Evaluation of Aerobic Propagation of Yeasts as Additional Step in Production Process of Corn Ethanol
Abstract
1. Introduction
2. Results
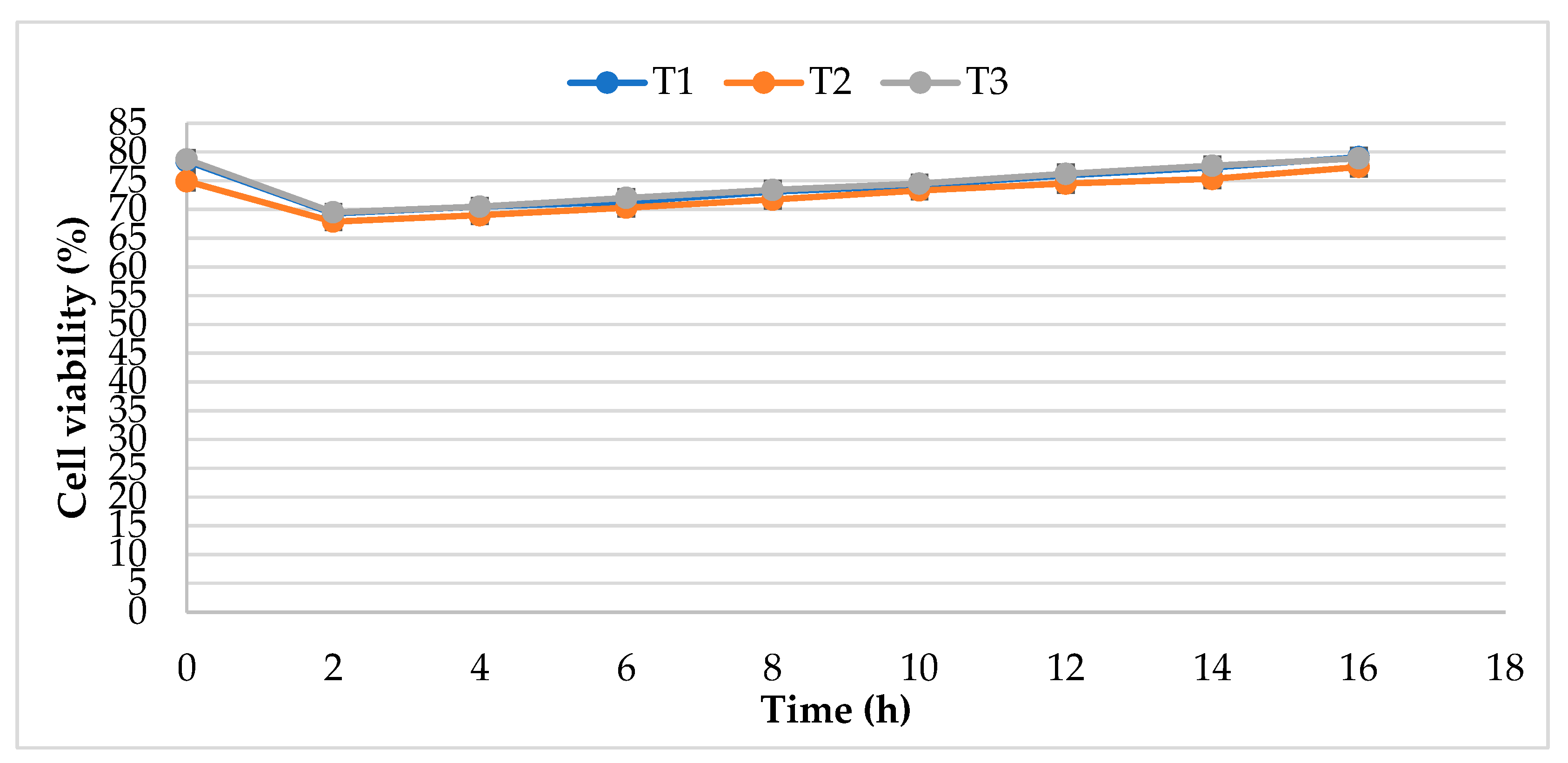
2.1. Cell Viability
2.2. Sugar Assimilation
2.3. Cellular Biomass
2.4. Alcohol Content
2.5. Cellular Yield (Y x/s)
2.6. Organic Acids
2.7. Trehalose
2.8. Glycerol
2.9. Protein
3. Discussion
4. Materials and Methods
4.1. Preparation of Wort
4.2. Treatments
4.3. Yeast Acclimation and Rehydration
4.4. Fed-Batch Cell Growth
- Fs (t) is a function of the total biomass of yeast occupying a given volume, [X (t) V (t)];
- µ (t): the specific growth rate;
- Yx/s (t): cell yield coefficient;
- Sf: the feed substrate concentration (g L−1);
- Sm: the substrate concentration at which the yield is the maximum (g L−1).
4.5. Cell Viability of Yeast
4.6. Determination of Cell Biomass and Protein
4.7. Determination of Total Sugars and Glycerol
4.8. Determination of Alcohol Content
4.9. Organic Acids
4.10. Trealose
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amata, I.A. Yeast a single cell protein: Characteristics and metabolism. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 158–170. [Google Scholar]
- Food and Agricultural Organization of the United Nations. How to Feed the World in 2050. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 26 April 2024).
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Stewart, G.G. The Yeast Handbook: Brewing and Distilling Yeasts; Energy Metabolism by the Yeast Cell; Springer International Publishing: Cham, Switzerland, 2017; Chapter 6; pp. 77–107. [Google Scholar]
- Imura, M.; Iwakiri, R.; Bamba, T.; Fukusaki, E. Metabolomics approach to reduce the Crabtree effect in continuous culture of Saccharomyces cerevisiae. J. Biosci. Bioeng. 2018, 126, 183–188. [Google Scholar] [CrossRef]
- Berg, J.M.; Stryer, L.; Tymoczko, J.L. Bioquímica; Guanabara Koogan: Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Belyea, R.L.; Rausch, K.D.; Tumbleson, M.E. Composition of corn and distillers dried grains with solubles from dry grind ethanol processing. Bioresour. Technol. 2004, 94, 293–298. [Google Scholar] [CrossRef]
- Lopes, M.L.; Paulillo, S.C.D.L.; Godoy, A.; Cherubin, R.A.; Lorenzi, M.S.; Giometti, F.H.C.; Bernardino, C.D.; Amorim Neto, H.B.D.; Amorim, H.V.D. Ethanol Production in Brazil: A Bridge between Science and Industry. Braz. J. Microbiol. 2016, 47, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Radosavljević, M.; Terzic, D.; Milašinović-Šeremešić, M.; Mojovic, L. Dried distillers’ grains with solubles (ddgs) produced from different maize hybrids as animal feed. J. Process. Energy Agric. 2014, 18, 80–83. [Google Scholar]
- Dewasme, L.; Srinivasan, B.; Perrier, M.; Vande Wouwer, A. Extremum-seeking algorithm design for fed-batch cultures of microorganisms with overflow metabolism. J. Process Control. 2011, 21, 1092–1104. [Google Scholar] [CrossRef]
- Mears, L.; Stocks, S.M.; Sin, G.; Gernaey, K.V. A review of control strategies for manipulating the feed rate in fed-batch fermentation processes. J. Biotechnol. 2017, 245, 34–46. [Google Scholar] [CrossRef]
- Ibáñez, F.; Saa, P.A.; Bárzaga, L.; Duarte-Mermoud, M.A.; Fernández-Fernández, M.; Agosin, E.; Pérez-Correa, J.R. Robust Control of Fed-Batch High-Cell Density Cultures: A Simulation-Based Assessment. Comput. Chem. Eng. 2021, 155, 107545. [Google Scholar] [CrossRef]
- Walker, G.M. Yeast Physiology and Biotechnology; Wiley: Chichester, UK, 1998; 321p. [Google Scholar]
- Pencheva, T.; Hristozov, I.; Huell, D.; Hitzmann, B.; Tzonkov, S. Modelling of Functional States during Saccharomyces cerevisiae Fed-batch Cultivation. Int. J. Bioautomation 2005, 2, 8–16. [Google Scholar]
- Assawajaruwan, S.; Kuon, F.; Funke, M.; Hitzmann, B. Feedback control based on NADH fluorescence intensity for Saccharomyces cerevisiae cultivations. Bioresour. Bioprocess. 2018, 5, 24. [Google Scholar] [CrossRef]
- Woehrer, W.; Roehr, M. Regulatory aspects of bakers’ yeast metabolism in aerobic fed-batch cultures. Biotechnol. Bioeng. 1981, 23, 567–581. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Enshasy, H.A. Effects of Different Aeration Rates and Feeding Strategies on Cell Growth and Invertase Production Kinetics by Saccharomyces boulardii. J. Sci. Ind. Res. 2018, 77, 575–582. [Google Scholar]
- Lim, H.C.; Shin, H.S. Fed-Batch Cultures. Principles and Applications of Semi-Batch Bioreactors; Cambridge University Press: New York, NY, USA, 2013; 457p. [Google Scholar]
- Hantelmann, K.; Kollecker, M.; Hüll, D.; Hitzmann, B.; Scheper, T. Two-dimensional fluorescence spectroscopy: A novel approach for controlling fed batch cultivations. J. Biotechnol. 2006, 121, 410–417. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Landi, C.; Cartenì, F.; Alteriis, E.; Giannino, F.; Paciello, L.; Parascandola, P. A novel process-based model of microbial growth: Self-inhibition in Saccharomyces cerevisiae aerobic fed-batch cultures. Microb. Cell Factories 2015, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Cooney, C.; Wang, D.I.C. Computer control of bakers’ yeast production. Biotechnol Bioeng. 1979, 21, 975–995. [Google Scholar] [CrossRef]
- Abbott, D.A.; Suir, E.; Duong, G.H.; de Hulster, E.; Pronk, J.T.; van Maris, A.J. Catalase overexpression reduces lactic acid-induced oxidative stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, P.J.; Depraetere, S.A.; Winderickx, J.; Delvaux, F.R.; Delvaux, F. The influence of yeast oxygenation prior to brewery fermentation on yeast metabolism and the oxidative stress response. FEMS Yeast Res. 2009, 9, 226–239. [Google Scholar] [CrossRef]
- Sonnleitner, B.; Käppeli, O. Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity: Formulation and verification of a hypothesis. Biotechnol Bioeng. 1986, 28, 927–937. [Google Scholar] [CrossRef]
- Postma, E.; Verduyn, C.; Scheffers, W.A.; Van Dijken, J.P. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1989, 55, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Basso, T.O.; Dario, M.G.; Tonso, A.; Stambuk, B.U.; Gombert, A.K. Insufficient uracil supply in fully aerobic chemostat cultures of Saccharomyces cerevisiae leads to respiro-fermentative metabolism and double nutrient-limitation. Biotechnol. Lett. 2010, 32, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 1992, 8, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tsurugi, K. A potential mechanism of energy-metabolism oscillation in an aerobic chemostat culture of the yeast Saccharomyces cerevisiae. FEBS J. 2006, 273, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Nissen, T.L.; Hamann, C.W.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 2000, 16, 463–474. [Google Scholar]
- Andre, Â.L.; Hemming, A.; Adler, L. Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol 3- phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Thevelein, J.M. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 1984, 48, 42–59. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Crumplen, R.; Stewart, G.G. The involvement of trehalose in yeast stress tolerance. J. Ind. Microbiol. 1991, 7, 191–195. [Google Scholar] [CrossRef]
- Aiba, S.; Nagai, S.; Nishizawa, Y. Fed batch culture of Saccharomyces cerevisiae: A perspective of computer control to enhance the productivity in baker’s yeast cultivation. Biotechnol. Bioeng. 1976, 18, 1001–1016. [Google Scholar] [CrossRef]
- Gélinas, P. Aeration and Foam Control in Baker’s Yeast Production: Mapping Patents. Compr. Rev. Food Sci. Food Saf. 2016, 15, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.J.; Rausch, K.D.; Yang, P.; Shapouri, H.; Belyea, R.L.; Tumbleson, M.E. Modified Dry Grind Ethanol Process; Publication of the Agricultural Engineering Department, University of Illinois: Urbana, IL, USA, 2001. [Google Scholar]
- Liu, K. Chemical Composition of Distillers Grains, a Review. J. Agric. Food Chem. 2011, 59, 1508–1526. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.; Nagodawithana, T.W. Yeast Technology; Van Nostrand Reinhold: New York, NY, USA, 1991; 378p. [Google Scholar]
- Kaspar von Meyenburg, H. Energetics of the budding cycle of Saccharomyces cerevisiae during glucose limited aerobic growth. Arch. Mikrobiol. 1969, 66, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, W.M. The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries; Nottingham University Press: Nottingham, UK, 2009; 541p. [Google Scholar]
- Douradinho, R.; Sica, P.; Oliveira, M.; Uchoa Pinto, A.; Mota, L.; Mattos, E.; Perecin, D.; Garcilasso, V.; de Almeida, J.M.A.R.; Piedade, S.; et al. Assessing Ionizing Radiation and Chlorine Dioxide (ClO2) as Potential Aseptization Treatments for Yeast Recycling on Mixed Wort of Corn and Sugarcane in Brazil. Stresses 2024, 4, 155–171. [Google Scholar] [CrossRef]
- Sica, P.; Prado, L.M.L.M.; Granja, P.; Carvalho, E.M.d.; Mattos, E.d.C.; Calegari, R.P.; Silverio, M.; Martins, B.C.; Baptista, A.S. Effects of Energy Cane (Saccharum spp.) Juice on Corn Ethanol (Zea mays) Fermentation Efficiency: Integration towards a More Sustainable Production. Fermentation 2021, 7, 30. [Google Scholar] [CrossRef]
- Pierce, J.S. Analysis committee measurement of yeast viability. J. Inst. Brew. 1970, 76, 442–443. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Eith, C.; Kolb, M.; Rumi, A.; Seubert, A.; Viehweger, K.H. Practices in Ion Chromatography: An Introduction; Metrohm: Herisau, Switzerland, 2006. [Google Scholar]
- Zago, E.A.; Silva, L.F.L.F.; Bernardino, C.D.; Amorim, H.V. Analytical Methods for Controlling Alcohol and Sugar Production; Fermentec: Piracicaba, Brazil, 1996. [Google Scholar]
- Silva, A.P.M.d.; Sica, P.; Pires, L.d.A.N.; Spironello, L.; Mota, L.A.; Peixoto, G.T.; Calegari, R.P.; Basso, T.O.; Tonso, A.; Gomes, M.P.; et al. Integration of Corn and Cane for Ethanol Production: Effects of Lactobacilli Contamination on Fermentative Parameters and Use of Ionizing Radiation Treatment for Disinfection. Fermentation 2023, 9, 89. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. 5. The trehalose content of baker’s yeast during anaerobic fermentation. Biochem. J. 1956, 62, 177–183. [Google Scholar] [CrossRef]
- Brin, M. Transketolase: Clinical aspects. Methods Enzymol. 1966, 9, 506–514. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006; Available online: https://www.R-project.org/ (accessed on 26 March 2024).

| Parameters | Treatments | |||
|---|---|---|---|---|
| Initial | T1 Final | T2 Final | T3 Final | |
| Cellular biomass (g L−1) | 9.00 d (±0.10) | 19.86 a (±0.30) | 18.07 b (±0.40) | 14.60 c (±0.30) |
| Sugar content in the reactor (g L−1) | 0.10 b (±0.02) | 0.00 a (±0.00) | 0.00 a (±0.00) | 0.00 a (±0.00) |
| Alcohol content (v v−1) | 0.00 d (±0.00) | 1.08 b (±0.10) | 1.35 a (±0.10) | 0.53 c (±0.10) |
| Cellular yield (Y x/s) (g g−1) | 0.00 d (±0.00) | 0.25 a (±0.00) | 0.23 b (±0.00) | 0.18 c (±0.00) |
| Ethanol yield (Y p/s) (g g−1) | 0.00 b (±0.00) | 0.01 a (±0.00) | 0.02 a (±0.00) | 0.01 a (±0.00) |
| Succinic acid (g L−1) | 0.00 d (±0.00) | 0.70 c (±0.05) | 0.96 b (±0.06) | 1.16 a (±0.05) |
| Acetic acid (g L−1) | 0.00 d (±0.00) | 0.11 c (±0.05) | 0.33 b (±0.05) | 0.84 a (±0.06) |
| Lactic acid (g L−1) | 0.00 a (±0.00) | 0.00 a (±0.00) | 0.00 a (±0.00) | 0.00 a (±0.00) |
| Trehalose (g 100 mL−1) | 9.09 d (±0.00) | 11.11 c (±0.20) | 11.67 b (±0.20) | 13.41 a (±0.25) |
| Glycerol (g L−1) | 0.00 c (±0.00) | 4.08 b (±0.12) | 4.12 b (±0.15) | 4.88 a (±0.17) |
| Protein (%) | 52.50 b (±0.00) | 53.00 a (±0.20) | 53.20 a (±0.20) | 53.30 a (±0.30) |
| Treatments | |
|---|---|
| T1 | Cell growth at 0.5 volume of air per volume of wort per minute (v v−1 min−1) |
| T2 | Cell growth at 1.0 volume of air per volume of wort per minute (v v−1 min−1) |
| T3 | Cell growth at 1.5 volume of air per volume of wort per minute (v v−1 min−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.R.B.; Douradinho, R.S.; Sica, P.; Mota, L.A.; Pinto, A.U.; Faria, T.M.; Baptista, A.S. Evaluation of Aerobic Propagation of Yeasts as Additional Step in Production Process of Corn Ethanol. Stresses 2024, 4, 380-392. https://doi.org/10.3390/stresses4020025
Oliveira MRB, Douradinho RS, Sica P, Mota LA, Pinto AU, Faria TM, Baptista AS. Evaluation of Aerobic Propagation of Yeasts as Additional Step in Production Process of Corn Ethanol. Stresses. 2024; 4(2):380-392. https://doi.org/10.3390/stresses4020025
Chicago/Turabian StyleOliveira, Matheus Ribeiro Barbosa, Rafael Soares Douradinho, Pietro Sica, Layna Amorim Mota, Alana Uchôa Pinto, Tamires Marques Faria, and Antonio Sampaio Baptista. 2024. "Evaluation of Aerobic Propagation of Yeasts as Additional Step in Production Process of Corn Ethanol" Stresses 4, no. 2: 380-392. https://doi.org/10.3390/stresses4020025
APA StyleOliveira, M. R. B., Douradinho, R. S., Sica, P., Mota, L. A., Pinto, A. U., Faria, T. M., & Baptista, A. S. (2024). Evaluation of Aerobic Propagation of Yeasts as Additional Step in Production Process of Corn Ethanol. Stresses, 4(2), 380-392. https://doi.org/10.3390/stresses4020025






