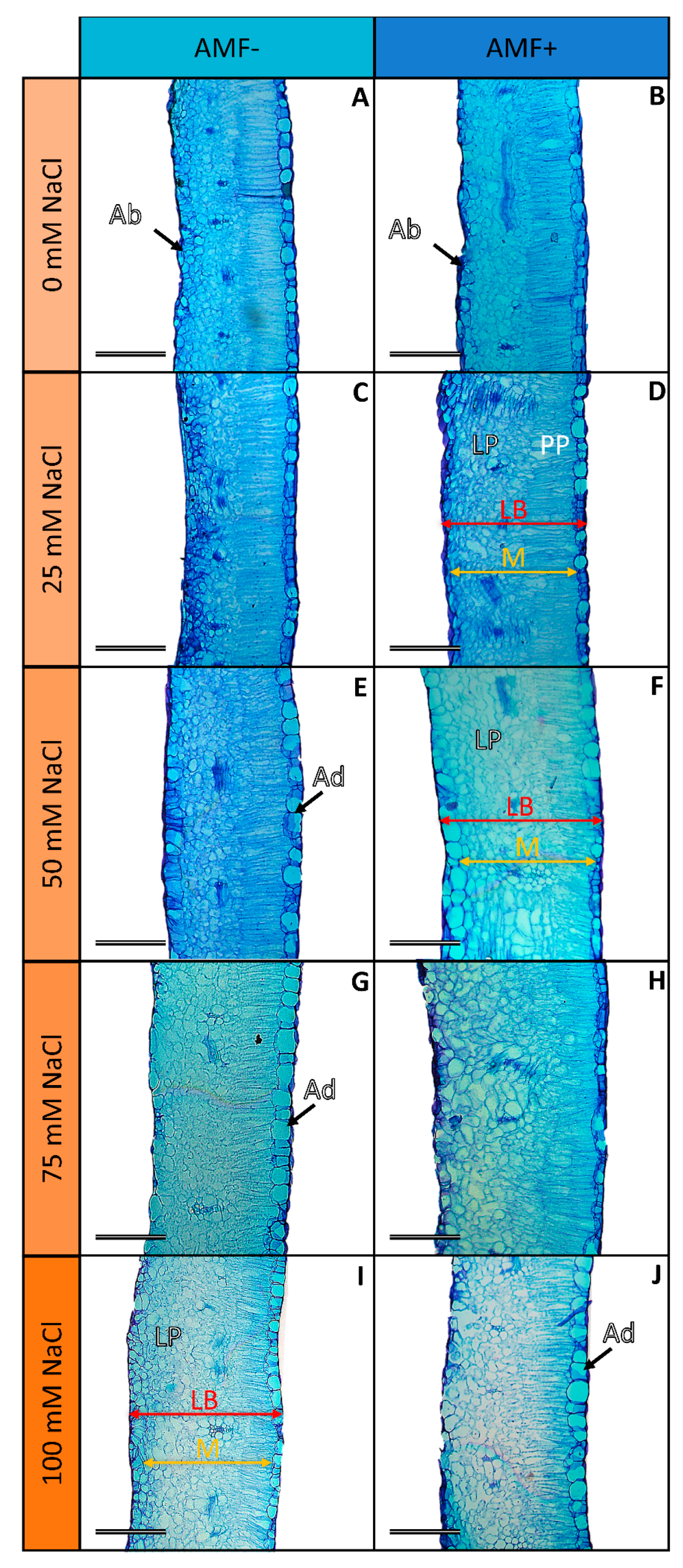
2.3. Leaf Anatomy
2.4. Nutrient Concentration in Leaves
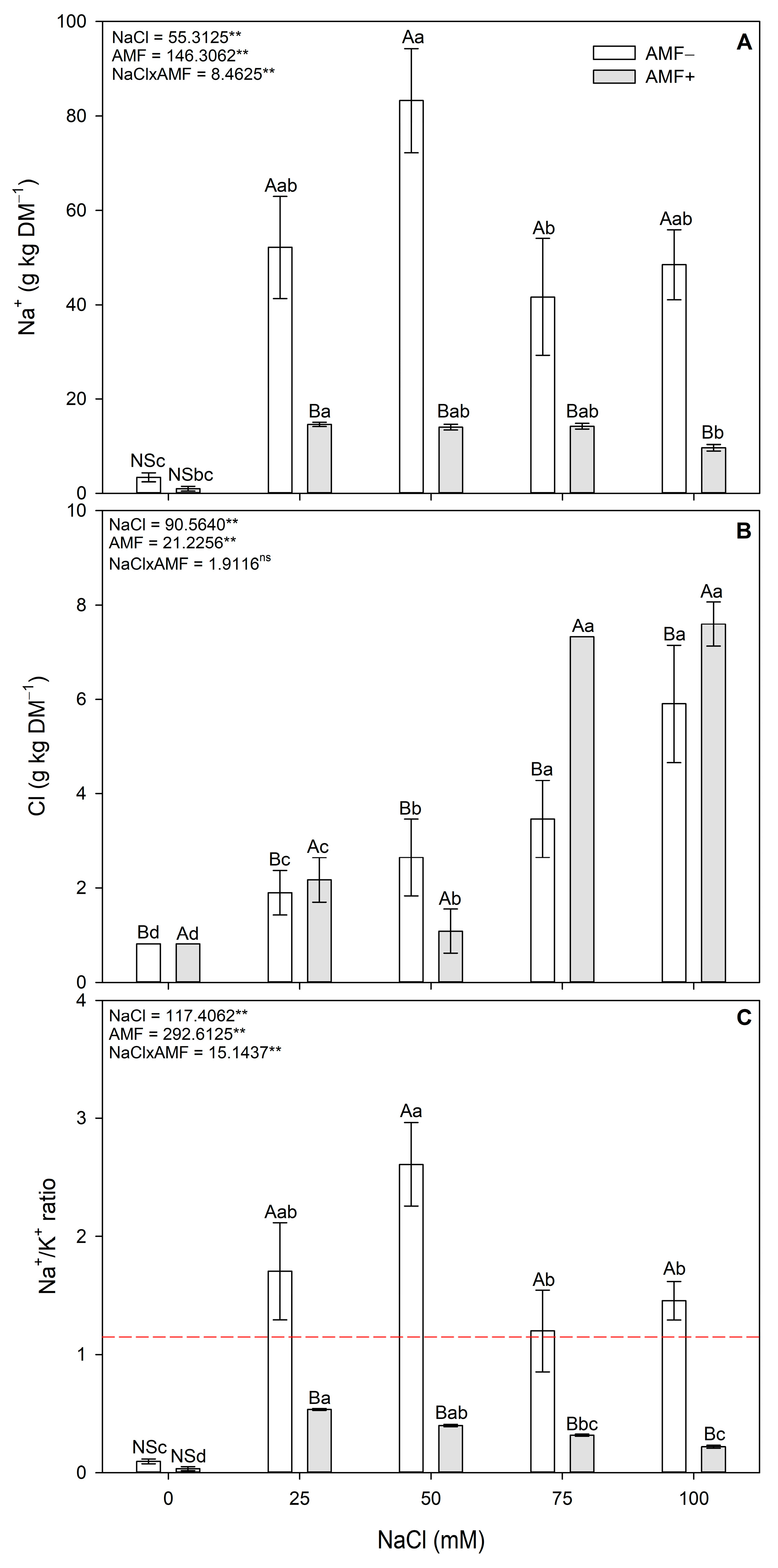
2.5. Ion Stress Markers
2.6. Grain and Biomass Yields
3. Material and Methods
3.1. Experimental Conditions
3.2. Evaluations
3.2.1. Mycorrhizal Colonization and Biochemical, Anatomical, and Nutritional Evaluations
3.2.2. Grain and Biomass Yields
3.3. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Pulses for Food Security and Nutrition: How Can Their Full Potential Be Tapped? FAO: Rome, Italy, 2016; Volume 2. [Google Scholar]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-perles, R.; Trindade, H.; Rosa, E.A.S.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef]
- Enyiukwu, D.; Amadioha, A.; Ononuju, C. Nutritional significance of cowpea leaves for human consumption. Greener Trends Food Sci. Nutr. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Omuto, C.; Vargas, R.; El Mobarak, A.; Mohamed, N.; Viatkin, K.; Yigini, Y. Mapping of Salt-Affected Soils—Technical Manual; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. CRC Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of Salt Stress on Growth, Physiological Parameters, and Ionic concentration of water dropwort (Oenanthe javanica) Cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Yao, X.-C.; Meng, L.-F.; Zhao, W.-L.; Mao, G.-L. Changes in the morphology traits, anatomical structure of the leaves and transcriptome in Lycium barbarum L. under salt stress. Front. Plant Sci. 2023, 14, 1090366. [Google Scholar] [CrossRef]
- Akcin, A.; Yalçın, E. The effect of salinity on anatomical characteristics of two halophyte species from Turkey. Bot. Lett. 2023, 170, 581–590. [Google Scholar] [CrossRef]
- Tagliaferre, C.; Guimarães, D.U.G.; Gonçalves, L.J.; Amorim, C.H.F.; Matsumoto, S.N.; D’arêde, L.O. Produtividade e tolerância do feijão caupi ao estresse salino. Irriga 2018, 23, 168–179. [Google Scholar] [CrossRef]
- Manaf, H.H.; Zayed, M.S. Productivity of cowpea as affected by salt stress in presence of endomycorrhizae and Pseudomonas fluorescens. Ann. Agric. Sci. 2015, 60, 219–226. [Google Scholar] [CrossRef]
- Kapoor, R.; Evelin, H.; Devi, T.S.; Gupta, S. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 450967. [Google Scholar] [CrossRef]
- Bisht, A.; Sharma, V.; Garg, N. Deciphering the role of arbuscular mycorrhizal fungi in mitigating the negative effects of abiotic stresses in legume crops. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Springer Nature: Singapore, 2024; pp. 337–361. [Google Scholar]
- Rabie, G.H.; Aboul-Nasr, M.B.; Al-Humiany, A. Increased salinity tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungus Glomus clarum and a nitrogen-fixer Azospirillum brasilense. Mycobiology 2005, 33, 51. [Google Scholar] [CrossRef][Green Version]
- de Carvalho Neta, S.J.; Araújo, V.L.V.P.; Fracetto, F.J.C.; da Silva, C.C.G.; de Souza, E.R.; Silva, W.R.; Lumini, E.; Fracetto, G.G.M. Growth-promoting bacteria and arbuscular mycorrhizal fungus enhance maize tolerance to saline stress. Microbiol. Res. 2024, 284, 127708. [Google Scholar] [CrossRef]
- Silva, G.A.E.; Siqueira, J.O.; Stürmer, S.L.; Moreira, F.M.S. Effectiveness of arbuscular mycorrhizal fungal isolates from the land uses of Amazon region in symbiosis with cowpea. An. Acad. Bras. Cienc. 2018, 90, 357–371. [Google Scholar] [CrossRef]
- Tavares, D.S.; Fernandes, T.E.K.; Rita, Y.L.; Rocha, D.C.; Sant’Anna-Santos, B.F.; Gomes, M.P. Germinative metabolism and seedling growth of cowpea (Vigna unguiculata) under salt and osmotic stress. S. Afr. J. Bot. 2021, 139, 399–408. [Google Scholar] [CrossRef]
- Dionísio, J.A.; Pimentel, I.C.; Signor, D.; de Paula, A.M.; Maceda, A.; Mattana, A.N.A.L. Guia Prático de Biologia do Solo, 1st ed.; de Lima, M.R., Neri, A., Grange, L., Genú, A.M., Moreira, A., Eds.; Sociedade Brasileira de Ciência do Solo/Núcleo Estadual Paraná: Curitiba, Brazil, 2016; ISBN 9788569146001. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Gomes, M.P.; Gonçalves, C.A.; Brito, J.C.M.; Souza, A.M.; Cruz, F.V.S.C.S.; Bicalho, E.M.; Figueredo, C.C.; Garcia, Q.S. Ciprofloxacin induces oxidative stress in duckweed (Lemna minor L.): Implications for energy metabolism and antibiotic-uptake ability. J. Hazard. Mater. 2017, 328, 140–149. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sant’Anna-Santos, B.F.; dos Santos, S.A.; Nunes, E.L.P.; Francino, D.M.T.; Carvalho Júnior, W.G.O. Does leaf anatomy aid in species identification of Butia (Arecaceae)? AoB Plants 2018, 10, ply046. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1940. [Google Scholar]
- Aguiar, T.V.; Sant’anna-Santos, B.F.; Azevedo, A.A.; Ferreira, R.S. ANATI QUANTI: Software de análises quantitativas para estudos em anatomia vegetal. Planta Daninha 2007, 25, 649–659. [Google Scholar] [CrossRef]
- Martins, A.P.L.; Reissmann, C.B. Material vegetal e as rotinas laboratoriais nos procedimentos químico-analíticos. Sci. Agrar. 2007, 8, 1–17. [Google Scholar] [CrossRef]
- Silva, E.B.; Nogueira, F.D.; Guimarães, P.T.G. Análise de Cloreto em Tecido Vegetal; UFLA: Lavras, Brazil, 1991; pp. 5–20. [Google Scholar]
- Mini, M.L.; Sathya, M.; Essa, M.M.; Al-sadi, A.M.; Jayachandran, K.S.; Anusuyadevi, M. Identification of salt-tolerant cowpea genotypes using ISSR markers and proteome analysis. Front. Biosci. 2019, 11, 130–149. [Google Scholar]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of salinity stress on chloroplast structure and function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-dependent inhibition of photosynthesis in willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Crutchik, D.; Sánchez, A.; Garrido, J.M. Simulation and experimental validation of multiple phosphate precipitates in a saline industrial wastewater. Sep. Purif. Technol. 2013, 118, 81–88. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Miransari, M. Arbuscular mycorrhizal fungi and soil salinity. In Mycorrhizal Mediation of Soil; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 263–277. ISBN 9780128043127. [Google Scholar] [CrossRef]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity response in chloroplasts: Insights from gene characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Nobel, P.S. Salinity effects on leaf anatomy: Consequences for photosynthesis. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef]
- Tuffi Santos, L.D.; Sant’Anna-Santos, B.F.; Meira, R.M.S.A.; Ferreira, F.A.; Tiburcio, R.A.S.; Machado, A.F.L. Leaf anatomy and morphometry in three eucalypt clones treated with glyphosate. Braz. J. Biol. 2009, 69, 129–136. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; Rabie, G.H.; Lamis, D.S.; Rabab, A.M. The impact of the arbuscular mycorrhizal fungi on growth and physiological parameters of cowpea plants grown under salt stress conditions. Int. J. Appl. Sci. Biotechnol. 2016, 4, 372–379. [Google Scholar] [CrossRef][Green Version]
- Abeer, H.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Induction of salt stress tolerance in cowpea [Vigna unguiculata (L.) Walp.] by arbuscular mycorrhizal fungi. Legum. Res. 2015, 38, 579–588. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Gomes, M.P.; Marques, R.Z.; Nascentes, C.C.; Scotti, M.R. Synergistic effects between arbuscular mycorrhizal fungi and rhizobium isolated from As-contaminated soils on the As-phytoremediation capacity of the tropical woody legume Anadenanthera peregrina. Int. J. Phytoremediation 2020, 22, 1362–1371. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Ruiz-LOZANO, J.M. Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant. Cell Environ. 2015, 38, 1613–1627. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, G.S.D.; Gheyi, H.R.; Melo, A.S.D.; Silva, P.C.C.; Soares, L.A.D.A.; Paiva, F.J.D.S.; Silva, S.S.D. Effect of combined potassium-phosphorus fertilization on gas exchange, antioxidant activity and fruit production of West Indian cherry under salt stress. Arid L. Res. Manag. 2022, 36, 163–180. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na + root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Posta, K.; Marschner, H.; Römheld, V. Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. Mycorrhiza 1994, 5, 119–124. [Google Scholar] [CrossRef]
- Gheyi, H.R.; da Dias, N.S.; de Lacerda, C.F.; Filho, E.G. Manejo da Salinidade Na Agricultura: Estudos Básicos e Aplicados [Internet]. 2016. Available online: https://ppgea.ufc.br/wp-content/uploads/2018/04/manejo-da-salinidade-na-agricultura.pdf (accessed on 30 April 2024).



| NaCl (mM) | P | K | Ca | Mg | S | Cu | Fe | Mn | Zn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | |
| 0 | 5.65 Bab | 8.50 Aa | 35.43 Aa | 30.06 Bc | 16.14 NSa | 16.31 NSa | 17.29 Aab | 14.56 Bab | 3.40 NSa | 3.57 NSa | 0.003 Bc | 0.012 Aa | 0.15 Bab | 1.23 Aa | 0.084 NScd | 0.075 NSab | 0.039 NSb | 0.034 NSc |
| 25 | 6.39 NSa | 6.33 NSbc | 30.80 Ab | 27.42 Bc | 14.61 NSab | 14.02 NSb | 16.59 Ab | 12.93 Bbc | 3.45 NSa | 3.29 NSa | 0.009 NSbc | 0.006 NSc | 0.15 Bb | 0.25 Aa | 0.135 Aa | 0.082 Ba | 0.034 NSbc | 0.038 NSb |
| 50 | 5.58 NSab | 6.69 NSbc | 31.91 Bb | 35.44 Ab | 14.6 NSab | 15.87 NSa | 18.66 Aa | 11.86 Bc | 2.36 NSb | 2.40 NSb | 0.009 NSab | 0.009 NSb | 0.15 NSab | 0.15 NSb | 0.109 Ab | 0.044 Bbc | 0.031 Bc | 0.038 Ab |
| 75 | 5.10 Bab | 7.58 Aab | 34.65 Ba | 44.98 Aa | 13.70 NSb | 12.90 NSb | 17.68 Aa | 15.55 Ba | 1.95 NSc | 2.09 NSc | 0.010 NSa | 0.008 NSbc | 0.15 NSb | 0.18 NSab | 0.102 Abc | 0.057 Bb | 0.053 Aa | 0.033 Bc |
| 100 | 4.67 Bb | 6.01 Ac | 33.19 Bab | 44.46 Aa | 12.98 Ac | 10.35 Bc | 17.79 Aa | 12.47 Bc | 1.70 NSc | 2.04 NSc | 0.01 NSa | 0.009 NSb | 0.21 Aa | 0.14 Bb | 0.078 Ad | 0.033 Bc | 0.037 Bb | 0.060 Aa |
| NaCl (mM) | SFM | SDM | RFM | RDM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | AMF− | AMF+ | |||||||||
| 0 | 14.49 | NSa | 14.21 | NSc | 2.14 | NSns | 2.01 | NSab | 10.56 | Ab | 4.87 | Bab | 1.35 | Aab | 0.41 | Bb |
| 25 | 13.74 | Bab | 21.25 | Aa | 2.13 | NSns | 3.35 | NSa | 15.54 | NSa | 11.41 | NSa | 1.49 | NSab | 1.36 | NSa |
| 50 | 13.30 | Bbc | 17.20 | Aab | 1.96 | NSns | 2.32 | NSa | 14.20 | NSab | 12.19 | NSa | 1.36 | NSab | 1.38 | NSa |
| 75 | 14.48 | Aa | 11.96 | Bc | 2.18 | NSns | 1.43 | NSb | 16.77 | Aa | 8.50 | Ba | 1.78 | Aa | 0.76 | Bab |
| 100 | 13.20 | Abc | 11.61 | Bc | 2.06 | NSns | 1.53 | NSb | 10.79 | Ab | 3.59 | Bb | 1.10 | NSb | 0.32 | NSb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, D.S.; Sant’Anna-Santos, B.F.; Gomes, M.P. Unleashing the Power of Fungi: Utilizing the Arbuscular Mycorrhizal Fungi Rhizophagus clarus to Mitigate Salinity Stress and Boost Cowpea Bean Productivity for Food Security. Stresses 2024, 4, 393-410. https://doi.org/10.3390/stresses4020026
Tavares DS, Sant’Anna-Santos BF, Gomes MP. Unleashing the Power of Fungi: Utilizing the Arbuscular Mycorrhizal Fungi Rhizophagus clarus to Mitigate Salinity Stress and Boost Cowpea Bean Productivity for Food Security. Stresses. 2024; 4(2):393-410. https://doi.org/10.3390/stresses4020026
Chicago/Turabian StyleTavares, Davi Santos, Bruno Francisco Sant’Anna-Santos, and Marcelo Pedrosa Gomes. 2024. "Unleashing the Power of Fungi: Utilizing the Arbuscular Mycorrhizal Fungi Rhizophagus clarus to Mitigate Salinity Stress and Boost Cowpea Bean Productivity for Food Security" Stresses 4, no. 2: 393-410. https://doi.org/10.3390/stresses4020026
APA StyleTavares, D. S., Sant’Anna-Santos, B. F., & Gomes, M. P. (2024). Unleashing the Power of Fungi: Utilizing the Arbuscular Mycorrhizal Fungi Rhizophagus clarus to Mitigate Salinity Stress and Boost Cowpea Bean Productivity for Food Security. Stresses, 4(2), 393-410. https://doi.org/10.3390/stresses4020026